Key Points
MYD88 L265P is a widely expressed somatic mutation in WM patients that supports NF-κB signaling through stimulation of BTK and IRAK 1/4.
Combined suppression of BTK and IRAK in MYD88 L265P expressing WM cells promotes synergistic inhibition of NF-κB signaling and WM cell killing.
Abstract
Myeloid differentiation factor 88 (MYD88) L265P somatic mutation is highly prevalent in Waldenström macroglobulinemia (WM) and supports malignant growth through nuclear factor κB (NF-κB). The signaling cascade(s) by which MYD88 L265P promotes NF-κB activation in WM remain unclear. By lentiviral knockdown or use of a MYD88 inhibitor, decreased phosphorylation of the NF-κB gatekeeper IκBα and survival occurred in MYD88 L265P-expressing WM cells. Conversely, WM cells engineered to overexpress MYD88 L265P showed enhanced survival. Coimmunoprecipitation studies identified Bruton tyrosine kinase (BTK) complexed to MYD88 in L265P-expressing WM cells, with preferential binding of MYD88 to phosphorylated BTK (pBTK). Increased pBTK was also observed in WM cells transduced to overexpress L265P vs wild-type MYD88. Importantly, MYD88 binding to BTK was abrogated following treatment of MYD88 L265P-expressing cells with a BTK kinase inhibitor. Inhibition of BTK or interleukin-1 receptor-associated kinase 1 and 4 (IRAK-1 and -4) kinase activity induced apoptosis of WM cells, and their combination resulted in more robust inhibition of NF-κB signaling and synergistic WM cell killing. The results establish BTK as a downstream target of MYD88 L265P signaling, and provide a framework for the study of BTK inhibitors alone, and in combination with IRAK inhibitors for the treatment of WM.
Introduction
Waldenström macroglobulinemia (WM) is a distinct clinicopathological entity resulting from the accumulation, predominantly in the bone marrow, of clonally related lymphocytes, lymphoplasmacytic cells, and plasma cells, which secrete a monoclonal immunoglobulin M (IgM) protein.1 This condition is considered to correspond to the lymphoplasmacytic lymphoma (LPL) as defined by the World Health Organization lymphoma classification system.2 We recently described the finding of a highly recurrent somatic mutation (myeloid differentiation factor 88 [MYD88] L265P) in WM patients using whole-genome sequencing, and subsequently confirmed its presence by Sanger DNA sequencing.3 In total, 91% of WM/LPL patients expressed MYD88 L265P. The high prevalence of MYD88 L265P in WM patients has also been confirmed by others using Sanger sequencing and allele-specific polymerase chain reaction (PCR).4-8 By Sanger or allele-specific PCR, MYD88 L265P is detected in up to one-half of patients with IgM monoclonal gammopathy of undetermined significance, and its presence, as well as expression level, are associated with malignant progression.5-7,9,10 In addition, MYD88 L265P has also been reported in ABC-type diffuse large B-cell lymphoma (DLBCL) (14%-29%), primary central nervous system lymphoma (33%), mucosa-associated lymphoid tissue lymphoma (9%), and chronic lymphocytic leukemia (2.9%) by either whole-genome, whole-exome, or Sanger DNA sequencing.4-7,11-14
MYD88 is an adaptor molecule for Toll-like receptors (TLRs) with the exception of TLR-3 and interleukin-1 receptor (IL-1R) signaling.15,16 Following TLR or IL-1R stimulation, MYD88 is recruited to the activated receptor complex as a homodimer which then complexes with interleukin-1 receptor-associated kinase 4 (IRAK4) and activates IRAK1 and IRAK2.17,18 Tumor necrosis factor receptor–associated factor 6 is then activated by IRAK1 leading to nuclear factor κB (NF-κB) activation via IκBα phosphorylation.19 Despite extensive previous work delineating the signaling pathways for wild-type (WT) MYD88, the impact of MYD88 L265P on cell signaling remains to be fully appreciated. Ngo et al11 demonstrated that survival of ABC DLBCL cells was sustained by presence of the MYD88 L265P, but not WT MYD88. Additionally, their studies showed that MYD88 L265P stimulated IRAK1 phosphorylation and NF-κB signaling. As part of these efforts, we tried to clarify the functional impact of MYD88 L265P in WM cells. The findings reported in this manuscript show that MYD88 L265P signals through IRAK1 and Bruton tyrosine kinase (BTK) to mediate the activation of NF-κB independently. Moreover, we show that MYD88 L265P promotes survival of WM through the activation of NF-κB, thereby providing a framework for the therapeutic targeting of the MYD88 signaling pathway in WM.
Methods and materials
Primary cells and cell lines
Genotyped CD19-selected bone marrow mononuclear cells (BMMCs) from WM patients and peripheral blood mononuclear cells (PBMCs) from healthy donors for MYD88 L265P and WT, respectively, along with MYD88 L265P expressing BCWM.1 and MWCL-1 WM cells, and OCI-LY3 diffuse large B-cell cells (DLBCL), as well as MYD88 WT expressing Ramos, MEC-1, U266 and OCI-Ly19 cells were used in these studies. All cells were confirmed for the presence of MYD88 L265P by Sanger DNA sequencing. Use of patient cells was obtained after informed consent approved by the institutional review board at Dana-Farber Cancer Institute (DFCI). This study was conducted in accordance with the Declaration of Helsinki. CD19-selected bone BMMCs and PBMCs were obtained after sorting using magnetic beads (Miltenyi Biotec). The purity of isolated B cells (CD19+) and clonality as assessed by light-chain restriction for WM tumor cells is in excess of 90%.
Lentiviral transduction studies
Knockdown of endogenous MYD88 was performed in MYD88 L265P-expressing BCMW.1 and MWCL-1 cells, and MYD88 WT-expressing cell lines. Vectors were constructed to encode individual small hairpin RNAs (shRNAs) directed against the 3′ untranslated region (3′ UTR) of MYD88 (MYD88 shRNA#1 target sequence 5′-GACCCAATGTACCAGTATT-3′, MYD88 shRNA#2 target sequence 5′-GCATATTCATCAAGTATGA-3′) and enhanced green fluorescence protein (GFP). A scrambled shRNA, was used as a control (control shRNA). BCWM.1 and MWCL-1 cells were also transduced to overexpress N-terminal flag-tagged MYD88 WT or L265P and red fluorescence protein. After sorting, cells were transduced with either MYD88 shRNA#1 or control shRNA. Knockdown of endogenous MYD88, and overexpression of exogenous MYD88 WT or L265P was confirmed by real-time reverse transcription–PCR using primers targeting the 3′ UTR and open reading frame of MYD88, as well as MYD88 protein expression levels by western blot analysis. Four days posttransduction, cells were sorted using a FACSAria III Cell Sorter (Beckton Dickinson).
Inhibitors of MYD88 signaling
BCWM.1, MWCL-1, and primary patient WM cells genotyped for MYD88 L265P, as well as MYD88 WT healthy donor CD19-selected PBMCs, and MYD88 WT cell lines were treated with and without inhibitors of MYD88 homodimerization (IMGENEX), IRAK 1 and 4 kinase function (EMDJ),20,21 and/or BTK (ibrutinib; Pharmacyclics, Inc.). Cells were incubated at 37°C with either 100 μM of the MYD88 inhibitor or control peptides or 0.01 to 20 μM of the IRAK 1 and 4 or BTK kinase inhibitors. Drug interactions were assessed by Calcusyn 2.0 software (Biosoft) and an online software tool (http://107.21.242.36/synergy/) based on Chou.22,23
Survival analysis
Cell survival was assessed following lentiviral knockdown of MYD88 or treatment with inhibitors. For studies assessing viability of cells over time (days 0-12 after sorting), cells were sorted by GFP expression at day 4 following lentiviral transduction with shRNA#1 MYD88 knockdown vector and then maintained in culture. Viability was determined by the fraction of viable shRNA-expressing cells relative to the shRNA-negative fraction, normalized to day 0 values.11 Apoptosis analysis was also performed following lentiviral transduction using annexin V/propidium iodide (PI) staining with the Apoptosis Detection Kit I (BD Pharmingen). Cells (1 × 106/well) were treated in 24-well plates for 6∼24 hours with inhibitors or corresponding controls. A minimum of 10 000 events were acquired using a BD FACSCanto II flow cytometer and analyzed with BD FACSDIVA software. The CellTiter-Glo Luminescent cell viability assay (Promega) was used to assess cell survival following treatment with a BTK inhibitor (ibrutinib) alone or in combination with an IRAK1/IRAK4 inhibitor. Cells were seeded into 384-well plates with the EL406 Combination Washer Dispenser (BioTek Instruments, Inc.) and inhibitors were injected into the cell-culture media with the JANUS Automated Workstation (PerkinElmer, Inc.). Cells were treated with a series of diluted inhibitors (IRAK1/4 inhibitor: 60∼0.6 μM, ibrutinib: 4∼0.04 μM) for 72 hours at 37°C. Luminescent measurement was performed using the 2104 Envision Multilabel Reader (PerkinElmer, Inc.).
Coimmunoprecipitation studies
For coimmunoprecipitation, cells were lysed with 1% NP-40 lysis buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1% NP-40, 1 mM sodium orthovanadate, 10 mM NaF, 1× protease inhibitors cocktail) and then centrifuged at 2600g for 5 minutes. The protein concentration was determined with the protein assay kit (Bio-Rad). The supernatants (2 mg total protein) were incubated with 2 μg of BTK-pY223 or BTK monoclonal antibodies (Epitomics, Inc) or 4 μg of MYD88 polyclonal antibody (Cell Signaling, Inc.) at 4°C for 30 minutes, followed by incubation with Protein A/G-coated magnetic beads (EMD Millipore) for another 30 minutes at 4°C. Samples were then washed 4 times with ice-cold lysis buffer on a magnetic stand; the proteins were eluted using sodium dodecyl sulfate–polyacrylamide gel electrophoresis loading buffer for further analysis.
Western blot analysis
Western blotting was performed to delineate expression levels of total protein (MYD88, IRAK1, IκBα, BTK) and phospho-specific isoforms of IRAK1-The209 (Abcam), IκBα-Ser32 (Cell Signaling, Inc.), BTK-Y223 (Epitomics, Inc), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control (Santa Cruz Biotechnology).
Phospho-flow cytometric analysis
The phosphorylation state of MYD88 pathway signaling proteins were assessed by phospho-flow cytometric analysis. Cells were fixed with Phosflow Fix Buffer I (BD Pharmingen), at 37°C for 10 minutes. Cells were washed twice with Phosflow Perm/Wash Buffer I (BD Pharmingen) then incubated in the presence of unlabeled primary antibodies, IRAK1-pT209, BTK-pY223 (Abcam), or fluorescent-labeled antibodies, Pcy7-NF-κB-p65-pS529, PE-BTK-pY223 (BD Pharmingen), for 30 minutes at room temperature in the dark. Cells were then washed thrice and resuspended in Phosflow Perm/Wash Buffer I. Secondary antibodies (DyLight-labeled donkey anti-rabbit IgG; BD Pharmingen) were added in the tubes with unlabeled primary antibodies and then incubated at room temperature for 20 minutes. Cells were washed once and resuspend cells in 500 μL of fluorescence-activated cell sorter buffer (BD) before analysis.
NF-κB luciferase reporter assay
Lentiviral NF-κB luciferase reporter and control virus (QIAGEN, Inc.) were transfected into BCWM.1 cells and puromycin selection was carried out to generate a homogenous population for further analysis. Cells were treated at 37°C for 6 hours with either the IRAK 1 and 4 inhibitor, the BTK inhibitor ibrutinib, or both. Luminescence was measured with the Bright-Glo Luciferase Assay System (Promega).
Statistical analysis
Comparison of pre- and posttreatment parameters was performed using a 2-tailed Student t test on Microsoft Excel software. For nonparametric testing of pre- and posttreatment responses, the Fisher exact probability test (VassarStats) was used. A P value < .05 was deemed to be significant.
Results
Knockdown of MYD88 is associated with decreased survival, while overexpression of MYD88 L265P promotes survival of WM cells
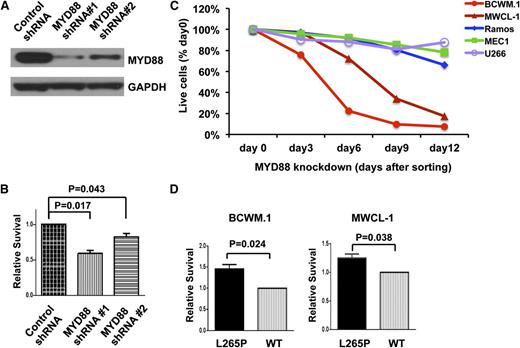
To clarify the potential oncogenic role of MYD88 L265P in WM, we first performed a lentiviral knockdown experiment in MYD88 L265P-expressing BCWM.1 WM cells. MYD88 protein levels were effectively reduced by both shRNA#1 and shRNA#2, with more efficient knockdown by shRNA#1 (Figure 1A). Conversely, expression of MYD88 was not impacted by scrambled control shRNA. Transduction with shRNA#1 also resulted in a greater decrease in survival of BCWM.1 WM cells that corresponded with the protein knockdown efficiency for shRNA#1 (Figure 1B). We next examined the impact of shRNA#1 knockdown on BCWM.1 and MWCL-1 and MYD88 WT-expressing MEC-1, Ramos, and U266 cells. Knockdown of MYD88 transcription and protein expression was also confirmed by real-time PCR and western blot analysis, respectively (data not shown). Knockdown of MYD88 with shRNA#1 showed a time-dependent decrease in survival of both MYD88 L265P-expressing cell lines, whereas little or no impact in survival was observed in MYD88 WT cell lines (Figure 1C). To delineate the contribution of MYD88 L265P vs WT on WM cell survival, their respective coding sequences were overexpressed in BCWM.1 and MCWL-1 cells followed by knockdown of endogenous MYD88 using 3′ UTR targeting shRNA#1 or a control vector. Both BCWM.1 and MWCL-1 cells showed enhanced survival for cells overexpressing MYD88 L265P vs WT (Figure 1D).
Inhibition of MYD88 is associated with decreased survival, while overexpression of MYD88 L265P promotes survival of WM cells. (A) The efficiency of MYD88 knockdown by 2 lentiviral shRNAs in BCWM.1 cells was confirmed by western blot using GAPDH as a loading control. (B) Relative survival of MYD88 L265P-expressing WM cells, BCWM.1, following knockdown of MYD88 by 2 lentiviral shRNAs targeting the 3′ UTRs of MYD88. P values are labeled compared with control shRNA. (C) Time-dependent survival of MYD88 L265P-bearing BCWM.1 and MWCL-1 cells, and MYD88 WT-expressing MEC-1, Ramos, and U266 cells following knockdown of MYD88. Cells were sorted by GFP for shRNA#1 expression at day 4 after lentiviral transduction. The percentage of GFP+, shRNA#1-expressing cells relative to the GFP−, shRNA#1-negative fraction at the indicated days after sorting is plotted and normalized to day 0 (right after sorting) values. (D) Relative survival of BCWM.1 and MWCL-1 WM cells engineered to overexpress WT or L265P MYD88 coding regions, with concurrent knockdown of endogenous MYD88 expression by 3′ UTR targeting shRNA#1. All experiments were performed at least in triplicate; P values are shown as labeled.
Inhibition of MYD88 is associated with decreased survival, while overexpression of MYD88 L265P promotes survival of WM cells. (A) The efficiency of MYD88 knockdown by 2 lentiviral shRNAs in BCWM.1 cells was confirmed by western blot using GAPDH as a loading control. (B) Relative survival of MYD88 L265P-expressing WM cells, BCWM.1, following knockdown of MYD88 by 2 lentiviral shRNAs targeting the 3′ UTRs of MYD88. P values are labeled compared with control shRNA. (C) Time-dependent survival of MYD88 L265P-bearing BCWM.1 and MWCL-1 cells, and MYD88 WT-expressing MEC-1, Ramos, and U266 cells following knockdown of MYD88. Cells were sorted by GFP for shRNA#1 expression at day 4 after lentiviral transduction. The percentage of GFP+, shRNA#1-expressing cells relative to the GFP−, shRNA#1-negative fraction at the indicated days after sorting is plotted and normalized to day 0 (right after sorting) values. (D) Relative survival of BCWM.1 and MWCL-1 WM cells engineered to overexpress WT or L265P MYD88 coding regions, with concurrent knockdown of endogenous MYD88 expression by 3′ UTR targeting shRNA#1. All experiments were performed at least in triplicate; P values are shown as labeled.
Inhibition of MYD88/IRAK signaling induces apoptosis of MYD88 L265P-expressing WM cells
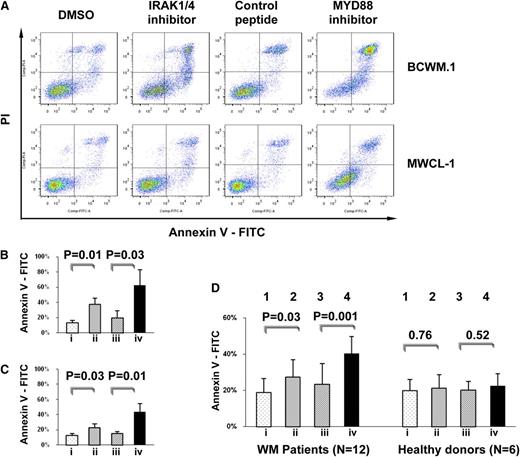
To further assess the impact of MYD88 L265P, we used inhibitors known to impede the MYD88 pathway signaling, including a cell-permeating peptide which blocks MYD88 homodimerization which is essential for Myddosome formation and IRAK 1 and 4 signaling, as well as a direct kinase inhibitor of IRAK 1 and 4. Treatment with these compounds resulted in significant apoptosis in the WM cell lines BCWM.1 and MWCL-1 (Figure 2A-C), as well as in primary WM patient cells (Figure 2D). In contrast, these inhibitors did not significantly impact survival of healthy donor CD19-selected B cells (Figure 2D), nor on any of the MYD88 WT cells lines (data not shown).
Inhibition of MYD88/IRAK signaling induces apoptosis of MYD88 L265P-expressing WM cells. (A) Survival assessed by annexin V/PI staining for MYD88 L265P-expressing WM cell lines. (B-D) Statistical analysis for annexin V–positive cells following treatment with (lane i) DMSO, (lane ii) an IRAK 1 and 4 kinase inhibitor, (lane iii) a control peptide, or (lane iv) peptide inhibitor of MYD88 homodimerization with results of 3 repeat experiments for (B) BCWM.1 and (C) MWCL-1 cells and (D) MYD88 L265P-genotyped primary WM patient LPL cells and MYD88 WT healthy donor B cells depicted. P value and numbers of patients are shown as labeled. DMSO, dimethyl sulfoxide.
Inhibition of MYD88/IRAK signaling induces apoptosis of MYD88 L265P-expressing WM cells. (A) Survival assessed by annexin V/PI staining for MYD88 L265P-expressing WM cell lines. (B-D) Statistical analysis for annexin V–positive cells following treatment with (lane i) DMSO, (lane ii) an IRAK 1 and 4 kinase inhibitor, (lane iii) a control peptide, or (lane iv) peptide inhibitor of MYD88 homodimerization with results of 3 repeat experiments for (B) BCWM.1 and (C) MWCL-1 cells and (D) MYD88 L265P-genotyped primary WM patient LPL cells and MYD88 WT healthy donor B cells depicted. P value and numbers of patients are shown as labeled. DMSO, dimethyl sulfoxide.
Impact of MYD88 inhibition on IRAK1 and IκBα phosphorylation in WM cells possessing the L265P variant
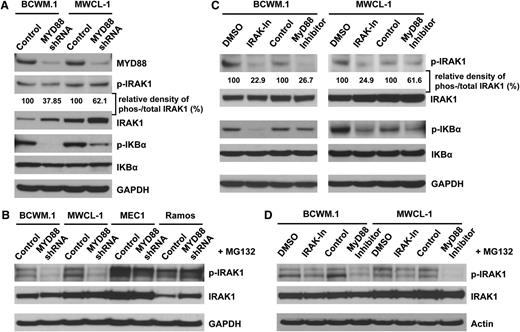
Since inhibitors of MYD88 homodimerization, and IRAK 1 and 4 kinase function, induced apoptosis of MYD88 L265P WM cells, we next sought to delineate the pathways responsible for this effect. MYD88 is known to trigger IRAK1 phosphorylation leading to downstream IκBα phosphorylation, and promotion of NF-κB signaling which is important for WM cell growth and survival.20,21 Moreover, inhibition of MYD88 signaling in L265P WM cell lines and primary WM patient cells leads to inhibition of NF-κB p65 phosphorylation and nuclear translocation.3 We therefore examined the IRAK pathway to further clarify its impact on WM survival. Both BCWM.1 and MWCL-1 WM cell lines constitutively expressed phosphorylated isoforms of IRAK1 and IκBα (Figure 3A,C). Since phosphorylation of IRAK1 leads to rapid ubiquitination and protein degradation, we first measured the ratio of phosphorylated IRAK 1 (p-IRAK1) to total IRAK1 (t-IRAK1) in these experiments.24 Knockdown of MYD88 led to decreased p-IRAK-1/t-IRAK1 expression, as well as IκBα phosphorylation, but not total IκBα levels in both BCWM.1 and MWCL-1 cells. Decreases in p-IRAK-1/t-IRAK1 expression, as well as IκBα phosphorylation, were more pronounced in BCWM.1 cells (Figure 3A). The rapid degradation of activated IRAK1 in WM cells was confirmed when the proteasome inhibitor MG-132 was used. In the presence of MG-132, the reduced IRAK1 phosphorylation following MYD88 knockdown was observed in MYD88 L265P-expressing WM cells, BCWM.1 and MWCL-1, but not in MYD88 WT-expressing MEC1 and Ramos cells (Figure 3B). The use of inhibitors blocking MYD88 homodimerization and IRAK 1 and 4 kinase function also led to reduction of IRAK1 and IκBα phosphorylation in BCWM.1 and MWCL-1 WM cells (Figure 3C). Similarly, in the presence of MG-132, reduction of IRAK1 phosphorylation following exposure to the MYD88 inhibitory peptide or the IRAK 1 and 4 inhibitor was also observed (Figure 3D).
MYD88 regulation of IRAK 1 and IκBα activity in WM cells-expressing the L265P mutation. (A) Western blot analyses were performed using antibodies which detect total and phosphorylated IRAK1 (The-209) and IκBα (Ser-32) following lentiviral transduction with either MYD88 knockdown (shRNA#1) or control lentiviral vectors. The relative density of phosphorylated vs total IRAK1 was analyzed by densitometry measurements and normalized to the values of control vectors. (B) To confirm the rapid degradation of activated IRAK1 that leading the reduction of total IRAK1 proteins, cells were treated with the proteasome inhibitor MG132 for 4 hours after MYD88 knockdown (shRNA#1). The phosphorylated and total IRAK1 were checked by western blot. (C) The effects of IRAK 1 and 4 inhibitor and MYD88 homodimerization inhibitory peptide were also evaluated by the phosphorylation of IRAK1 and IKBα with western blot. (D) Changes in phosphorylated IRAK1 in the presence of MG132 following treatment with either an IRAK 1 and 4 or MYD88 peptide inhibitor.
MYD88 regulation of IRAK 1 and IκBα activity in WM cells-expressing the L265P mutation. (A) Western blot analyses were performed using antibodies which detect total and phosphorylated IRAK1 (The-209) and IκBα (Ser-32) following lentiviral transduction with either MYD88 knockdown (shRNA#1) or control lentiviral vectors. The relative density of phosphorylated vs total IRAK1 was analyzed by densitometry measurements and normalized to the values of control vectors. (B) To confirm the rapid degradation of activated IRAK1 that leading the reduction of total IRAK1 proteins, cells were treated with the proteasome inhibitor MG132 for 4 hours after MYD88 knockdown (shRNA#1). The phosphorylated and total IRAK1 were checked by western blot. (C) The effects of IRAK 1 and 4 inhibitor and MYD88 homodimerization inhibitory peptide were also evaluated by the phosphorylation of IRAK1 and IKBα with western blot. (D) Changes in phosphorylated IRAK1 in the presence of MG132 following treatment with either an IRAK 1 and 4 or MYD88 peptide inhibitor.
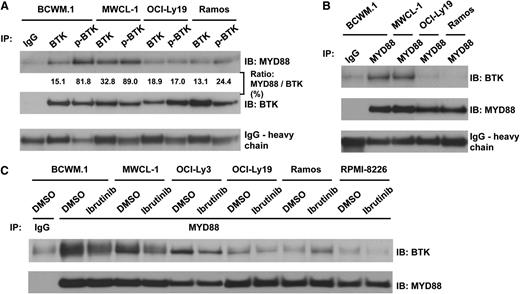
Coimmunoprecipitation studies identify BTK as a binding partner of MYD88 L265P in WM
BTK is an important downstream adapter for B-cell receptor signaling that triggers NF-κB activation. BTK is constitutively activated in BCWM.1 and MWCL-1 cells, though the mechanism responsible for its activation remains unclear.25 Additionally, it was previously reported that BTK interacted with MYD88 WT in transfected HEK-293 cells, and coimmunoprecipitated with BTK in lipopolysaccharide (LPS)–stimulated but not untreated macrophages.26 We therefore examined whether BTK complexes with MYD88 in L265P as well as WT-expressing cells. Using both BTK and phospho-BTK isoform-specific antibodies, we observed more robust MYD88 coimmunoprecipitation with phospho-BTK in L265P-expressing WM cells. In contrast, MYD88 WT cells showed little coimmunoprecipitation with either form of BTK (Figure 4A). Reverse coimmunoprecipitation studies confirmed the binding of BTK with MYD88 only in cell lines possessing the MYD88-L265P genotype (Figure 4B). MYD88 and BTK also coimmunoprecipitated in OCI-Ly3 DLBCL cells which carry the L265P mutation, whereas little or no coimmunoprecitation was observed in OCI-Ly19, Ramos, or RPMI 8226 cells which are WT for MYD88 (Figure 4C) and express similar levels of BTK and MYD88 proteins (data not shown). Importantly, treatment with the BTK inhibitor ibrutinib (4 μM for 90 minutes) reduced the binding of BTK with MYD88 in BCWM.1, MWCL-1 WM cells as well as OCI-Ly3 DLBCL cells (Figure 4C).
Coimmunoprecipitation studies identifying phospho-BTK as a binding partner of MYD88 in L265P-expressing WM cells, and abrogation of MYD88-BTK binding following treatment with ibrutinib in MYD88 L265P-expressing cells. (A) Immunoprecipitation experiments using pull-down (IP) anti-BTK– or phospho-BTK–specific antibodies followed by IB with an anti-MYD88 antibody in lysates from MYD88 L265P heterozygous-expressing BCWM.1 and MWCL-1 cells, and MYD88 WT-expressing OCI-Ly19 and Ramos cells. The ratio of MYD88 vs total BTK was analyzed by densitometry showing the amount of MYD88 protein that was pulled down by BTK. (B) Depicts experiment in which IP was performed with anti-MYD88 antibody and IB with an anti-BTK antibody. (C) Impact of ibrutinib pretreatment (4 μM for 90 minutes) on coimmunoprecipitation of BTK with MYD88 in MYD88 L265P-expressing BCWM.1, MWCL-1, and OCI-Ly3 cells, and MYD88 WT-expressing OCY-Ly19, Ramos, and RPMI 8226 cells. IB, immunoblotting antibody; IP, immunoprecipitation antibody.
Coimmunoprecipitation studies identifying phospho-BTK as a binding partner of MYD88 in L265P-expressing WM cells, and abrogation of MYD88-BTK binding following treatment with ibrutinib in MYD88 L265P-expressing cells. (A) Immunoprecipitation experiments using pull-down (IP) anti-BTK– or phospho-BTK–specific antibodies followed by IB with an anti-MYD88 antibody in lysates from MYD88 L265P heterozygous-expressing BCWM.1 and MWCL-1 cells, and MYD88 WT-expressing OCI-Ly19 and Ramos cells. The ratio of MYD88 vs total BTK was analyzed by densitometry showing the amount of MYD88 protein that was pulled down by BTK. (B) Depicts experiment in which IP was performed with anti-MYD88 antibody and IB with an anti-BTK antibody. (C) Impact of ibrutinib pretreatment (4 μM for 90 minutes) on coimmunoprecipitation of BTK with MYD88 in MYD88 L265P-expressing BCWM.1, MWCL-1, and OCI-Ly3 cells, and MYD88 WT-expressing OCY-Ly19, Ramos, and RPMI 8226 cells. IB, immunoblotting antibody; IP, immunoprecipitation antibody.
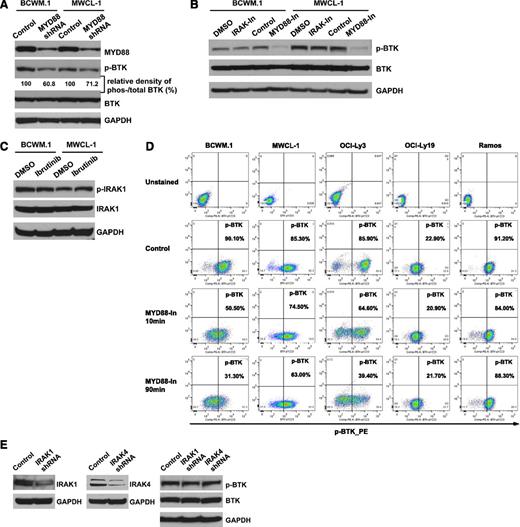
MYD88 is a regulator of BTK activation in L265P-expressing WM cells
To further clarify whether MYD88 L265P affects BTK signaling in WM cells, we performed knockdown experiments of MYD88 in the BCWM.1 and MWCL-1 WM cell lines and examined the impact on BTK activity. Both BCWM.1 and MWCL-1 cells showed decreased phospho-BTK expression in cells transduced with MYD88 shRNA#1 vs control shRNA while total BTK levels remained unchanged (Figure 5A). Similarly, phospho-BTK but not total BTK expression was decreased in BCWM.1 and MWCL-1 WM cell lines following treatment with an inhibitor of MYD88 signaling (Figure 5B). Decreased phospho-BTK levels were also confirmed by phospho-flow cytometric studies following inhibition of MYD88 in both BCWM.1 and MWCL-1 cells. Similar findings were also observed in OCI-Ly3 DLBCL cells that are homozygous for MYD88 L265P, but not in cell lines expressing WT MYD88. Decreased phospho-BTK levels were observed as early as 10 minutes following treatment with the MYD88 inhibitor in BCWM.1 cells (Figure 5D). Treatment with an inhibitor which blocked both IRAK 1 and 4 kinase activities did not impact phosphorylation or total BTK levels in BCWM.1 and MWCL-1 cells (Figure 5B). Conversely, use of a BTK kinase inhibitor had no impact on IRAK 1 phosphorylation or total IRAK1 levels (Figure 5C).
MYD88 is a regulator of BTK activity in L265P-expressing WM cells. (A) Phospho-BTK and total BTK levels following the knockdown of MYD88 in L265P-expressing WM cells. The relative density of phosphorylated vs total BTK was analyzed by densitometry measurements and normalized to the values of control vectors. (B) Phospho-BTK and total BTK levels were examined after the use of inhibitors of IRAK 1 and 4 or MYD88 by western blot. (C) Phospho-IRAK1 and total IRAK1 levels following ibrutinib treatment of BCWM.1 and MWCL-1 cells. (D) Phospho-BTK levels following use of an MYD88 inhibitor (100 μM) by phospho-flow cytometric analysis in BCWM.1, MWCL-1, OCI-Ly3, OCI-Ly19, and Ramos cells. (E) Phosphorylated BTK and BTK levels as determined by western blot analysis following the knockdown of IRAK 1, and IRAK 4 in BCWM.1 cells.
MYD88 is a regulator of BTK activity in L265P-expressing WM cells. (A) Phospho-BTK and total BTK levels following the knockdown of MYD88 in L265P-expressing WM cells. The relative density of phosphorylated vs total BTK was analyzed by densitometry measurements and normalized to the values of control vectors. (B) Phospho-BTK and total BTK levels were examined after the use of inhibitors of IRAK 1 and 4 or MYD88 by western blot. (C) Phospho-IRAK1 and total IRAK1 levels following ibrutinib treatment of BCWM.1 and MWCL-1 cells. (D) Phospho-BTK levels following use of an MYD88 inhibitor (100 μM) by phospho-flow cytometric analysis in BCWM.1, MWCL-1, OCI-Ly3, OCI-Ly19, and Ramos cells. (E) Phosphorylated BTK and BTK levels as determined by western blot analysis following the knockdown of IRAK 1, and IRAK 4 in BCWM.1 cells.
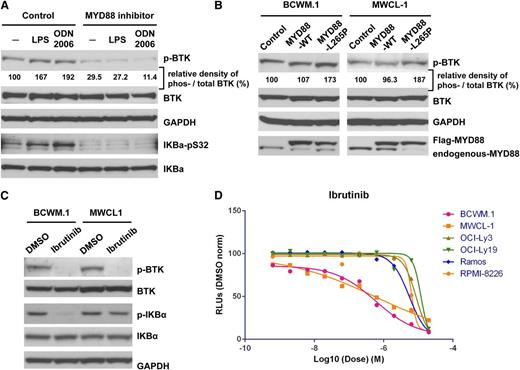
TLR receptor stimulation induces BTK activation through MYD88 in WM cells
Since both BCWM.1 and MWCL-1 cells each possess one copy of L265P, and one copy of WT MYD88, we next tried to clarify whether MYD88 triggered BTK activity following TLR stimulation in WM cells. For these experiments, we treated WM cells with LPS or CpG oligonucleotides (ODN 2006) given their established role as triggers of TLR-directed NF-κB signaling through MYD88.27 Treatment of BCWM.1 cells with either LPS or ODN 2006 increased both BTK and IκBα phosphorylation, whereas concurrent treatment with a MYD88 inhibitor blocked both BTK and IκBα constitutive as well as LPS or ODN 2006 induced phosphorylation by western blot analysis (Figure 6A).
MYD88 signals through BTK in response to TLR stimulation, and BTK is stimulated by MYD88 L265P in WM cells. (A) The impacts of TLR4 ligand, LPS, and TLR9 ligand, ODN 2006 (a type B CpG oligonucleotide; InvivoGen), on the phosphorylation of BTK and IκBα in BCWM.1 WM cells in the presence or absence of a MYD88 inhibitor. (B) Total and phospho-BTK levels in WM cells transduced with a lentiviral control vector, or vectors engineered to overexpress flag-tagged MYD88 WT or L265P coding regions. (C) The impact of ibrutinib on the phosphorylation state of BTK and IκBα depicted by western blot analysis. (D) Dose-response curve for ibrutinib on the cell viability of MYD88 L265P-bearing BCWM.1 and MWCL-1 WM cells, and OCI-Ly3 DLBCL cells vs MYD88 WT-expressing Ramos, OCI-Ly19, and RPMI 8226.
MYD88 signals through BTK in response to TLR stimulation, and BTK is stimulated by MYD88 L265P in WM cells. (A) The impacts of TLR4 ligand, LPS, and TLR9 ligand, ODN 2006 (a type B CpG oligonucleotide; InvivoGen), on the phosphorylation of BTK and IκBα in BCWM.1 WM cells in the presence or absence of a MYD88 inhibitor. (B) Total and phospho-BTK levels in WM cells transduced with a lentiviral control vector, or vectors engineered to overexpress flag-tagged MYD88 WT or L265P coding regions. (C) The impact of ibrutinib on the phosphorylation state of BTK and IκBα depicted by western blot analysis. (D) Dose-response curve for ibrutinib on the cell viability of MYD88 L265P-bearing BCWM.1 and MWCL-1 WM cells, and OCI-Ly3 DLBCL cells vs MYD88 WT-expressing Ramos, OCI-Ly19, and RPMI 8226.
MYD88 L265P overexpression stimulates BTK activation in WM cells
We next sought to clarify the impact of MYD88 L265P vs WT expression on BTK activation in WM cells in the absence of TLR pathway stimulation. BCWM.1 and MWCL-1 cells were transduced with MYD88 L265P and showed more robust BTK phosphorylation vs control vector-transduced cells. Total BTK levels remained unaltered in these experiments. In contrast, WM cells transduced to overexpress MYD88 WT did not show any differences in either BTK phosphorylation or total BTK levels vs control transduced cells (Figure 6B).
The BTK inhibitor ibrutinib blocks phosphorylation of IκBα and leads to enhanced tumor cell killing in MYD88 L265P-expressing WM cells
Since BTK is a component of MYD88 signaling, we next examined the impact of ibrutinib on downstream IκBα phosphorylation and tumor cell killing in MYD88 L265P-expressing WM cells. These studies showed that ibrutinib blocked phosphorylation of IκBα without imparting changes in total BTK or IκBα levels (Figure 6C). Ibrutinib also induced higher levels of killing in MYD88 L265P-expressing WM vs MYD88 WT-expressing cells (Figure 6D), as well as MYD88 L265P-expressing OCI-Ly3 DLBCL cells which carry a mutation in CARD11 downstream of BTK.
Dual inhibition of BTK and IRAK leads to synergistic killing in WM cells, and more robust suppression of NF-κB activity
Because MYD88 triggers both IRAK and BTK activation in L265P-expressing WM cells, and since both IRAK and BTK lead to IκBα activation in WM cells, we next sought to clarify the impact of blocking both IRAK 1 and 4, and BTK kinase activity on WM cell survival and NF-κB activity. The use of dual IRAK 1 and 4 and BTK kinase inhibitors resulted in increased killing of BCWM.1 and MWCL-1 WM cell lines. Combination index (CI) analysis in BCWM.1 and MWCL-1 cells denoted synergistic tumor cell killing for ibrutinib and the IRAK1 and 4 kinase inhibitor at most dose levels for BCWM.1, and at higher doses (>1.9 μM) levels of IRAK 1 and 4 kinase inhibitor for MWCL-1 (Figure 7A-D). Increased tumor cell killing in response to dual BTK and IRAK 1 and 4 kinase inhibition was associated with more robust inhibition of NF-κB p65 luciferase reporter activity as well as more pronounced inhibition of IκBα phosphorylation in BCWM.1 cells (Figure 7E-F). Lastly, dual BTK and IRAK 1 and 4 kinase inhibition of CD19+ lymophoplasmacytic cells from BMMCs from 4 WM patients genotyped for MYD88 L265P showed increased tumor cell killing vs each agent alone, whereas no such effect was observed in primary CD19+ cells from 4 healthy donors genotyped for MYD88 WT expression (Figure 7G).
Dual inhibition of BTK and IRAK leads to synergistic killing in MYD88 L265P-expressing WM cells, and more robust suppression of NF-κB pathway activity. (A,C) BCWM.1 and MWCL-1 cells were treated with an inhibitor of BTK (ibrutinib; PCI 32765), IRAK 1 and 4, or both. Cell death was assessed by high-throughput CellTiter-Glo Luminescent cell viability assay. (B,D) Synergism was assessed by CI analysis, with the heat maps depicting the CI values at varying dosimetry for ibrutinib and the IRAK 1 and 4 kinase inhibitor. CI, combination index. (E) For these experiments, CI values <1 denote synergistic interactions. p65-NF-κB activity was assessed by a luciferase promoter assay at 6 hours in BCWM.1 cells. The doses were used as follows: ibrutinib (lane a, 5.000; lane b, 1.580; lane c, 0.500; lane d, 0.158; lane e, 0.050; lane f, 0.016 µM) and IRAK1/4 kinase inhibitor (lane g, 20.000; lane h, 6.325; lane i, 2.000; lane j, 0.633; lane k, 0.200; lane l, 0.063 µM). Experiments were performed in triplicate. A representative experimental set is shown. (F) The inhibition of NF-κB activity was also assessed by examination of phospho-IkBα following treatment with ibrutinib, IRAK 1 and 4 kinase inhibitor, or both using western blot analysis in BCWM.1 cells. (G) Primary BMMCs from 4 WM patients genotyped for MYD88 L265P patients, and PBMCs from 4 healthy donors genotyped for MYD88 WT were cultured with ibrutinib (4.0, 2.0, 1.0 μM) or IRAK 1 and 4 inhibitor (20.0, 10.0, 5.0 μM) or in combination for 24 hours. Cell apoptosis was assessed in CD19+-expressing cells following annexin V staining.
Dual inhibition of BTK and IRAK leads to synergistic killing in MYD88 L265P-expressing WM cells, and more robust suppression of NF-κB pathway activity. (A,C) BCWM.1 and MWCL-1 cells were treated with an inhibitor of BTK (ibrutinib; PCI 32765), IRAK 1 and 4, or both. Cell death was assessed by high-throughput CellTiter-Glo Luminescent cell viability assay. (B,D) Synergism was assessed by CI analysis, with the heat maps depicting the CI values at varying dosimetry for ibrutinib and the IRAK 1 and 4 kinase inhibitor. CI, combination index. (E) For these experiments, CI values <1 denote synergistic interactions. p65-NF-κB activity was assessed by a luciferase promoter assay at 6 hours in BCWM.1 cells. The doses were used as follows: ibrutinib (lane a, 5.000; lane b, 1.580; lane c, 0.500; lane d, 0.158; lane e, 0.050; lane f, 0.016 µM) and IRAK1/4 kinase inhibitor (lane g, 20.000; lane h, 6.325; lane i, 2.000; lane j, 0.633; lane k, 0.200; lane l, 0.063 µM). Experiments were performed in triplicate. A representative experimental set is shown. (F) The inhibition of NF-κB activity was also assessed by examination of phospho-IkBα following treatment with ibrutinib, IRAK 1 and 4 kinase inhibitor, or both using western blot analysis in BCWM.1 cells. (G) Primary BMMCs from 4 WM patients genotyped for MYD88 L265P patients, and PBMCs from 4 healthy donors genotyped for MYD88 WT were cultured with ibrutinib (4.0, 2.0, 1.0 μM) or IRAK 1 and 4 inhibitor (20.0, 10.0, 5.0 μM) or in combination for 24 hours. Cell apoptosis was assessed in CD19+-expressing cells following annexin V staining.
Discussion
MYD88 L265P is a highly prevalent somatic mutation which in some series has been observed in >90% of patients with WM.3-8 We therefore sought to clarify the functional role of the MYD88 L265P mutation in WM with these studies. By lentiviral knockdown of MYD88 studies, and/or use of inhibitors which block MYD88 and IRAK activity, we observed that MYD88 signaling was supportive of WM growth and survival. Overexpression of MYD88 L265P promoted enhanced survival of WM cells vs WT MYD88, consistent with a gain-of-function mutation as reported by Ngo et al11 in ABC DLBCL cells.
As reported by Ngo et al,11 we also observed the dependence of IRAK1 and IκBα signaling on MYD88 in L265P-expressing WM cells. IκBα serves as a critical determinant of NF-κB p65 nuclear translocation.28 Inhibition of MYD88 blocks IκBα phosphorylation in L265P-expressing ABC DLBCL,11 and also in WM cells as reported herein. Although IκBα is a downstream component of the IRAK signaling cascade, other pathways can also trigger its activation including BTK.29,30 BTK is constitutively activated in WM cells and its inhibition blocks IκBα activity in WM cells as shown in these studies. We therefore sought to determine whether MYD88 L265P was responsible for the BTK activation in WM cells. Our studies showed that MYD88 was preferentially complexed to phosphorylated BTK in both L265P-expressing WM cell lines, whereas little complexing was observed in MYD88 WT cells. Importantly, knockdown of MYD88 or the use of a MYD88 inhibitor abrogated BTK activity in L265P-expressing cell lines, while overexpression of MYD88 L265P showed more robust BTK activity. In contrast, overexpression of MYD88 WT did not show enhanced BTK activation over control vector-transduced cells. Finally, the use of ibrutinib, an inhibitor of BTK kinase activity, resulted in decreased MYD88-BTK complexing in MYD88 L265P-expressing cells. Taken together, these findings denote that BTK binds to, and is activated in response to, MYD88 L265P and promotes activation of IκBα. Moreover, the lack of effect of IRAK 1 and 4 inhibition on BTK activity, and vice versa, using inhibitors for each of the kinases suggests that these pathways are likely distinct in mediating MYD88-related NF-κB activation.
An interesting finding in these studies was the presence of an intact TLR signaling pathway in BCWM.1 and MWCL-1 cells, both of which express 1 WT MYD88 allele. Inhibition of MYD88 blocked IκBα signaling by both LPS and ODNs in these cells, consistent with prior studies which focused on WT MYD88 signaling. It remains unclear from our experiments whether MYD88 L265P is capable of transmitting TLR signaling, and such studies are now in progress in our laboratory to clarify this important point. These findings may, however, have important clinical implications because, not uncommonly, flares in WM disease can occur in patients who are septic or experiencing viral infections; they can also occur in patients after receiving vaccinations. The preservation of intact TLR signaling offered by either the MYD88 WT or MYD88 L265P could be etiologic for these observations.
Central to these findings are the implications for targeting the MYD88 pathway for WM therapy, including use of IRAK and BTK inhibitors. Advanced in the clinical development pathway are inhibitors of BTK kinase activity whose prototype ibrutinib is a potent inducer of apoptosis of MYD88 L265P-expressing WM cells,25 with encouraging activity observed in several WM patients included in a phase 1 study as well as in an ongoing phase 2 clinical trial.31,32 The induction of BTK activity by MYD88 L265P may therefore represent the underlying mechanism by which BTK inhibitors induce killing of malignant cells in WM patients, and provides further support for the investigation of BTK inhibitors in WM. It is interesting that ibrutinib-mediated inhibition of BTK activity did not affect activation state or protein levels of IRAK 1 and vice versa, supporting that MYD88 signaling through these pathways is likely to be distinct. Consistent with this observation, the combined use of IRAK and BTK inhibitors resulted in augmented inhibition of NF-κB signaling and more robust WM cell killing. These studies therefore provide the rationale for development of MYD88 inhibitors with the potential to more proximally block BTK and IRAK signaling, or conversely use a combination of BTK and IRAK inhibitors in WM patients, as well as in patients with other diseases dependent on MYD88 L265P signaling.
In summary, we demonstrate by lentiviral transduction and MYD88 pathway inhibitors that acquisition of MYD88 L265P confers a survival advantage in WM cells. Survival of WM cells is predicated by induction of both IRAK and BTK signaling, the latter of which represents a novel finding for MYD88 L265P signaling. Importantly, these studies provide the framework for the investigation of inhibitors targeting MYD88 and its downstream pathways for the treatment of WM.
Presented in part at the annual meetings of the American Society for Clinical Oncology, June 1-5, 2012, and the American Society for Hematology, December 7-11, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the contributions of Roodolph St. Pierre, Michael McKeown, and Sanjay Divakaran from Dr James Bradner’s Laboratory at DFCI for their help with high-throughput drug screening and synergism analyses, as well as Susan Lazo-Kallanian, John Daley, and Robert W. Smith from the DFCI Flow Cytometry Facility for their help in performing flow cytometric analyses.
The authors acknowledge the generous support of Peter and Helen Bing, the Coyote Fund for WM, the Edward and Linda Nelson Fund for WM Research, the Bauman Family Trust, the Tannenhauser Family Foundation, and the WM patients who provided samples for their support of these studies.
Authorship
Contribution: G.Y., Z.R.H., Y.Z., and S.P.T. conceived and designed the experiments; G.Y., Z.R.H., and S.P.T. performed data analysis and wrote manuscript; L.X., Y.C., X.L., G.Y., R.J.M., and C.J.P. procured and/or prepared samples; G.Y., Y.C., Y.-T.T., L.X., and S.J.B. performed functional assays and assessments; Y.Z. and X.L. performed lentiviral transduction experiments; S.P.T. provided patient care, obtained consent, and samples; R.J.M. collected patient data; and N.G. and K.C.A. provided input for assays and data analysis.
Conflict-of-interest disclosure: S.P.T. has received research funding and consulting fees from Pharmacyclics, Inc. and Jansen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström’s Macroglobulinemia, Dana-Farber Cancer Institute, 450 Brookline Ave, M548, Boston, MA 02215; e-mail: steven_treon@dfci.harvard.edu.