Key Points
Transient expansion of fetal megaerythroid progenitors in ERG/Gata1s mouse is biologically similar to Down syndrome TMD.
The N-terminal domain of GATA1 and the downregulation of ERG expression are essential for normal fetal erythropoiesis.
Abstract
Children with Down syndrome develop a unique congenital clonal megakaryocytic proliferation disorder (transient myeloproliferative disorder [TMD]). It is caused by an expansion of fetal megakaryocyte-erythroid progenitors (MEPs) triggered by trisomy of chromosome 21 and is further enhanced by the somatic acquisition of a mutation in GATA1. These mutations result in the expression of a short-isoform GATA1s lacking the N-terminal domain. To examine the hypothesis that the Hsa21 ETS transcription factor ERG cooperates with GATA1s in this process, we generated double-transgenic mice expressing hERG and Gata1s. We show that increased expression of ERG by itself is sufficient to induce expansion of MEPs in fetal livers. Gata1s expression synergizes with ERG in enhancing the expansion of fetal MEPs and megakaryocytic precursors, resulting in hepatic fibrosis, transient postnatal thrombocytosis, anemia, a gene expression profile that is similar to that of human TMD and progression to progenitor myeloid leukemia by 3 months of age. This ERG/Gata1s transgenic mouse model also uncovers an essential role for the N terminus of Gata1 in erythropoiesis and the antagonistic role of ERG in fetal erythroid differentiation and survival. The human relevance of this finding is underscored by the recent discovery of similar mutations in GATA1 in patients with Diamond-Blackfan anemia.
Introduction
Neonates with Down syndrome (DS) have hematologic abnormalities resulting from inappropriate fetal hematopoiesis,1 characterized by expansion of fetal liver (FL) megakaryocyte-erythroid progenitors (MEPs) in second-trimester fetuses.2,3 In ∼5% of children with DS, this expansion is dramatically enhanced by an acquired mutation in the X-chromosome transcription factor GATA1, resulting in a congenital transient clonal myeloproliferative disorder (TMD).4-6
GATA1 regulates the megakaryocytic-erythroid lineages.7,8 Inherited mutations in the N-terminal Zinc finger domains cause X-linked dyserythropoietic anemia and thrombocytopenia.9 The GATA1 mutations found in DS-TMD cause the expression of a shorter truncated isoform (GATA1s) lacking the N-terminal transactivation domain. This protein is normally coexpressed with full-length GATA1 due to an alternative translation initiation site or alternative splicing.10 Mice expressing only Gata1s exhibit transient early fetal megakaryocytic hyperproliferation without perinatal abnormalities.11 This hyperproliferative was recently attributed to removal of inhibitory activity of the Gata1 N-terminal domain on E2F.12
The unique TMD of DS suggests a specific collaboration between the expansion of fetal MEPs caused by trisomy 21 and the differentiation arrest and hyperproliferation conferred by Gata1s.13 Several chromosome 21 gene products have been suggested to cooperate with GATA1s in the generation of DS-TMD.2,6,12,14-18 Because a trisomy causes overexpression of many genes,19 it is possible that cooperation between several genes on the trisomic chromosome generates the hematopoietic phenotype.
The role of the chromosome 21 ETS transcription factor ERG20 in hematopoiesis21 and leukemia22 places it as a potential regulator of MEP expansion in DS fetuses and a collaborator with GATA1s in TMD. ERG is expressed in megakaryocytic and erythroid progenitors and is downregulated upon their differentiation.6 ERG is essential for maintenance of fetal hematopoietic stem cells (HSCs).21,23 It is one of a heptad of transcription factors, bound in combinatorial interactions to HSC enhancers.24 Developmental cooperation between ERG and GATA1 has been suggested by demonstrating their cobinding to regulatory elements of key hematopoietic genes such as Scl/Tal1.24 ERG is also a known oncogene involved in chromosomal translocations in leukemia, Ewing sarcoma, and prostate cancer.25,26 We demonstrated in vitro and in vivo that ERG is a megakaryocytic oncogene that cooperates with Gata1s in transforming mouse megakaryocytic progenitors.6,16,17 Further support to the hematopoietic role of ERG was recently given by an experiment in which reversing ERG trisomy to functional disomy in the Ts65Dn mouse model of DS corrected the myeloproliferative syndrome observed in these adult mice.27 Yet, unlike human DS fetuses, no prenatal hematopoietic abnormalities were reported in Ts65Dn mice.
To study the putative fetal developmental hematopoietic cooperation between ERG and Gata1s, we generated double transgenic mice expressing hERG and Gata1s. We show that similar to trisomy 21 in DS, increased expression of ERG by itself is sufficient to induce megakaryopoiesis and expansion of MEPs in FLs. We also demonstrate that expression of Gata1s together with ERG further enhances and prolongs hyperproliferation of fetal MEP and megakaryocytic progenitors, generating a human TMD-like gene expression profile and liver pathology. Strikingly, most male transgenic ERG/Gata1s did not survive beyond embryonic day 12.5 due to severe anemia, revealing the necessity of the N terminus of Gata1 and of downregulation of ERG for fetal erythropoiesis.
Materials and methods
Generation of double ERG/Gata1s transgenic mouse
The ERG transgene was generated as described previously.28 In brief, the human ERG3 hematopoietic isoform was cloned into the HS21/45-vav vector replacing hCD4.29 Double transgenic mice expressing both ERG and Gata1s were generated by crossing TgERG males with homozygous Gata1s knock-in females.11 Animal studies were approved by the institutional animal care and use committee of the Chaim Sheba Medical Center at Tel Hashomer.
Isolation and immunostaining of FL, hematopoietic stem, and progenitor cells
FL single-cell suspensions were washed in phosphate-buffered saline (PBS) with 0.05M EDTA, 0.1% bovine serum albumin. Lin progenitors were isolated using the lineage cell depletion kit (Miltenyi Biotec). Cells were stained with phycoerythrin–Cy7–conjugated C-kit, fluorescein isothiocyanate–conjugated Sca1, allophycocyanin (APC)–conjugated FCγR, and phycoerythrin-conjugated CD34 (eBioscience). Erythroid FL cells were stained with fluorescein isothiocyanate–conjugated Ter119 and APC-conjugated CD71 (eBioscience). For apoptosis assays, cells were stained with APC-conjugated annexin V (Enzo Life Sciences), washed with PBS, 1% fetal bovine serum, resuspended with propidium iodide (5 μg/mL), and were analyzed by flow cytometry (FACSCalibur; BD Biosciences). Flow cytometry data were analyzed by Flowjo software.
Colony-forming assay
FL single cells were cultured in methylcellulose (Stem Cell Technologies). For megakaryocytic colonies, cells were plated in Methocult 3231 supplemented with 50 ng/mL thrombopoietin (TPO) (PeproTech) and scored 5 to 7 days later. For erythroid colonies, cells were plated in Methocult 3334 containing 3 U/mL erythropoietin (EPO) and colonies scored 7 to 10 days later.
RNA preparation
Total cellular RNA was prepared using TRIzol (Life Technologies). Complementary DNA was generated using the Verso cDNA kit (Thermo Scientific). Real-time quantitative polymerase chain reaction (PCR) was done using SYBR Green and a Prism 7900HT Fast Sequence Detection System (Applied Biosystems).
Gene expression profiling
Experiments were performed using Affymetrix Mouse gene 1.0 ST oligonucleotide arrays (Affymetrix) with total RNA from each FL sample (GSE46481) (supplemental Methods, available on the Blood website).
G1ME and FL cell infection and western blots
G1ME cells30 and FL cells were infected with MIGR1-GATA1, MIGR1-GATA1s, or vector alone as described.31 Two days after infection, RNA was extracted and cell lysates were generated and the proteins were separated by gel electrophoresis. Anti–GATA-1 (sc-1234), HA-tag (sc-7392), FOG-1 (sc-9361), GATA-2 (sc-9008), and HSC70 (sc-7298) antibodies were purchased from Santa Cruz Biotechnology. The anti-Myb antibody (05-175) was purchased from Millipore.
Benzidine staining and cytospins
FL cells were stained with benzidine as described.32 Cytospins were generated by centrifuging 5 × 105 cells at 600 rpm for 10 minutes on glass slides and then Giemsa staining (Sigma-Aldrich).
Reticulin staining
FL tissues were fixed in 4% neutral-buffered formalin and paraffin-embedded. Reticulin staining was carried out using conventional techniques.
Results
Fetal expansion of megakaryocytic-erythroid and megakaryocytic progenitors in an ERG/Gata1s mouse model
We generated transgenic mice expressing the human ERG3 hematopoietic isoform6 regulated by the vav promoter that is expressed in all hematopoietic lineages starting at embryonic day 9.5 (E9.5).33 Four transgenic lines were generated with a similar phenotype. ERG expression was detected in yolk sacs from E10.5 and in FLs (supplemental Figure 1). The level of TgERG expression was comparable to the endogenous ERG expression levels in human cord blood CD34+ cells and in acute megakaryocytic leukemia (AMKL) cell lines (supplemental Figure 1). To model the fetal hematopoietic events of DS, TgERG male mice were crossed with homozygous Gata1s knock-in females.11 This cross generates 4 genotypes (Figure 1): females are heterozygous and males are hemizygous for Gata1s while 50% of the animals express the hERG transgene.
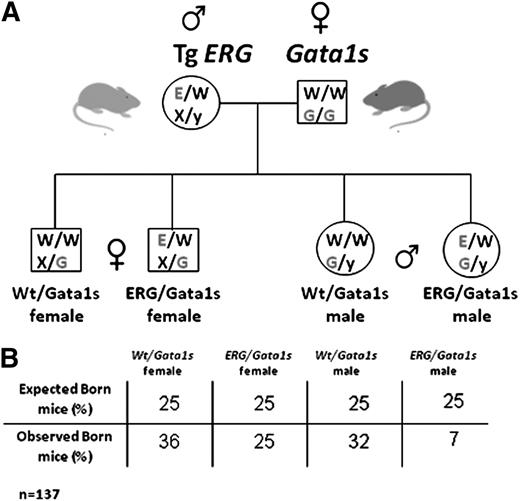
Generation of ERG/Gata1s mice. (A) TgERG males were mated with Gata1s knock-in homozygous females to generate animals that express the hERG transgene and the short isoform of GATA1, GATA1s. As GATA1 is located on the X chromosome; male offspring from this cross are all hemizygous and females are all heterozygous for GATA1s. Half of the animals carry the ERG transgene. (B) A table representing the observed vs the expected born mice from the different genotypes. Statistical significance was tested using the Fisher exact test (P < .01 for ERG/Gata1s males and P = .09 for ERG/Gata1s females; n(total) = 137) and the χ2 test (P = .0047).
Generation of ERG/Gata1s mice. (A) TgERG males were mated with Gata1s knock-in homozygous females to generate animals that express the hERG transgene and the short isoform of GATA1, GATA1s. As GATA1 is located on the X chromosome; male offspring from this cross are all hemizygous and females are all heterozygous for GATA1s. Half of the animals carry the ERG transgene. (B) A table representing the observed vs the expected born mice from the different genotypes. Statistical significance was tested using the Fisher exact test (P < .01 for ERG/Gata1s males and P = .09 for ERG/Gata1s females; n(total) = 137) and the χ2 test (P = .0047).
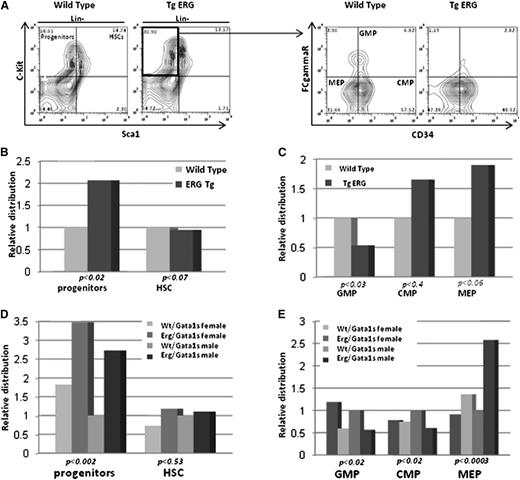
In humans, constitutional trisomy 21 causes a marked expansion of fetal MEPs preceding occurrence of the GATA1s mutation.2,3 We therefore examined whether increased ERG expression affects fetal hematopoietic progenitors. Lin− cells isolated from E14.5 FLs were immunostained for c-Kit and Sca1 to distinguish between HSCs (Lin−, Sca1+, c-Kit+) and hematopoietic progenitor cells (HPCs) (Lin−, Sca1−, c-Kit+).34 HPCs but not HSCs were significantly expanded in FLs from TgERG embryos compared with Wt littermates (Figure 2A-B). ERG overexpression in FLs of E14.5 embryos caused a nearly twofold expansion of MEPs (FcRγ−, CD34−)34 (Figure 2A,C) compared with Wt embryos and a smaller increase in common myeloid progenitors (CMPs) (FcRγ−, CD34+). MEP expansion was on the expense of granulocyte/monocyte progenitors (GMPs) (FcRγ+, CD34+) as a significant decrease in these progenitors was observed in TgERG embryos. Hence, increased expression of ERG during fetal hematopoiesis resulted in significant increase in MEPs, similar to the expansion observed in DS fetuses.2,3
Expansion of MEPs in TgERG embryo FLs is enhanced by Gata1s. (A) Representative flow cytometric analysis showing immunostaining of lineage-negative (Lin−) cells. HSCs are c-Kit+ and Sca1+ and HPCs are c-Kit+ and Sca−. HPCs were subclassified into GMPs (FcγR+ and CD34+), CMPs (FcγR− and CD34+), and MEPs (FcγR− and CD34−), respectively. (B) Relative progenitor and stem cell populations in wild-type and TgERG E14.5 FLs. (C) Distribution of myeloid progenitors in wild-type and TgERG E14.5 FLs. (D) Relative progenitor and stem cell populations in Wt/Gata1s and ERG/Gata1s E14.5 FLs. (E) Distribution of myeloid progenitors in Wt/Gata1s and ERG/Gata1s E14.5 FLs. The bar graphs represent the average of at least 3 independent experiments with n >10 embryos for each genotype. Statistical significant differences (t test for pairs and ANOVA for groups) are detailed in the figure. ANOVA, analysis of variance.
Expansion of MEPs in TgERG embryo FLs is enhanced by Gata1s. (A) Representative flow cytometric analysis showing immunostaining of lineage-negative (Lin−) cells. HSCs are c-Kit+ and Sca1+ and HPCs are c-Kit+ and Sca−. HPCs were subclassified into GMPs (FcγR+ and CD34+), CMPs (FcγR− and CD34+), and MEPs (FcγR− and CD34−), respectively. (B) Relative progenitor and stem cell populations in wild-type and TgERG E14.5 FLs. (C) Distribution of myeloid progenitors in wild-type and TgERG E14.5 FLs. (D) Relative progenitor and stem cell populations in Wt/Gata1s and ERG/Gata1s E14.5 FLs. (E) Distribution of myeloid progenitors in Wt/Gata1s and ERG/Gata1s E14.5 FLs. The bar graphs represent the average of at least 3 independent experiments with n >10 embryos for each genotype. Statistical significant differences (t test for pairs and ANOVA for groups) are detailed in the figure. ANOVA, analysis of variance.
To study the consequences of ERG and Gata1s interactions on expansion and distribution of E14.5 FL progenitors, these immunostainings were repeated on Lin− cells isolated from E14.5 FLs of Wt/Gata1s and ERG/Gata1s fetuses. Similar to the TgERG phenotype, ERG/Gata1s FLs exhibited extensive expansion of MEPs (Figure 2D-E). The magnitude of the expansion was higher in ERG/Gata1s fetuses than those expressing ERG alone (compare the scales in Figure 2, B-C to D-E; P < .05 for progenitors and P < .06 for MEPs), demonstrating an additive effect of the 2 transcription factors.
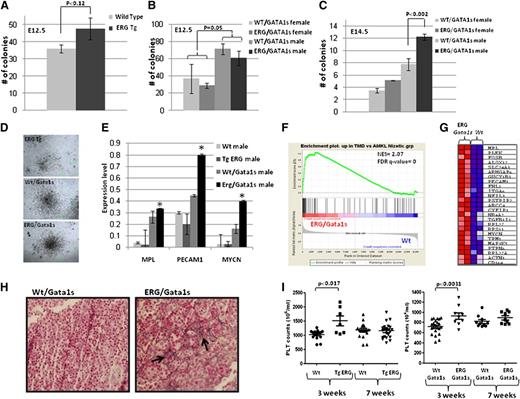
Both ERG and GATA1s are implicated in megakaryopoiesis.6,7,16,17,21 As TMD is a perinatal megakaryocytic proliferation syndrome, we next examined their effect on fetal megakaryopoiesis. FL cells were isolated from TgERG and Wt littermates and from Wt/Gata1s and ERG/Gata1s mice, and cultured in methylcellulose supplemented with TPO for growth of megakaryocytic colonies (colony forming unit megakaryocyte [CFU-MK]) (Figure 3A-D). Consistent with previous reports, we observed increased formation of CFU-MK colonies in E12.5 FL cells expressing Gata1s, as males (which are hemizygous for Gata1s) formed twice the number of colonies compared with females that express the Gata1 Wt isoform (Figure 3B). At E12.5, the presence of the ERG transgene led to a slight increase in MK colonies on a Gata1 wt background (Figure 3A) and caused no further enhancement of the effect of Gata1s (Figure 3B). This may result from the low level of ERG expression at E12.5 (supplemental Figure 1). In contrast, at E14.5, ERG significantly enhanced the number and size of Gata1s CFU-MK (Figure 3C-D). These observations indicate a synergistic effect of the 2 proteins in promoting fetal megakaryocytic proliferation.
ERG synergizes with Gata1s to promote transient megakaryocytic proliferation presenting a human TMD expression profile. (A-C) FL cells were isolated from E12.5 (A-B) and E14.5 (C) wild-type, TgERG embryos and males and females of Wt/Gata1s and ERG/Gata1s embryos. The cells were plated on methylcellulose supplemented with TPO to promote the growth of megakaryocytic colonies (CFU-MK). MK colonies were counted 5 to 7 days following plating. Each bar graph represents the average of at least 3 independent experiments. (D) Representative figure of megakaryocytic colonies from (top) TgERG, (middle) Wt/Gata1s, and (bottom) ERG/Gata1s FLs. (E) Real-time PCR of common human TMD genes (11). *Statistical significance was tested using the t test (P = .028 for Mpl; P = .009 for Pecam1; and P = .0 for Mycn). (F) Gene set enrichment analysis (GSEA) using gene expression of E14.5 ERG/Gata1s FL cells (GSE46481) compared with the respective Wt control shows significant enrichment of genes that were upregulated in human TMD (GSE4119). NES and FDR q values are shown. (G) Heat map showing the core enrichment genes from the GSEA presented in panel F. (H) Hepatic fibrosis in ERG/Gata1s male FL. Reticulin staining (arrowheads) of (left) Wt/Gata1s and (right) ERG/Gata1s FL tissues (magnification, ×400). (I) Transient thrombocytosis in TgERG and ERG/Gata1s mice. Platelet counts were retrieved from male and female TgERG and ERG/Gata1s and their Wt and Wt/Gata1s littermates at 3 and 7 weeks of age; n= at least 6 for each group. A significant difference was found between TgERG and Wt and between ERG/Gata1s and Wt/Gata1s (t test: P < .017, P < .0031, respectively). FDR, false detection rate; NES, normalized enrichment score.
ERG synergizes with Gata1s to promote transient megakaryocytic proliferation presenting a human TMD expression profile. (A-C) FL cells were isolated from E12.5 (A-B) and E14.5 (C) wild-type, TgERG embryos and males and females of Wt/Gata1s and ERG/Gata1s embryos. The cells were plated on methylcellulose supplemented with TPO to promote the growth of megakaryocytic colonies (CFU-MK). MK colonies were counted 5 to 7 days following plating. Each bar graph represents the average of at least 3 independent experiments. (D) Representative figure of megakaryocytic colonies from (top) TgERG, (middle) Wt/Gata1s, and (bottom) ERG/Gata1s FLs. (E) Real-time PCR of common human TMD genes (11). *Statistical significance was tested using the t test (P = .028 for Mpl; P = .009 for Pecam1; and P = .0 for Mycn). (F) Gene set enrichment analysis (GSEA) using gene expression of E14.5 ERG/Gata1s FL cells (GSE46481) compared with the respective Wt control shows significant enrichment of genes that were upregulated in human TMD (GSE4119). NES and FDR q values are shown. (G) Heat map showing the core enrichment genes from the GSEA presented in panel F. (H) Hepatic fibrosis in ERG/Gata1s male FL. Reticulin staining (arrowheads) of (left) Wt/Gata1s and (right) ERG/Gata1s FL tissues (magnification, ×400). (I) Transient thrombocytosis in TgERG and ERG/Gata1s mice. Platelet counts were retrieved from male and female TgERG and ERG/Gata1s and their Wt and Wt/Gata1s littermates at 3 and 7 weeks of age; n= at least 6 for each group. A significant difference was found between TgERG and Wt and between ERG/Gata1s and Wt/Gata1s (t test: P < .017, P < .0031, respectively). FDR, false detection rate; NES, normalized enrichment score.
The transient prenatal proliferation of megakaryoblasts in DS newborns represents a later stage of the progenitor expansion observed in DS FL.3,35 Therefore, we hypothesized that expanded fetal progenitors in ERG/Gata1s mice may have similar biological properties to human DS-TMD. To test this hypothesis, we profiled gene expression (supplemental Figure 2A) of FL RNA derived from E12.5 and E14.5 Wt, TgERG, Gata1s, and ERG/Gata1s pooled embryos. E14.5 FL cells from ERG/Gata1s mice demonstrated significantly increased expression of megakaryocytic genes (supplemental Table 1), including the Mpl gene and Pecam1 in addition to Mycn, that were previously associated with DS-TMD36 (Figure 3E). GSEA demonstrated significant enrichment of the ERG/Gata1s gene signature in DS-AMKL compared with non–DS-AMKL14 (supplemental Figure 2B) and in TMD36 vs DS-AMKL (Figure 3F-G), which was higher than the enrichment observed with Gata1s alone (supplemental Figure 2C).
Hepatic fibrosis is a major complication of human DS-TMD.37 Accordingly, liver fibrosis was demonstrated by reticulin stain in ERG/Gata1s FLs (Figure 3H). Postnatally, the transient feature of this fetal megakaryocytic progenitor expansion is evident by a transient increase in platelet counts in 3-week-old TgERG and ERG/Gata1s mice that was reduced to normal levels by 7 weeks (Figure 3I). Together, these phenotypic and genomic studies suggest that the coexpression of ERG and Gata1s causes a prenatal syndrome that is biologically similar to the TMD associated with DS.
Constitutive ERG expression uncovers a vital role for the N-terminal domain of GATA1 in fetal erythropoiesis
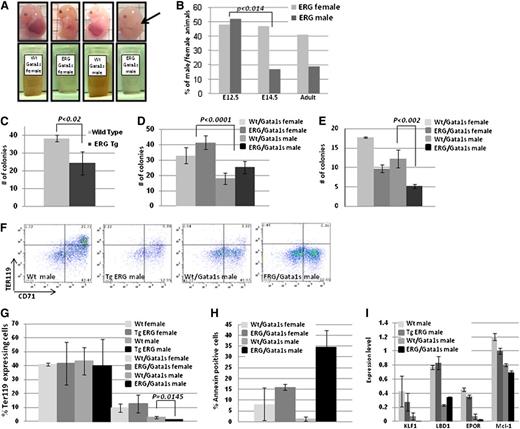
Genotyping offspring born to TgERG males and Gata1s homozygous females (Figure 1A) revealed a dramatic decrease in ERG/Gata1s live males (7%, expected 25%, P < .01) (Figure 1B). Gross examination of FLs at E14.5 revealed that the ERG/Gata1s males suffer from severe anemia; ERG/GATA1s FLs were considerably smaller than littermate FLs (Figure 4A arrow) and exhibited a paler cellular suspension (Figure 4A). Genotyping confirmed early embryonic death of ERG/Gata1s males between E12.5 and E14.5 (Figure 4B).
Requirement of the N terminus of GATA1 in fetal erythropoiesis. (A) Representative pictures of E14.5 embryos. The FL of ERG/Gata1s males is smaller and paler (arrow). A total of 1 × 107 FL cells were resuspended in PBS. The light color of the ERG/Gata1s FL cells points to the decrease number in mature erythrocytes in those embryos. (B) ERG/Gata1s males die between E12.5 and E14.5. Females and males generated from crossing heterozygous TgERG males with homozygous Gata1s KI females were tested for the presence of ERG transgene on E12.5, E14.5, and adults (n = 85, 98, and 137 for E12.5, E14.5, and adults, respectively). A significant difference in the TgERG male population was found between E12.5 and E14.5 (t test: P < .014). (C-E) ERG and Gata1s inhibit erythroid colony formation. FL cells were isolated from (C) E12.5 TgERG embryos and Wt littermates and (D-E) E12.5 and E14.5 embryos of ERG/Gata1s embryos and Wt/Gata1s littermates and were plated on methylcellulose supplemented with EPO to promote growth of erythroid colonies (BFU-E). Erythroid colonies (BFU-E) were counted 7 to 10 days after plating. Each bar graph represents the average of at least 3 independent experiments. Statistical significance was tested using the t test. (F) Representative figures of flow cytometric analysis of male E12.5 FL cells using the erythroid markers Ter119 and CD71. (G) Expression of Gata1s impairs erythropoiesis. Expression of Ter119 erythroid marker as measured by flow cytometry analysis in FL cells generated from E12.5 females and males of Wt, TgERG, Gata1s, and ERG/Gata1s animals. (H) Increase apoptosis in ERG/Gata1s males. Annexin V levels were measured by flow cytometry in Ter119-positive FL cells for the detection of apoptosis. FL cells were isolated from E12.5 Wt, TgERG, Wt/Gata1s, and ERG/Gata1s males. Significant increase in apoptosis in ERG/Gata1s males compared with Wt/Gata1s males was measured using the t test (P = .017). (I) Decreased expression of erythroid and antiapoptotic genes in ERG/Gata1s males. Expression level of the different genes was obtained from the Affymetrix mouse gene 1.0 ST chip array and confirmed by real-time PCR on at least 2 additional samples for each genotype.
Requirement of the N terminus of GATA1 in fetal erythropoiesis. (A) Representative pictures of E14.5 embryos. The FL of ERG/Gata1s males is smaller and paler (arrow). A total of 1 × 107 FL cells were resuspended in PBS. The light color of the ERG/Gata1s FL cells points to the decrease number in mature erythrocytes in those embryos. (B) ERG/Gata1s males die between E12.5 and E14.5. Females and males generated from crossing heterozygous TgERG males with homozygous Gata1s KI females were tested for the presence of ERG transgene on E12.5, E14.5, and adults (n = 85, 98, and 137 for E12.5, E14.5, and adults, respectively). A significant difference in the TgERG male population was found between E12.5 and E14.5 (t test: P < .014). (C-E) ERG and Gata1s inhibit erythroid colony formation. FL cells were isolated from (C) E12.5 TgERG embryos and Wt littermates and (D-E) E12.5 and E14.5 embryos of ERG/Gata1s embryos and Wt/Gata1s littermates and were plated on methylcellulose supplemented with EPO to promote growth of erythroid colonies (BFU-E). Erythroid colonies (BFU-E) were counted 7 to 10 days after plating. Each bar graph represents the average of at least 3 independent experiments. Statistical significance was tested using the t test. (F) Representative figures of flow cytometric analysis of male E12.5 FL cells using the erythroid markers Ter119 and CD71. (G) Expression of Gata1s impairs erythropoiesis. Expression of Ter119 erythroid marker as measured by flow cytometry analysis in FL cells generated from E12.5 females and males of Wt, TgERG, Gata1s, and ERG/Gata1s animals. (H) Increase apoptosis in ERG/Gata1s males. Annexin V levels were measured by flow cytometry in Ter119-positive FL cells for the detection of apoptosis. FL cells were isolated from E12.5 Wt, TgERG, Wt/Gata1s, and ERG/Gata1s males. Significant increase in apoptosis in ERG/Gata1s males compared with Wt/Gata1s males was measured using the t test (P = .017). (I) Decreased expression of erythroid and antiapoptotic genes in ERG/Gata1s males. Expression level of the different genes was obtained from the Affymetrix mouse gene 1.0 ST chip array and confirmed by real-time PCR on at least 2 additional samples for each genotype.
We therefore studied early fetal erythropoiesis in the transgenic embryos. E12.5 and E14.5 FL cells were isolated from females and males of Wt, TgERG, Wt/Gata1s, and ERG/Gata1s embryos and cultured in methylcellulose supplemented with EPO to promote erythroid colonies (burst-forming unit-erythroid [BFU-E]). In E12.5 Gata1s FLs, regardless of ERG expression, the number of erythroid colonies (BFU-E) was significantly lower in male FL cells, that are hemizygous for Gata1s (Figure 4D). The number of erythroid colonies (BFU-E) that were formed with E12.5 TgERG FLs was lower compared with Wt FL (Figure 4C). In E14.5, Gata1s and constitutive ERG expression synergized to inhibit BFU-E formation (Figure 4E). There was a significant drop in BFU-E colony number in FLs that express both Gata1s and ERG compared with the other genotypes.
To further study the effect of the 2 transcription factors on erythropoiesis, E12.5 FL cells were immunostained for expression of the erythroid marker Ter119 (Figure 4F-G). Gata1s expressing FLs had significantly reduced Ter119-expressing cells compared with their level in Wt FLs. The decrease in Ter119 cells was larger in hemizygous males (lacking a Wt Gata1 protein). Although the presence of ERG with a Wt Gata1 background had no effect on Ter119 cells, it added to the effect of Gata1s, leading to an almost complete absence of erythroid cells at E12.5 ERG/Gata1s males with only 1.4% of Ter119-expressing cells compared with 2.8% in Wt/Gata1s males (P = .0145) (Figure 4G). Furthermore, about one-third of Ter119 cells in ERG/Gata1s male FLs were apoptotic compared with 2% apoptosis found in the absence of the ERG transgene (P = .017) (Figure 4H). These phenotypes were reflected in gene expression profiles of E12.5 FL cells displaying a reduction in expression of erythroid genes (supplemental Tables 2-3), key erythroid transcription factors (Klf1, Ldb1), Epo receptor, and the antiapoptotic gene Mcl1 (Figure 4I).
Together, these observations demonstrate that the lack of the N terminus of Gata1 coupled with the constitutive expression of ERG block fetal erythropoiesis, resulting in severe anemia below the survival threshold for most ERG/Gata1s male embryos.
ERG synergizes with GATA1s to attenuate terminal erythroid differentiation
We next studied erythropoiesis in embryos surviving the initial block in erythropoiesis. Similar to E12.5 embryos, in E14.5 FL cells the generation of BFU colonies and the number of Ter119-positive cells were lower in Gata1s mice and were further reduced by the expression of ERG (Figure 4E, supplemental Figure 3A). These observations were accompanied by a decrease in the expression of erythroid genes (supplemental Figure 3B).
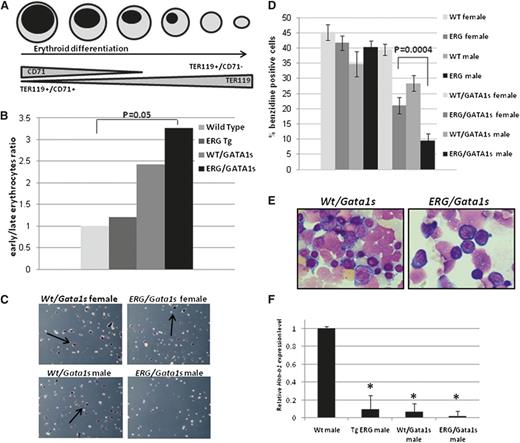
The combined expression of TER119 and CD71 can discriminate early and late differentiated erythroid cells (Figure 5A). The ratio between early (TER119+/CD71+) and late (TER119+/CD71−) erythroid cells was significantly higher in E14.5 ERG/Gata1s male FL cells (Figure 5B). Benzidine staining that measures hemoglobin carrying cells32 also showed that male ERG/Gata1s FL cells contain less differentiated erythrocytes as <10% of the cells were positive for benzidine staining compared with 20% observed in ERG/Gata1s FL female cells (P = .0004) (Figure 5C-D). Accordingly, cytospins show that ERG/Gata1s male FL cells contain mainly immature proerythroblasts (Figure 5E). Furthermore, the expression of the hemoglobin major β-chain, Hbb-b1, was reduced by 10-fold in the FLs of ERG- and Gata1s-expressing animals compared with Wt FLs (Figure 5F). The block in fetal erythroid differentiation was manifested by transient reduction in red blood cell and hemoglobin levels in peripheral blood of 3-week-old TgERG and ERG/Gata1s mice compared with Wt and Wt/Gata1s mice (supplemental Figure 4). Consistently, GSEA analysis using gene expression of human erythroid cells at different stages of differentiation38 shows enrichment of Wt/Gata1s and, more significantly, the ERG/Gata1s E14.5 FL gene signature in early erythroid cells (Figure 6A-B). Taken together, these results show that constitutive expression of ERG throughout erythroid differentiation and expression of Gata1s blocks terminal erythroid maturation.
Erythroid differentiation arrest in ERG/GATA1s mice. (A) Schematic representation of erythroid differentiation indicating early erythrocytes as Ter119+/CD71+ and late differentiated erythrocytes as Ter119+/CD71−. (B) Increase in early erythroblast population compared with late differentiated erythrocytes in ERG/Gata1s males. FL cells were isolated from E14.5 Wild-type, TgERG, Wt/Gata1s, and ERG/Gata1s male embryos and stained with Ter119 and CD71. Statistical significance between Wt embryos and the remaining genotypes was measured using the t test. (C-D) Reduced benzidine-stained cells in ERG/Gata1s males. Representative images of benzidine-stained FL cells (C) and a graph averaging the ratio of benzidine-positive cells in FLs isolated from E14.5 Wt, TgERG, Wt/Gata1s, and ERG/Gata1s male and female embryos (D). The bar graph represents the average of at least 3 experiments. (E) Giemsa stain of cytospins of E14.5 FL cells show a decrease in maturing erythroblasts and increase in immature proerythroblasts in ERG/Gata1s males compared with Wt/Gata1s males. (F) Expression of hemoglobin. Adult hemoglobin β major chain (Hbb-b1) expression was measured by real-time PCR in E14.5 FL cells from Wt, TgERG, Wt/Gata1s, and ERG/Gata1s males. *Significant difference from Wt hemoglobin expression was measured using the t test (P = .00037 for TgERG; P < .000001 for Wt/Gata1s; and P < .00001 for ERG/Gata1s).
Erythroid differentiation arrest in ERG/GATA1s mice. (A) Schematic representation of erythroid differentiation indicating early erythrocytes as Ter119+/CD71+ and late differentiated erythrocytes as Ter119+/CD71−. (B) Increase in early erythroblast population compared with late differentiated erythrocytes in ERG/Gata1s males. FL cells were isolated from E14.5 Wild-type, TgERG, Wt/Gata1s, and ERG/Gata1s male embryos and stained with Ter119 and CD71. Statistical significance between Wt embryos and the remaining genotypes was measured using the t test. (C-D) Reduced benzidine-stained cells in ERG/Gata1s males. Representative images of benzidine-stained FL cells (C) and a graph averaging the ratio of benzidine-positive cells in FLs isolated from E14.5 Wt, TgERG, Wt/Gata1s, and ERG/Gata1s male and female embryos (D). The bar graph represents the average of at least 3 experiments. (E) Giemsa stain of cytospins of E14.5 FL cells show a decrease in maturing erythroblasts and increase in immature proerythroblasts in ERG/Gata1s males compared with Wt/Gata1s males. (F) Expression of hemoglobin. Adult hemoglobin β major chain (Hbb-b1) expression was measured by real-time PCR in E14.5 FL cells from Wt, TgERG, Wt/Gata1s, and ERG/Gata1s males. *Significant difference from Wt hemoglobin expression was measured using the t test (P = .00037 for TgERG; P < .000001 for Wt/Gata1s; and P < .00001 for ERG/Gata1s).
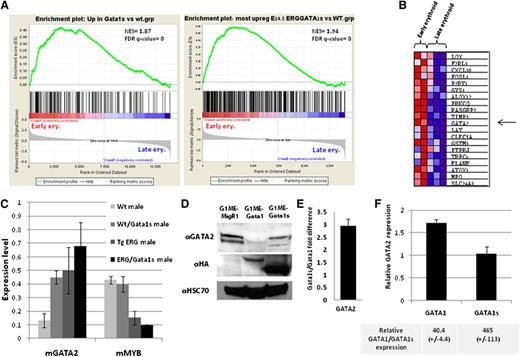
ERG/Gata1s FL cells present an early erythroid expression profile. (A) GSEA using gene expression of human erythroid cells at different stages of differentiation38 shows enrichment of Wt/Gata1s and ERG/GATA1s E14.5 FL gene signature (GSE46481) in early erythroid cells. (B) Heat map showing the top 20 core enrichment genes from the GSEA presented in panel A, right. (C) Expression level of Gata2 and Myb genes as obtained from the mouse gene 1.0 ST chip array and confirmed by real-time PCR on at least 2 additional samples for each genotype. (D) Representative western blot from G1ME cells transduced with MigR1, MIGR1-GATA1, and MIGR1-GATA1s using the indicated antibodies. (E) Average difference in GATA2 expression from 3 independent G1ME expreriments presented in panel D. (F) An average repression of GATA2 mRNA by real-time PCR in E14.5 GATA1s KI FL cells infected with MIGR1-GATA1and MIGR1-GATA1s relative to MigR1-infected cells. Relative GATA1 and GATA1s RNA expression levels are indicated below (n = 3).
ERG/Gata1s FL cells present an early erythroid expression profile. (A) GSEA using gene expression of human erythroid cells at different stages of differentiation38 shows enrichment of Wt/Gata1s and ERG/GATA1s E14.5 FL gene signature (GSE46481) in early erythroid cells. (B) Heat map showing the top 20 core enrichment genes from the GSEA presented in panel A, right. (C) Expression level of Gata2 and Myb genes as obtained from the mouse gene 1.0 ST chip array and confirmed by real-time PCR on at least 2 additional samples for each genotype. (D) Representative western blot from G1ME cells transduced with MigR1, MIGR1-GATA1, and MIGR1-GATA1s using the indicated antibodies. (E) Average difference in GATA2 expression from 3 independent G1ME expreriments presented in panel D. (F) An average repression of GATA2 mRNA by real-time PCR in E14.5 GATA1s KI FL cells infected with MIGR1-GATA1and MIGR1-GATA1s relative to MigR1-infected cells. Relative GATA1 and GATA1s RNA expression levels are indicated below (n = 3).
Altered expression of the early erythroid genes GATA2 and Myb in ERG/Gata1s FL cells
Using the GSEA described in the previous paragraph for ERG/Gata1s FL (Figure 6A right panel), we identified Gata2 among the top 20 core enrichment genes (Figure 6B). Gata2 expression levels, as measured by expression array and confirmed by real-time PCR on separate embryos, was elevated in ERG/Gata1s E14.5 FLs (Figure 6C). GATA2 expression is directly activated by ERG and repressed by GATA1.23,39 In G1ME cells,30 Gata2 expression was reduced by an average of threefold upon expression of Gata1 compared with Gata1s-expressing G1ME cells (Figure 6D-E). In E14.5 Wt/Gata1s FL cells transduced with GATA1, GATA2 expression was reduced by an average of 1.7-fold compared with its expression in E14.5 FL cells that expressed only GATA1s (Figure 6F). Both in G1ME and in the FL cells, GATA2 expression was significantly reduced when GATA1 was expressed, despite the much higher expression levels of GATA1s (Figure 6D-F). This implies that repression by GATA1s is less efficient than repression by GATA1 and that even small amount of GATA1 is sufficient to repress GATA2.
Another potential cause of the persistent high levels of Gata2 and the fetal anemia of ERG/Gata1s FL cells is the repression of c-myb by ERG. c-myb suppresses GATA2 during erythroid differentiation.38 Low levels of c-myb cause anemia and enhance megakaryopoiesis.40,41 ERG binds to c-myb promoter24 and represses c-myb expression in transduced primary FL hematopoietic cells regardless of GATA1 expression.17 Consistent with this data, we observed a fourfold reduction in c-myb expression in TgERG FL cells (Figure 6C). Together, these results suggest that persistent expression of Gata2 and suppressed expression of c-myb contribute to the block in erythroid differentiation in ERG/Gata1s FLs.
Discussion
We present here a detailed analysis of the developmental interactions between ERG and Gata1s during fetal hematopoiesis. The collaboration between these 2 mutated genes results in marked suppression of the generation of fetal erythroid cells, their differentiation and their survival, coupled with expansion of megaerythroid and megakaryocytic progenitors similar to those observed in DS-TMD.
DS-TMD is characterized by the transient expansion of fetal megakaryoblasts that continue in the first 3 months of life. Although often asymptomatic, some of the patients develop liver failure caused by liver fibrosis secondary to the infiltration of pathological fetal megakaryoblasts. Up to 30% of the DS neonates recovering from the TMD develop AMKL before the age of 4 years. The syndrome we observed in ERG/Gata1s transgenic mice is similar in the phenotype and gene expression of the expanded fetal cells, its transient nature, its association with liver fibrosis, and the progression to megaerythroid progenitor leukemia (supplemental Figure 5). Yet, it differs in the timing of the TMD which is prenatal in the mice and perinatal in humans.
The current model for DS-TMD assumes 2 cooperating processes.13 First trisomy 21 causes expansion of fetal MEPs. Then, GATA1s, created by a somatic mutation in GATA1, blocks their terminal megakaryocytic differentiation and enhances their proliferation, leading to the perinatal TMD. Here we show that increased expression of a single chromosome 21 gene, ERG, can cooperate with Gata1s to create a TMD-like fetal hematopoietic state in a mouse model.
ERG was recently demonstrated to be required for maintenance of the FL HSCs.23 We show here for the first time that constitutive hematopoietic expression of human ERG causes expansion of FL MEPs in a mouse model with no other genetic aberrations related to DS. Although Gata1s enhanced early fetal megakaryopoiesis, as previously reported,11 it did not affect the percentage of MEPs. These results support previous observations in human FLs2,3,35 that the expansion of fetal MEPs is caused by trisomy 21 preceding the “second hit” of the mutations in GATA1.
Constitutive transgenic expression of hERG, to a similar level observed in normal human hematopoietic precursors (supplemental Figure 1) cooperated with Gata1s to further expand the same fetal megakaryocytic precursor observed in human TMD as demonstrated by gene expression profiling. However, in humans, it is likely that the same effect is achieved by cooperation of several Hsa21 genes, such as ERG, ETS2,17,42 DYRK1A18 or miR-125-b2.12 Yet, consistent with our results, Ng et al27 reported that the addition of 1 copy of Erg was both necessary and sufficient for the postnatal myeloproliferation in a mouse model of DS.
The gene expression profile of Gata1s/ERG fetal cells was similar to both human TMD and DS AMKL, consistent with the development of DS leukemias from the same cells causing TMD. Specifically, we saw increased expression of Mycn in FLs of the double transgene ERG/Gata1s (Figure 3E). Mycn is highly expressed in DS-TMD36 and was suggested to participate in the elimination of Gata1-mediated cell-cycle arrest via Cdkn2c.29 Therefore, elevated Mycn levels may contribute to the hyperproliferation phenotype by repressing Cdkn2, similar to what has been previously shown for cMyc.29 Indeed, we see about a twofold decrease in Cdkn2c expression in Gata1s mice compared with animals expressing Wt Gata1, with the highest repression observed in ERG/Gata1s male FLs (supplemental Figure 6).
GATA1 transcription factor plays a key role in differentiation and maturation of erythroid cells by regulating numerous erythroid genes.43-45 However, the role of the N-terminal “transactivation” domain in erythropoiesis is unclear. The initial report of the Gata1s “knock in” mouse, used in our study, reported reduced fetal E12.5 erythropoiesis without any detailed analysis.11
We show here that the expression of ERG dramatically enhances the fetal erythropoiesis defect caused by the elimination of the N terminus of Gata1. Embryos that express both ERG and Gata1s suffer from severe anemia as demonstrated by low cellularity in their FLs (Figure 4A) and almost complete absence of erythroid cells (Figure 4G), leading to death of ERG/Gata1s males at embryonic day E13.5 (Figure 4B). Our detailed analysis reveals a block in all stages of erythropoiesis and decreased numbers and survival of erythroid progenitors coupled with arrest in erythroid differentiation. The erythroid phenomenon is not observed in children with DS because the GATA mutation in DS is somatic, occurring only in the TMD cells and not in the germline as in the Gata1s mouse.
ERG expression is normally decreased with erythroid differentiation6 (supplemental Figure 7). However, in this transgenic mouse model, ERG constitutive expression is controlled by the hematopoietic vav promoter. We demonstrate here that the differential regulation of ERG expression along erythroid differentiation is critical, and that lack of its normal downregulation causes a block in final erythroid maturation (Figure 7).
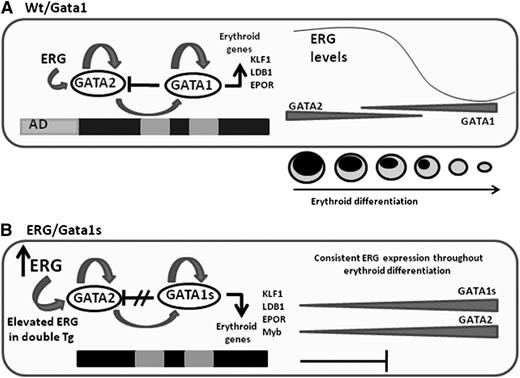
Schematic representation of the main events leading to impaired fetal erythropoiesis. (A) Gata2 which is expressed in erythroid progenitors, positively regulates Gata1 and its own expression.39 Gata1 also positively regulates its own expression but represses Gata2. Progression in erythroid differentiation becomes possible due to the Gata1/Gata2 switch on the promoter of erythroid genes resulting in their activation. Gata2 repression is an essential step in erythroid differentiation and is executed by several factors including Gata1 and the decline in ERG expression. (B) In the ERG/Gata1s mice, several factors block erythroid differentiation. In the absence of the N-terminal domain of Gata1, Gata1s fails to repress Gata2, erythroid genes are not activated, and differentiation is blocked. Furthermore, the Gata2 repressor Myb is downregulated by ERG and Gata1s. Because Myb functions as a Gata2 repressor, decreased Myb levels result in an increase in Gata2 expression. In the ERG transgenic model, the ERG expression level fails to decline with erythroid differentiation, thus further maintaining Gata2 expression and preventing erythroid cells to fully differentiate to erythrocytes.
Schematic representation of the main events leading to impaired fetal erythropoiesis. (A) Gata2 which is expressed in erythroid progenitors, positively regulates Gata1 and its own expression.39 Gata1 also positively regulates its own expression but represses Gata2. Progression in erythroid differentiation becomes possible due to the Gata1/Gata2 switch on the promoter of erythroid genes resulting in their activation. Gata2 repression is an essential step in erythroid differentiation and is executed by several factors including Gata1 and the decline in ERG expression. (B) In the ERG/Gata1s mice, several factors block erythroid differentiation. In the absence of the N-terminal domain of Gata1, Gata1s fails to repress Gata2, erythroid genes are not activated, and differentiation is blocked. Furthermore, the Gata2 repressor Myb is downregulated by ERG and Gata1s. Because Myb functions as a Gata2 repressor, decreased Myb levels result in an increase in Gata2 expression. In the ERG transgenic model, the ERG expression level fails to decline with erythroid differentiation, thus further maintaining Gata2 expression and preventing erythroid cells to fully differentiate to erythrocytes.
Consistent with these findings, we observed significant enrichment in early erythroid genes in E14.5 ERG/Gata1s FLs (Figure 6A right panel). Gata2 which was among the top core enrichment genes (Figure 6B), is a key regulator of early erythropoiesis, and promotes the survival and proliferation of immature cells.46 Gata2 downregulation is required for erythroid differentiation.47 In K562 cells overexpressing Gata2, a reduced growth rate and inhibition of erythroid differentiation was observed. More significantly, primary adult erythroid progenitor cells that highly expressed Gata2 showed reduced growth, decrease in the number and proportion of mature erythroid cells, and reduced hemoglobin A and F levels in a dose-dependent manner with increasing amounts of GATA2 expression, suggesting that the blockage in erythroid maturation is due to increased expression of GATA2.47
The regulation of erythroid gene expression and erythroid differentiation is governed by the interplay between GATA1 and GATA2, which share a common DNA-binding motif.39 GATA2 directly activates GATA1 expression in early erythroid progenitors, and positively regulates its own expression, whereas GATA1 accelerates its own expression and represses GATA2,39 allowing occupancy of regulated erythroid genes by GATA1 during erythroid maturation39 (Figure 7). The absence of the transactivation domain in Gata1s alleviates the repression of Gata2 by Gata1; Gata2 levels were higher in Gata1s FL cells compared with those that express wt Gata1 (Figure 6C). Moreover, induction of GATA1 but not GATA1s in G1ME cells and in GATA1s FLs results in GATA2 repression (Figure 6D-F). These results indicate a specific role for the N-terminal domain of Gata1 in Gata2 transcriptional regulation and thus in regulating the transcriptional network in early erythropoiesis.
As shown by chromatin immunoprecipitation studies, Gata2 is a direct target of ERG, which positively regulates its expression in fetal megaerythroid progenitors.23 The constitutive expression of vav-driven transgenic hERG throughout erythropoiesis may further explain the consistent high levels of Gata2.
The upregulation of Gata2 in ERG/Gata1s transgenic FL cells may also be explained by the repression of c-myb in these mice. c-Myb is expressed in early erythroid differentiated cells and is downregulated with differentiation by Gata1 in association with the transcription co-repressor FOG1.48 It was proposed that c-Myb may also function as a Gata2 repressor because Gata2 expression levels were increased by fivefold in c-MybKD/KD E14.5 FL cells, inducing an immature erythroid phenotype.40 Furthermore, ERG is a direct repressor of c-myb as previously shown by ChiP-Seq and gene expression studies.17,24 Thus, we suggest that persistent expression of Gata2 coupled with the repression of c-myb play a central role in the fetal erythropoiesis defects observed in ERG/Gata1s embryos.
In summary, we present here an ERG/Gata1s double transgenic mouse model of TMD. We show that moderate increased expression of ERG was sufficient to generate expansion of the MEP compartment in FLs and that expression of Gata1s, together with ERG, enhances this expansion. We also demonstrated the requirement of ERG downregulation and of the N-terminal domain of Gata1 for embryonic and fetal erythropoiesis. The relevance of these findings to human diseases is highlighted by a previous case report of anemia in humans with GATA1s germline mutation49 and the recent discovery of GATA1s mutations in some patients with the congenital Diamond-Blackfan anemia.50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Z. Li and S. Orkin for providing the Gata1s knock-in mice, M. Weiss for the G1ME cells, and D. Levanon, Y. Groner, and L. Rainis for their assistance in the generation of the TgERG mice.
This work was supported by Children with Cancer (United Kingdom), the Israel Science Foundation, the Waxman Cancer Research Foundation (New York), the USA-Israel Binational Science Foundation, the Clinical Genetics Foundation, iCORE Israel 41/11, and National Institutes of Health R01CA120772-01A2. Y.B. is a European Hematology Association fellow, and L.G. was supported by a postdocotoral grant from the Israel Cancer Research Foundation.
Authorship
Contribution: Y.B. designed and performed experiments, analyzed data, and wrote the paper; L.G. performed experiments, analyzed data, and helped write the paper; T.M.C., B.G., and G.S. performed experiments; I.M. provided research assistance; J.J.-H. performed array experiment and analysis; G.R. provided guidance; J.D.C. assisted in manuscript preparation; and S.I. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shai Izraeli, Sheba Medical Center Tel-Hashomer, Ramat Gan, Israel 52621; e-mail: shai.izraeli@sheba.health.gov.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal