Key Points
The JAK-inhibitor ruxolitinib affects dendritic cell differentiation, phenotype, and function leading to impaired T-cell activation.
Abstract
The Janus kinase (JAK)–inhibitor ruxolitinib decreases constitutional symptoms and spleen size of myelofibrosis (MF) patients by mechanisms distinct from its anticlonal activity. Here we investigated whether ruxolitinib affects dendritic cell (DC) biology. The in vitro development of monocyte-derived DCs was almost completely blocked when the compound was added throughout the differentiation period. Furthermore, when applied solely during the final lipopolysaccharide-induced maturation step, ruxolitinib reduced DC activation as demonstrated by decreased interleukin-12 production and attenuated expression of activation markers. Ruxolitinib also impaired both in vitro and in vivo DC migration. Dysfunction of ruxolitinib-exposed DCs was further underlined by their impaired induction of allogeneic and antigen-specific T-cell responses. Ruxolitinib-treated mice immunized with ovalbumin (OVA)/CpG induced markedly reduced in vivo activation and proliferation of OVA-specific CD8+ T cells compared with vehicle-treated controls. Finally, using an adenoviral infection model, we show that ruxolitinib-exposed mice exhibit delayed adenoviral clearance. Our results demonstrate that ruxolitinib significantly affects DC differentiation and function leading to impaired T-cell activation. DC dysfunction may result in increased infection rates in ruxolitinib-treated patients. However, our findings may also explain the outstanding anti-inflammatory and immunomodulating activity of JAK inhibitors currently used in the treatment of MF and autoimmune diseases.
Introduction
Myelofibrosis (MF) is a clonal stem cell disorder characterized by splenomegaly, constitutional symptoms originating from elevated proinflammatory cytokines, and progressive anemia.1 Half of MF patients carry the Janus kinase 2 (JAK2) V617F mutation2 which is closely related to deregulated JAK-STAT (signal transducer and activator of transcription) signaling.3
Ruxolitinib, a selective JAK inhibitor, was recently approved for treatment of primary and secondary MF. Impressive symptom control in MF patients on ruxolitinib treatment is predominantly mediated by profound suppression of inflammation, mirrored by a significant reduction in proinflammatory and proangiogenic cytokines.4 Reduction of these cytokines is paralleled by a significant shrinkage of the spleen. Thus, although not being curative, ruxolitinib induces marked clinical benefits, but clinical responses are independent of the JAK2 mutational status.5,6 Subtle changes of the allelic disease burden despite clinical benefits support the idea that the drug exerts only marginal anticlonal activity. These observations suggest that potential off-target effects as well as JAK inhibition in nondiseased cells (eg, immune cells) might at least in part be responsible for clinical improvement.
Dendritic cells (DCs) are the most powerful antigen-presenting cells (APCs) and are critical regulators orchestrating adaptive immune responses in vivo.7,8 Efficient activation of naive CD8+ T cells requires, in addition to presence of the specific antigen, appropriate expression of costimulatory signals by APCs. These allow proper T-cell activation, inducing cytotoxic CD8+ T cells to destroy infected or transformed cells.
The JAK protein family is a group of cytoplasmatic tyrosine kinases comprising JAK1, JAK2, JAK3, and TYK2. JAKs mediate cytokine signaling via association with type I and type II cytokine receptors. Activation of JAKs results in the phosphorylation of downstream STAT proteins, followed by their nuclear translocation and activation of target genes. Although all JAKs are essential in common cellular signaling pathways involved in the regulation of the immune system, each JAK exerts distinct additional functions.
JAK1 is crucial in the response to interferon (IFN), interleukin-10 (IL-10), IL-2/IL-4, and IL-6, whereas JAK2 is associated with the response to single-chain receptors (ie, Epo-R, GH-R, Prl-R), the IL-3 cytokine family, and the IFN-γ receptor.9 A specific role for JAKs in DCs has long been proposed, but remains poorly understood. Zhong et al recently suggested that JAK2 selectively regulates the capacity of DCs to initiate immune responses, although their ability to stimulate T-cell activation was not impaired.10 Others demonstrated that IFN-γ–induced JAK1 in human APCs enhances IL-12p70 and inhibits IL-10 release, thus representing a potential target for immunosuppressive strategies.11
However, until now, potential effects of the clinically used JAK-inhibitor ruxolitinib on the development and functional activity of immune cells were not completely understood. We here establish a role for ruxolitinib as modulator of DC function, which subsequently inhibits proper T-cell activation. Our study might set the stage for further testing of JAK inhibitors in diseases characterized by deregulated immune activation mediated by DCs (eg, in lupus erythematodes).
Methods
Buffy coats for human monocyte isolation were obtained from voluntary blood donors at the University Hospital Bonn. Approval was obtained from the institutional ethics committee of the University of Bonn. Informed consent was obtained from participants in accordance with the Declaration of Helsinki.
Media and reagents
Cells were cultured in RPMI 1640 containing glutamax-I, supplemented with 10% inactivated fetal calf serum (RP10 medium), and 1% penicillin/streptomycin (Invitrogen). Ruxolitinib was obtained from Selleckchem, TG101348 (SAR302503) from Axon Medchem. Thioate-stabilized CpG-motive containing oligodeoxynucleotide 1668 (TCCATGACGTTCCTGATGCT) was purchased from TibMolbiol. All reagents not otherwise indicated were purchased from Sigma-Aldrich.
Generation of DCs
Human monocyte-derived DCs (moDCs) were generated from peripheral blood by plastic adherence as described previously.12,13 Adherent monocytes were cultured in RP10 medium supplemented with granulocyte macrophage–colony-stimulating factor (GM-CSF; 100 ng/mL; Leukine, Liquid Sargramostim) and IL-4 (20 ng/mL; R&D Systems). Cytokines were added to differentiate DCs every other day.
Murine bone marrow–derived DCs (bmDCs) were generated using GM-CSF as described previously.14,15 For most experiments, stimulation with Toll-like receptor 4 (TLR4) ligand (100 ng/mL Escherichia coli lipopolysaccharide [LPS]; Sigma-Aldrich) in combination with ovalbumin (OVA; Sigma-Aldrich) was carried out at day 6. At day 7, cells were harvested for further experiments.
For isolation of murine splenic DCs, spleens were removed from C57/BL6N mice and digested after perfusion with 0.4 mg of collagenase/DNAse (Sigma-Aldrich) per milliliter of phosphate-buffered saline (PBS). Cells were incubated with anti-CD11c–conjugated magnetic microbeads (Miltenyi Biotec) according to the manufacturer’s protocol and isolated with a magnetic-activated cell sorting column.
Ruxolitinib and TG101348 were dissolved in dimethyl sulfoxide (DMSO) and added to the culture media in concentrations varying from 0.1 to 10 μM, which corresponds to serum levels achieved in treated patients. In each case, equal amounts of DMSO were added as a control.
Isolation and fluorochrome labeling of murine OT-I or OT-II cells
Spleen and lymph nodes of OT-I or OT-II mice were removed and homogenized through a metal cell strainer. After centrifugation, OT-I or OT-II cells were isolated by magnetic cell sorting using the CD4+ or CD8+ T-cell negative selection kit according to the manufacturer’s protocol (Miltenyi Biotec). For carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling, 10 × 106 to 20 × 106 cells per milliliter were suspended in PBS, followed by the addition of CFSE to a final concentration of 5 μM, followed by incubation for 10 minutes at 37°C and washing with PBS/1% bovine serum albumin.16
Immunostaining
Murine and human DCs were stained using commercially available monoclonal antibodies from BD Biosciences, Dako Diagnostika, Immunotech, R&D Systems, and eBioscience.
Intracellular staining was performed as described previously16 after adding SIINFEKL peptide and GolgiPlug before cells were stained with CD8 monoclonal antibody. After fixation and permeabilization with saponin buffer, intracellular staining was performed.
Determination of cytokine production
Secretion of IL-12 in DC culture supernatants was determined using a commercially available enzyme-linked immunosorbent assay from R&D Systems, following the manufacturer’s instructions. The readout was performed using a Synergy2 microplate reader (BioTek).
In vitro migration assay
A total of 1 × 105 cells were seeded into a transwell chamber (8 μm, BD Falcon; BD Biosciences) in a 24-well plate, and migration to CCL19/MIP-3β was analyzed after 4 hours by counting gated DCs for 1 minute in a fluorescence-activated cell sorter (FACS) cytometer.
Mixed lymphocyte reactions
A variable number of irradiated stimulator DCs were cultured with a total of 1 × 105 responding allogeneic peripheral blood mononuclear cells. Tritium-labeled thymidine incorporation was measured on day 5 by a 16-hour pulse with [3H]-thymidine (18.5 kBq/well; GE Healthcare).
Detection of apoptosis
Apoptosis in DCs was detected by live-dead staining using the propidium iodide or 7-aminoactinomycin D–annexin V staining kit from eBioscience.
Polyacrylamide gel electrophoresis and western blotting
Whole-cell lysates were prepared as described previously.17-19 Protein concentrations were determined using a bicinchoninic acid assay (Pierce, Perbio Science). For analysis of the activation and expression status of pro-Caspase-320 (purchased from Santa Cruz Biotechnology), 20 μg of whole-cell lysates were separated on a polyacrylamide gel and transferred on a nitrocellulose membrane. The blot was probed with monoclonal antibodies against pro-Caspase-3 as well as glyceraldehyde-3-phosphate dehydrogenase (10B8; Santa Cruz Biotechnology) as loading control. Protein bands were detected using an enhanced chemiluminescence kit (GE Healthcare).
Animal keeping
C57BL/6N mice were purchased from Elevage Janvier (France). Transgenic mice were obtained from breedings at the University of Bonn. All mice were bred under specific pathogen-free conditions and were used at 8 to 12 weeks of age. In vivo experiments were approved by the animal care commissions of North Rhine-Westphalia, Germany, and Innsbruck, Austria.
Drug treatment of mice
Ruxolitinib was dissolved to make a stock solution in DMSO and further diluted for oral gavage in water containing 0.5% methylcellulose (w/v) and 0.1% Tween 80.
In vivo bmDC migration
Mycobacterium tuberculosis (TB; final concentration = 0.125 mg; Voigt Global Distribution Inc.) in Freund incomplete adjuvants (FIA; Sigma-Aldrich) was injected into the hock of recipients. Two days after TB application, 5 × 106 ruxolitinib-exposed, 2 μM CFSE-labeled immature bmDCs and vehicle-exposed 0.5 μM Dye eFluor670 (eBioscience)–labeled bmDCs were subcutaneously injected in a total injection volume of 50 μL in the hock of TB-FIA–injected recipients as well as solvent-injected recipients as control for spontaneous migration. Twenty-four hours thereafter, migration of the fluorescent-labeled bmDCs in the draining popliteal lymph node was measured by flow cytometry.
Induction of Ag-specific T-cell responses in mice
Mice were immunized intravenously with soluble OVA (200 μg per 20 g mouse) in a total volume of 200 μL, accompanied by 20 μg of CpG oligodeoxynucleotide 1668.16
In vitro and in vivo proliferation assay
OT-I or OT-II cells were labeled with CFSE as described previously.16 For in vitro testing, 1 × 105 OT-I or OT-II cells were cocultured with DCs at a ratio of 2:1 in a total volume of 200 μL per well. Before coculture, DCs were pulsed either with SIINFEKL peptide (20 μg/mL for 20 minutes at 37°C) or with OVA protein (500 μg/mL for 2 hours at 37°C) followed by antigen washout. For in vivo proliferation assays, 2 × 106 purified CD8+ CFSE-labeled T cells were transferred into naive C57/BL6N recipient mice which were vaccinated with OVA/CpG 12 hours later. Spleens were extracted and proliferation and activation of transgenic T cells was assessed by FACS 2.5 days later.
In vivo cytotoxicity assay
In vivo cytotoxicity assays were performed as previously described.16
Recombinant adenovirus
AdLGO was a kind gift from Dirk Wohlleber and Percy Knolle (Institutes of Molecular Medicine and Experimental Immunology, University Bonn, Bonn, Germany). AdLGO was propagated on human embryonic kidney (HEK 293) cells and purified by cesium chloride density-gradient centrifugation as described previously.21-23
Bioluminescence imaging
AdLGO load in C57BL/6N mice was quantified by in vivo bioluminescence using the IVIS Imaging System 200 (Xenogen Corp)23 after intravenous injection of mice with 5 × 106 plaque-forming units of AdLGO. Luciferase activity was detectable upon injection of 2.5 mg of luciferin (S039; Synchem). Data were analyzed using Living Image 2.50 software (Xenogen Corp).
Statistical analysis
All experiments were performed at least 3 times, with representative experiments shown. To analyze statistical significance, a Student t test was used. Comparisons were made as indicated with the Mann-Whitney test or analysis of variance (ANOVA) and Dunnett’s using Prism 5 software (Graphpad Software).
Results
Exposure of human monocytes to ruxolitinib inhibits differentiation into DCs
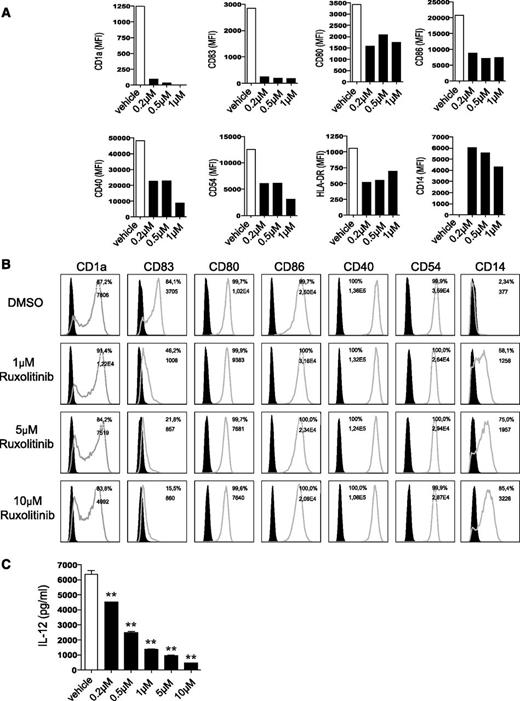
We first tested the toxic effects of ruxolitinib on DCs and did not detect any significant induction of apoptosis by ruxolitinib doses up to 5 μM. However, when the drug is used at higher concentrations or if it is repeatedly applied, a significant amount of cell death can be observed (supplemental Figure 1A-B, available on the Blood website). To gain insight into the dose-dependent effects of the compound on DC generation from human monocytes, we next applied increasing concentrations of ruxolitinib starting from day 0 on. Monocytes differentiated in the presence of ruxolitinib did not develop any morphologic DC features, but rather resembled monocytes. Accordingly, CD14 expression was maintained and the cells lacked upregulation of the bona fide DC marker CD1a, which is induced when monocytes are differentiated in the presence of vehicle. Moreover, LPS-matured moDCs expressed markedly less CD40, CD54, CD80, CD83, CD86, and HLA-DR when compared with vehicle-exposed cells (Figure 1A).
Ruxolitinib impairs phenotype and function of DCs. (A) Human monocytes cultured under DC-driving conditions with final LPS stimulation were exposed every other day to different concentrations of ruxolitinib (0.2 µM, 0.5 µM, and 1 µM on day 0, 2, 4, 6; ▪) or DMSO (□) throughout the differentiation period and analyzed for expression of DC and activation markers. Results are from 1 experiment representative of at least 3. (B) Expression of DC and activation markers after exposure of moDCs to ruxolitinib (1 µM, 5 µM, and 10 µM) on day 5, followed by subsequent final maturation with LPS on day 6, are shown. Filled black graphs represent negative controls. (C) Monocytes were cultured under DC-driving conditions and treated with ruxolitinib once on day 5, followed by LPS activation on day 6. Supernatants were collected on day 7 and analyzed for IL-12 production using a commercially available enzyme-linked immunosorbent assay. Results of 1 representative donor are shown. The significance was calculated according to the 1-way ANOVA Dunnett multiple comparison test and is related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Ruxolitinib impairs phenotype and function of DCs. (A) Human monocytes cultured under DC-driving conditions with final LPS stimulation were exposed every other day to different concentrations of ruxolitinib (0.2 µM, 0.5 µM, and 1 µM on day 0, 2, 4, 6; ▪) or DMSO (□) throughout the differentiation period and analyzed for expression of DC and activation markers. Results are from 1 experiment representative of at least 3. (B) Expression of DC and activation markers after exposure of moDCs to ruxolitinib (1 µM, 5 µM, and 10 µM) on day 5, followed by subsequent final maturation with LPS on day 6, are shown. Filled black graphs represent negative controls. (C) Monocytes were cultured under DC-driving conditions and treated with ruxolitinib once on day 5, followed by LPS activation on day 6. Supernatants were collected on day 7 and analyzed for IL-12 production using a commercially available enzyme-linked immunosorbent assay. Results of 1 representative donor are shown. The significance was calculated according to the 1-way ANOVA Dunnett multiple comparison test and is related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Ruxolitinib impairs human and murine DC activation
In the next set of experiments, we analyzed the effect of ruxolitinib on LPS-induced activation of moDCs generated in the presence of IL-4 and GM-CSF for 5 days without ruxolitinib exposure. We chose slightly higher concentrations of ruxolitinib (1-10 µM) at this time, due to the single addition of the compound. A sole application of ruxolitinib on day 5 re-induced expression of CD14 in previously CD14-negative DC cultures and downregulated CD1a expression. Subsequent activation on day 6 revealed that ruxolitinib dose-dependently impaired LPS-induced upregulation of CD80 and CD83 (Figure 1B). Consistent with our findings in human DCs, primary murine splenic or bmDCs exhibited reduced LPS-induced upregulation of CD40, CD80, CD86, and major histocompatibility complex (MHC) II in vitro in a concentration-dependent manner when exposed to the JAK inhibitor (data not shown).
Ruxolitinib modulates cytokine levels in DCs
Cytokines are critical in the regulation of DC function as well as their capacity to prime T-cell responses. Thus, we analyzed cytokine secretion in ruxolitinib-treated human moDCs in response to TLR stimulation. The most prominent reduction was detectable for IL-12 (Figure 1C), which is critical for T-cell activation.
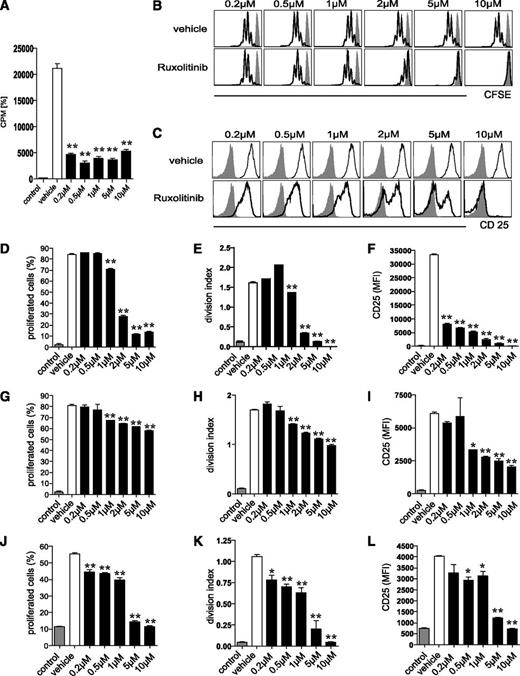
Ruxolitinib reduces human moDC-mediated allogeneic T-cell activation in vitro
We next assessed the ability of ruxolitinib-pretreated human moDCs to prime allogeneic T-cell responses in vitro using the mixed lymphocyte reaction (MLR). A sole application of ruxolitinib on day 5 dose-dependently reduced moDC capacity to induce allogeneic T-cell proliferation when compared with vehicle-treated controls (Figure 2A).
Ruxolitinib impairs the T-cell stimulatory function of DCs in vitro. (A) The ability of human moDCs, treated once with ruxolitinib (0.2-10 µM; ▪) on day 5 and activated with LPS on day 6, to prime allogeneic T-cell responses in vitro, was assessed using a MLR assay. Irradiated stimulator DCs were cultured with responding allogeneic peripheral blood mononuclear cells. Tritium-labeled thymidine incorporation was measured 5 days later. □, vehicle control. (B-I) CFSE-labeled OT-I cells were cocultured with murine OVA-loaded bmDCs. Ruxolitinib was added once on the first day of culture and resulted in (B,D) reduced OT-I proliferation, (C,F) decreased expression of CD25 as well as (E) a reduced division index. Pretreatment of bmDCs with ruxolitinib followed by repeated washout of the compound still resulted in (G) reduced OT-I proliferation, (H) division index, and (I) CD25 expression. (J-K) CFSE-labeled OT-II cells were cocultured with murine OVA-loaded bmDCs, followed by a single addition of ruxolitinib on the first day of culture. OT-II proliferation and (L) CD25 expression are shown. Results are from 1 experiment representative of 3. The significance was calculated according to the 1-way ANOVA Dunnett multiple comparison test and is always related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Ruxolitinib impairs the T-cell stimulatory function of DCs in vitro. (A) The ability of human moDCs, treated once with ruxolitinib (0.2-10 µM; ▪) on day 5 and activated with LPS on day 6, to prime allogeneic T-cell responses in vitro, was assessed using a MLR assay. Irradiated stimulator DCs were cultured with responding allogeneic peripheral blood mononuclear cells. Tritium-labeled thymidine incorporation was measured 5 days later. □, vehicle control. (B-I) CFSE-labeled OT-I cells were cocultured with murine OVA-loaded bmDCs. Ruxolitinib was added once on the first day of culture and resulted in (B,D) reduced OT-I proliferation, (C,F) decreased expression of CD25 as well as (E) a reduced division index. Pretreatment of bmDCs with ruxolitinib followed by repeated washout of the compound still resulted in (G) reduced OT-I proliferation, (H) division index, and (I) CD25 expression. (J-K) CFSE-labeled OT-II cells were cocultured with murine OVA-loaded bmDCs, followed by a single addition of ruxolitinib on the first day of culture. OT-II proliferation and (L) CD25 expression are shown. Results are from 1 experiment representative of 3. The significance was calculated according to the 1-way ANOVA Dunnett multiple comparison test and is always related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Ruxolitinib reduces murine DC-induced antigen-specific T-cell responses in vitro
We then analyzed the effect of ruxolitinib on the generation of antigen-specific CD4+ and CD8+ mediated T-cell responses. To accomplish this, we first used OT-I cells expressing a transgenic T-cell receptor against the OVA257-264 peptide (SIINFEKL) presented in the context of H-2kb. Ruxolitinib was added once on the first day of coculture of CFSE-labeled OT-I cells with SIINFEKL-pulsed DCs. This resulted in significantly reduced OT-I proliferation (Figure 2B,D-E) paralleled by markedly decreased CD25 (Figure 2C,F) and CD69 (data not shown) expression. To exclude a predominant effect of ruxolitinib on T cells, we preincubated DCs with ruxolitinib for 4 hours followed by extensive washing of the cells and subsequent addition of OT-I cells. This procedure also resulted in a significant, although slightly less intense, reduction of antigen-specific T-cell proliferation and impaired induction of activation markers (Figure 2G-I). In a second step, we aimed to determine the effect of ruxolitinib on CD4+ T-cell activation. In line with the results from the OT-I cells, addition of ruxolitinib to the culture in increasing concentrations similarly resulted in reduced OT-II cell proliferation (Figure 2J-K) as well as decreased activation-associated induction of CD25 expression (Figure 2L).
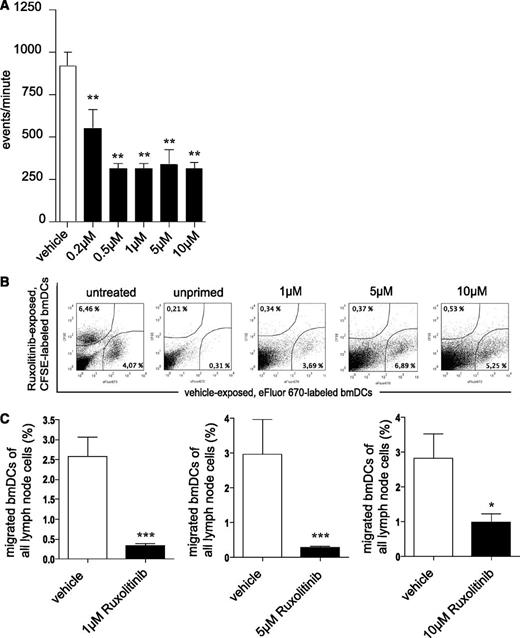
Ruxolitinib-treated DCs display reduced migratory capacity in vitro and in vivo
Mature moDCs express chemokine CC motif receptor 7 (CCR7), the receptor for CCL19/MIP-3β, which guides DC transit from peripheral tissue to draining local lymph nodes following a CCL19/MIP-3β gradient.8,24 Migration of day 5 ruxolitinib-treated and LPS-matured human moDCs toward MIP-3 was severely impaired (Figure 3A). However, FACS analyses revealed only a marginal reduction of LPS-induced CCR7 expression levels on moDCs, most likely not explaining the observed reduction of DC migration (data not shown). Inhibition of migration was corroborated using a murine in vivo model. Overnight ruxolitinib-exposed and CFSE-labeled ex vivo–generated bmDCs entered draining lymph nodes markedly less efficiently when compared with co-injected solvent-treated and eFluor670-labeled bmDCs (Figure 3B-C).
Ruxolitinib impairs migratory behavior of DCs. (A) Ruxolitinib-treated and LPS-stimulated human moDCs (▪) were assessed for their migratory behavior toward CCL19/MIP-3β in Transwell assays. □, Results of vehicle-exposed, LPS-stimulated DCs. Results are from 1 experiment representative of at least 3. The significance was calculated according to 1-way ANOVA Dunnett’s multiple comparison test and is related to the vehicle control. *P < .1; **P < .01; ***P < .001. (B) Overnight ruxolitinib-exposed, CFSE-labeled ex vivo–generated immature bmDCs (▪) were subcutaneously injected into the hock of TBI-FIA (M tuberculosis in FIA)–injected recipient mice together with vehicle-challenged, eFluor670-labeled immature bmDCs (□) and then quantified in the local draining lymph node by FACS. Both bmDC groups were further injected into solvent-injected recipients (“unprimed”) as control for spontaneous migration. Untreated (no ruxolitinib, no vehicle exposure), but CFSE- or eFluor670-labeled bmDCs were used as additional controls to exclude changes in migratory behavior due to the dying process (“untreated”). Numbers indicate percentage of migrated bmDCs of all lymph node cells. (C) Histograms represent results of pooled independent experiments with a total of n = 14 (10 µM), n = 8 (5 µM), n = 7 (1 µM) mice. The significance was calculated according to the Mann-Whitney test and is related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Ruxolitinib impairs migratory behavior of DCs. (A) Ruxolitinib-treated and LPS-stimulated human moDCs (▪) were assessed for their migratory behavior toward CCL19/MIP-3β in Transwell assays. □, Results of vehicle-exposed, LPS-stimulated DCs. Results are from 1 experiment representative of at least 3. The significance was calculated according to 1-way ANOVA Dunnett’s multiple comparison test and is related to the vehicle control. *P < .1; **P < .01; ***P < .001. (B) Overnight ruxolitinib-exposed, CFSE-labeled ex vivo–generated immature bmDCs (▪) were subcutaneously injected into the hock of TBI-FIA (M tuberculosis in FIA)–injected recipient mice together with vehicle-challenged, eFluor670-labeled immature bmDCs (□) and then quantified in the local draining lymph node by FACS. Both bmDC groups were further injected into solvent-injected recipients (“unprimed”) as control for spontaneous migration. Untreated (no ruxolitinib, no vehicle exposure), but CFSE- or eFluor670-labeled bmDCs were used as additional controls to exclude changes in migratory behavior due to the dying process (“untreated”). Numbers indicate percentage of migrated bmDCs of all lymph node cells. (C) Histograms represent results of pooled independent experiments with a total of n = 14 (10 µM), n = 8 (5 µM), n = 7 (1 µM) mice. The significance was calculated according to the Mann-Whitney test and is related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Exposure of human DCs to a more selective JAK2 inhibitor also profoundly affects DC biology
In vitro exposure of human moDCs to the more selective JAK2-inhibitor TG101348 during the final LPS-induced maturation phase confirms the data obtained with the less selective JAK-inhibitor ruxolitinib. Expression of activation markers (ie, CD80, CD83, CD86, and CD40) are dose-dependently reduced, whereas CD14 is re-expressed. In addition, TG101348 impairs allogeneic T-cell activation and reduces cytokine production and DC migration toward a CCL19 gradient (supplemental Figure 2A-E).
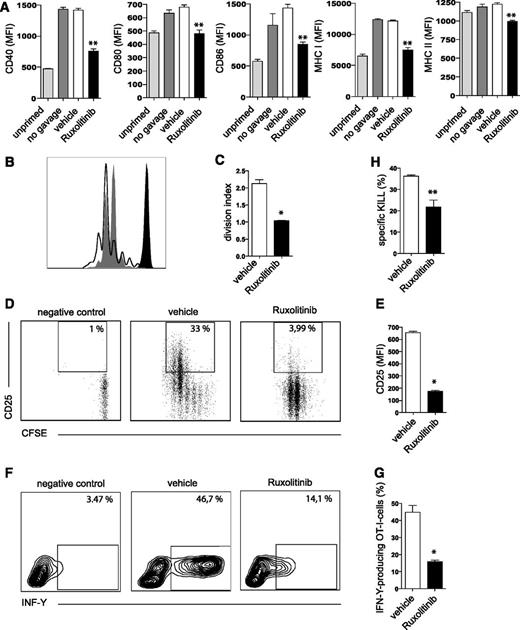
Ruxolitinib treatment reduces induction of cytotoxic T-cell responses in vivo
Mice receiving 75 mg/kg ruxolitinib or vehicle 6 hours prior to and 6 hours after injection of OVA/CpG were analyzed for expression of activation markers on CD11c+CD8+ splenic DCs. Indeed, lower expression levels of CD40, CD80, CD86 as well as MHC I and II molecules were detected in ruxolitinib-challenged animals (Figure 4A).
Ruxolitinib impairs the T-cell stimulatory function of DCs in vivo. (A) Mice were fed twice via oral gavage either ruxolitinib (▪) or vehicle (□) prior to and after injection of OVA/CpG, followed by an analysis of MHC expression and activation markers on CD11c+CD8+ splenic DCs 20 hours after priming. Gray columns represent mice vaccinated with OVA/CpG without receiving vehicle or ruxolitinib feeding, to exclude any effects of the oral gavage per se. (B-G) CFSE-labeled OT-I cells (2 × 106) were adoptively transferred to naive C57/BL6N recipient mice injected with OVA/CpG. Mice were fed 6 hours prior to as well as 6 and 18 hours after priming with OVA/CpG with ruxolitinib (gray shaded) or its vehicle (black line). (B) OT-I cell proliferation shown as histogram and (C) division index as well as (D-E) CD25 expression and (F-G) IFN-γ production (analyzed by intracellular cytokine staining) were assessed 2.5 days after priming with OVA/CpG. (H) Using the entirely endogenous T-cell repertoire of nontransgenic C57/B6N mice primed with OVA/CpG, OVA-specific cytotoxicity on day 5 was assessed. Mice had been treated either with ruxolitinib or its vehicle in the concentrations and schedule indicated above. Results are from 1 experiment (n = 4 per group) representative of at least 3. The significance was calculated according to (A) the 1-way ANOVA Dunnett multiple comparison test or (C,E,G,H) Mann-Whitney test and is always related to the vehicle control. *P < .1; **P < .01.
Ruxolitinib impairs the T-cell stimulatory function of DCs in vivo. (A) Mice were fed twice via oral gavage either ruxolitinib (▪) or vehicle (□) prior to and after injection of OVA/CpG, followed by an analysis of MHC expression and activation markers on CD11c+CD8+ splenic DCs 20 hours after priming. Gray columns represent mice vaccinated with OVA/CpG without receiving vehicle or ruxolitinib feeding, to exclude any effects of the oral gavage per se. (B-G) CFSE-labeled OT-I cells (2 × 106) were adoptively transferred to naive C57/BL6N recipient mice injected with OVA/CpG. Mice were fed 6 hours prior to as well as 6 and 18 hours after priming with OVA/CpG with ruxolitinib (gray shaded) or its vehicle (black line). (B) OT-I cell proliferation shown as histogram and (C) division index as well as (D-E) CD25 expression and (F-G) IFN-γ production (analyzed by intracellular cytokine staining) were assessed 2.5 days after priming with OVA/CpG. (H) Using the entirely endogenous T-cell repertoire of nontransgenic C57/B6N mice primed with OVA/CpG, OVA-specific cytotoxicity on day 5 was assessed. Mice had been treated either with ruxolitinib or its vehicle in the concentrations and schedule indicated above. Results are from 1 experiment (n = 4 per group) representative of at least 3. The significance was calculated according to (A) the 1-way ANOVA Dunnett multiple comparison test or (C,E,G,H) Mann-Whitney test and is always related to the vehicle control. *P < .1; **P < .01.
Next, ruxolitinib or vehicle was fed to mice 6 hours prior to as well as 6 hours and 18 hours after priming with OVA/CpG and adoptive transfer of CFSE-labeled OT-I cells. OVA is usually taken up by DCs in vivo and then cross-presented on MHC class I molecules. Analysis of transferred CFSE-labeled OT-I T cells revealed reduced proliferation (Figure 4B-C), CD25 expression (Figure 4D-E), and IFN-γ production (Figure 4F-G) in mice pretreated with ruxolitinib. We next evaluated the effects of ruxolitinib on the induction of cytotoxic activity of CD8+ T cells in a non–TCR-transgenic setting. To this end, we quantified the in vivo cytotoxic T-cell response against OVA in C57/BL6N mice primed with OVA/CpG either exposed to ruxolitinib or vehicle. Using this assay, we show that ruxolitinib markedly reduces OVA-specific cytotoxic activity (Figure 4H).
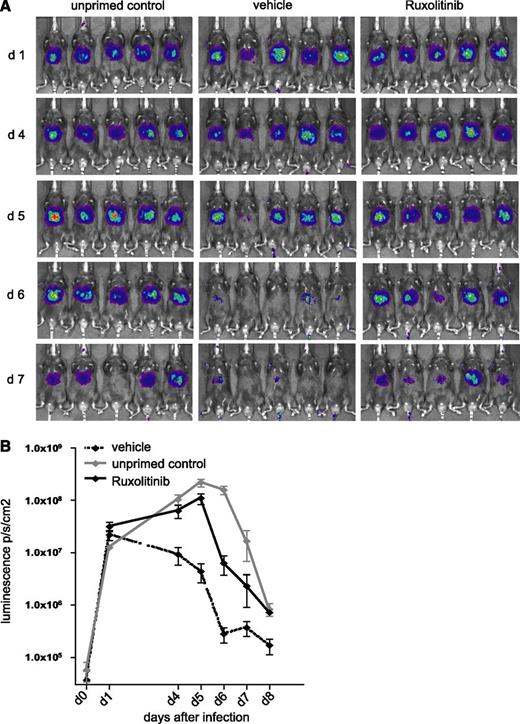
Ruxolitinib delays adenoviral clearance in vivo
We were finally interested in whether the observed immunomodulatory effects of ruxolitinib might also be of in vivo relevance. We therefore infected mice with AdLGO, a recombinant adenovirus expressing OVA, enhanced green fluorescent protein, and luciferase. It has been demonstrated that luminescence highly correlates with the number of viral copies per milligram of liver tissue,23 thus providing an ideal tool to study viral clearance in vivo. After infection with AdLGO, mice were vaccinated with OVA/CpG intravenously which usually results in clearance of the adenoviral infection within 6 days (data not shown). In contrast, AdLGO-infected, but nonvaccinated mice eliminate the nonreplicating adenovirus 2 to 3 days later. We now additionally fed AdLGO-infected mice vaccinated with OVA/CpG 6 hours before as well as 6 hours and 18 hours after vaccination with ruxolitinib or the respective vehicle. Using bioluminescence imaging, we could show that vehicle-exposed mice were able to clear the adenoviral infection within the expected time frame of 6 days. In contrast, mice receiving ruxolitinib retained higher viral loads as reflected by persisting bioluminescence signals in the liver (Figure 5).
Ruxolitinib-treated mice show a delay in clearing adenovirus infection. (A) C57/BL6N mice were infected with 5 × 106 plaque-forming units of AdLGO, a recombinant adenovirus expressing OVA, enhanced green fluorescent protein, and click-beetle luciferase, followed by vaccination with OVA/CpG or left unprimed as control. Mice were fed 6 hours before as well as 6 hours and 18 hours after vaccination ruxolitinib or the respective vehicle control. (B) Time course of bioluminescence measurements is shown on a logarithmic scale. Results are from 1 experiment (n = 5 per group) representative of 3. The significance is related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Ruxolitinib-treated mice show a delay in clearing adenovirus infection. (A) C57/BL6N mice were infected with 5 × 106 plaque-forming units of AdLGO, a recombinant adenovirus expressing OVA, enhanced green fluorescent protein, and click-beetle luciferase, followed by vaccination with OVA/CpG or left unprimed as control. Mice were fed 6 hours before as well as 6 hours and 18 hours after vaccination ruxolitinib or the respective vehicle control. (B) Time course of bioluminescence measurements is shown on a logarithmic scale. Results are from 1 experiment (n = 5 per group) representative of 3. The significance is related to the vehicle control. *P < .1; **P < .01; ***P < .001.
Discussion
The pathogenesis of MF is closely related to deregulation of the JAK-STAT signaling pathway.3 MF patients display a hyperinflammatory status, reflected by highly increased levels of proinflammatory (IL-1, IL-6, IL-8, and tumor necrosis factor-α) and proangiogenic (vascular endothelial growth factor and platelet-derived growth factor) cytokines.4 The JAK-inhibitor ruxolitinib normalizes hyperinflammation and improves constitutional symptoms. Interestingly, clinical responses occur independent of the JAK2 mutational status.5,6 Hence, JAK inhibition in nondiseased cells (ie, immune cells including DCs) as well as potential off-target effects may account for the observed therapeutic efficacy. Accordingly, JAK inhibitors are also applied as a systemic immunosuppressant in autoimmune diseases.25 So far, only limited mechanistic data on JAK inhibitor–mediated immune modulation are available.
Our report demonstrates that ruxolitinib profoundly affects DC differentiation and function in vitro and in vivo. Ruxolitinib exposure almost completely blocks in vitro DC development. Accordingly, the cells morphologically and phenotypically resemble monocytes rather than DCs, display markedly reduced IL-12 cytokine production, and decreased expression of costimulatory molecules upon TLR stimulation. This observation is most likely due to inhibition of cytokine signaling by ruxolitinib, as GM-CSF and IL-4 signals are critical factors driving DC differentiation.26 Our data are further corroborated by genetic data from mice with induced deletion of JAK2, which selectively modulates innate immune responses in a DC-dependent manner.10 This also induced impaired DC development, reduced upregulation of costimulatory markers upon TLR ligation finally leading to a reduced potency of JAK2-deficient DCs to secrete proinflammatory cytokines. We here complement these data by focusing on a scenario more closely reflecting the clinical situation, as we also exposed already differentiated DCs to ruxolitinib. In this setting, LPS-induced upregulation of CD83 during final maturation is predominantly reduced by ruxolitinib. Interestingly, even though IL-4– and GM-CSF–generated DCs almost completely expressed the bona fide DC marker CD1a and were negative for CD14, short-term ruxolitinib exposure of CD14-negative DCs stimulated with LPS for final maturation resulted in re-expression of CD14. In line with impaired final maturation of ruxolitinib-exposed DCs, the latter are significantly less potent in terms of inducing an allogeneic (ie, MLR) as well as a peptide-specific (OVA peptides and OT-I/OT-II cells) T-cell response. These data were also confirmed by using the more selective JAK2-inhibitor TG101348.
Thus, we provide first evidence that inhibition of JAKs profoundly modulates DC development and activation. In contrast to JAK3 and TYK2 loss-of-function mutations, which are associated with immunodeficiency, germline JAK1 and JAK2 mutations are not compatible with life, which limits the availability of proper genetic tools for studying the role of these kinases in selective immune cell populations. However, there is increasing evidence that JAK1 and 2 are involved in immune cell activation.27 As an example for an important regulatory role of JAKs in DCs, JAK1 regulates granzyme B expression in plasmocytoid DCs.28
Moreover, a recent report using mice with an inducible genetic JAK2 deletion10 demonstrated, in contrast to our pharmacologic approach, that DCs from JAK2-deleted mice appropriately activated T cells, although DC maturation was impaired. This might at least in part be explained by the fact that ruxolitinib does not only selectively inhibit JAK2, but also targets JAK1 in addition to yet undefined off-target effects. However, our observation that the more selective JAK2-inhibitor TG101348 mimics the ruxolitinib effects supports an important role of JAK2 as relevant target kinase.
Next, due to the importance of proper DC migration to secondary lymphoid organs in order to induce T-cell responses, we further focused on DC migration. JAK/STAT signaling has already been associated with cell migration and also affects chemokine production.29-32 Ruxolitinib-exposed, TLR-stimulated DCs exhibited a pronounced impairment of their migratory behavior in vitro and in vivo. This was, however, not due to impaired induction of CCR7. Recent data suggest that JAK3 is involved in DC migration,29 however, ruxolitinib does not significantly inhibit JAK3, but primarily blocks JAK1 and JAK2 activity, which makes it rather unlikely that JAK3 inhibition is responsible for the observed effect. Further studies are currently ongoing to explore the mechanistic background of the observed migration defect in more detail.
Our in vitro findings demonstrating ruxolitinib-induced DC dysfunction subsequently motivated us to explore its immune-modulatory effects in various in vivo models. First, ruxolitinib-exposed mice showed reduced upregulation of costimulatory markers on DCs, followed by decreased antigen-specific CD8+ T-cell activation, proliferation, and cytotoxic activity in both mice bearing TCR-transgenic or entirely endogenous T-cell repertoires. This suggests that patients treated with ruxolitinib might also display, at least during the time of maximum plasma levels, an impaired DC-mediated T-cell priming function. Even though recent clinical trials have not shown markedly increased infection rates, viral reactivation (eg, herpes zoster), urinary tract, and opportunistic infections may be more frequently observed.33 Thus, potential effects of the drug on infectious prevalence in patients during long-term JAK-inhibitor therapy have to be closely monitored in the future. This assumption is further supported by our second in vivo model, showing that clearance of a hepatic adenoviral infection is delayed by systemic ruxolitinib therapy. Even though DCs are critical for viral clearance in this setting, we cannot rule out the possibility that ruxolitinib also affects other immune cell populations involved in viral clearance in vivo. However, the direct effects of the compound on cytotoxic T lymphocyte responses are less pronounced, and doses that are required to inhibit cytotoxic T lymphocyte activity are higher when compared with the DC-modulating concentrations. In keeping with this concept, it has been shown that daily long-term administration of ruxolitinib at higher doses than we applied (2 × 90 vs 2 × 75 mg/kg per day) did not alter hematologic parameters.4
In summary, here we show that ruxolitinib is a potent immune-suppressive compound. These data at least in part explain its beneficial effects in T-cell–mediated diseases, as T cells require full activation by APCs to exert deleterious autoimmune effects. Inhibitory effects induced by JAK1 and JAK2 inhibition in DCs may be due to reduction of both signal 2 (costimulation) and signal 3 (cytokine production), whereas signal 1 (antigen presentation) was not modified in our study. Moreover, migration of treated DCs is markedly impaired, further supporting the profound immune-inhibitory effects by ruxolitinib.
Our results may help to understand in more detail the anti-inflammatory effects of ruxolitinib seen in MF patients and provide some explanation for the beneficial effects already observed in patients with diseases such as psoriasis34-36 or rheumatoid arthritis,30 in which DCs play a key role in disease pathogenesis. Furthermore, our data may set the stage for further testing of ruxolitinib as a novel immunomodulatory drug in patients with a deregulated immune response, including steroid-refractory graft-versus-host disease and sepsis. On the other hand, patients exposed to long-term ruxolitinib treatment should be monitored closely for potential infectious complications and the development of malignancies as the compound may impair protective T-cell responses against leukemia or tumor antigens presented by DCs.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the technical help of Kati Riethausen and Heidrun Struppeck. The authors thank Dirk Wohlleber and Percy Knolle for providing AdLGO as a kind gift. The authors also thank Novartis and Incyte for providing ruxolitinib.
This work was supported by Bonn forscht BONFOR-Forschungskommission Forschungsförderung (A.H.), Sonderforschungsbereich 704 (P.B.), Deutsche Krebshilfe (P.B.), Deutsche Forschungsgemeinschaft (D.W.). Industrial support from Novartis was received (Novartis provided ruxolitinib for some experiments).
Authorship
Contribution: A.H., S.A.E.H., S.N.D., S.W., and S.P.Y. performed experiments, analyzed results, and made the figures; A.H., D.W., and P.B. designed the research and wrote the paper; and C.K. designed the research and discussed results.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Brossart, Department of Hematology and Oncology, University Hospital Bonn, Sigmund-Freud-Straße 25, 53127 Bonn, Germany; e-mail: peter.brossart@ukb.uni-bonn.de; and Dominik Wolf, Department of Hematology and Oncology, University Hospital Bonn, Sigmund-Freud-Straße 25, 53127 Bonn, Germany; email: dominik.wolf@ukb.uni-bonn.de.
References
Author notes
A.H. and S.A.E.H. contributed equally.
D.W. and P.B. shared senior authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal