Key Points
CD107a protects cytotoxic lymphocytes from damage during degranulation.
Interference with CD107a expression can cause the death of cytotoxic lymphocytes during degranulation.
Abstract
Cytotoxic lymphocytes are important for immune responses against viral infections and cancer. They are able to kill target cells through the release of cytotoxic granules (CGs) without being harmed in the process. Because the lysosomal-associated membrane proteins (LAMPs) appear on the cell surface after CG exocytosis, we hypothesized that some of these proteins might be involved in transiently protecting cytotoxic lymphocytes from self-destruction. Intracellular expression of CD107a/LAMP-1, and to a lesser extent that of CD107b/LAMP-2, correlated with lymphocyte CG content. Engineered surface expression of CD107a/LAMP-1, but not of CD107b/LAMP-2, reduced the granule-mediated killing of transfected target cells. This was dependent on glycosylation of the CD107a/LAMP-1 hinge. Moreover, surface expression of CD107a/LAMP-1 reduced binding of perforin to cells. Importantly, knockdown of CD107a/LAMP-1 in primary human natural killer (NK) cells and deficiency of CD107a/LAMP-1 in mice resulted in increased NK cell apoptosis upon target cell–induced degranulation. Thus, our data support a novel role of CD107a/LAMP-1 in the protection of NK cells from degranulation-associated suicide, which may represent a general mechanism to transiently limit self-destruction by cytotoxic lymphocytes upon target cell killing.
Introduction
The killing of virally infected or transformed cells by cytotoxic lymphocytes involves firm adhesion to the target cell and the formation of an immunologic synapse (IS).1,2 Cytotoxic granules (CGs) are then polarized toward the IS and fuse with the cytotoxic lymphocyte’s membrane to release their content within the synaptic cleft, ultimately leading to the lysis of the target cell. Remarkably, cell death is specific and unidirectional.3
CGs are a specialized form of secretory lysosomes and contain granzymes, perforin, granulysin, cathepsins, and the lysosome-associated membrane proteins CD107a/LAMP-1, CD107b/LAMP-2, and CD63/LAMP-3.4-9 Perforin can bind to the target cell’s membrane and mediates the delivery of granzymes into the cytoplasm, where they induce apoptosis.10-12 Inside the CGs, granzymes form complexes with the proteoglycan serglycin and are kept inactive by low pH and calcium concentrations.13-16 These mechanisms are considered essential to prevent damage to cytotoxic cells from the content of their CGs.11 However, once secreted into the synaptic cleft, where Ca2+ concentrations are high and the pH is neutral, other mechanisms are required to facilitate unidirectional killing and to prevent cytotoxic lymphocyte self-destruction. To this end, cytotoxic lymphocytes endocytose granzyme B, reducing the concentration of active granzyme B17 and express the cytoplasmic serine protease inhibitor serpinB9, which can inactivate granzyme B that has leaked into the cytoplasm.18,19 Moreover, surface cathepsin B has been suggested to cleave perforin at the cytotoxic cell’s membrane,20 but cytotoxic T lymphocytes (CTLs) from cathepsin B–deficient mice survive normally after target cell encounter.21 Additional protective mechanisms may exist.
Because of their transient accumulation at the IS upon degranulation, we hypothesized that LAMPs might serve to protect cytotoxic lymphocytes upon degranulation. In particular, CD107a/LAMP-1 has been widely used as a marker for degranulating cytotoxic lymphocytes, because surface expression is induced upon degranulation.22-24 CD107a is a highly glycosylated protein comprising a 40-kDa backbone with predicted 17 N-glycosylation and 9 O-glycosylation sites. The intralysosomal part of CD107a comprises 2 highly N-glycosylated domains, separated by a proline and serine-rich hinge that is highly O-glycosylated.25 Here we show that engineered plasma membrane expression of CD107a could protect target cells from lysis by cytotoxic natural killer (NK) cells as well as by purified CGs. Small interfering RNA (siRNA)-mediated knockdown of CD107a in human NK cells or genetic deletion of CD107a in mice resulted in an increase of apoptotic NK cells after target cell–induced degranulation. These results provide evidence supporting a role of CD107a in the protection of cytotoxic cells from degranulation-associated suicide.
Material and methods
Cells
NK cell assays
The 51Cr release assay was performed as described.30 For fluorescence-activated cell sorting (FACS)–based killing assays 721.221 cells were mixed with NK cells at an E/T of 0.5 and coincubated for 4 hours. Thereafter, cells were stained with anti–CD56-FITC, followed by staining with anti–CD107a-PeCy5. Subsequently, cells were washed and stained with PE-conjugated Annexin-V according to the manufacturer’s protocol. For the killing assay with purified granules, HeLa cells were incubated with sonicated granules in medium containing 5 mM CaCl2. Cells were harvested and Annexin-V staining was performed. Where indicated, percentage of inhibition was calculated as follows: For each experiment, mean lysis of control-transfected cells at a given E/T was determined. Subsequently, each experimentally determined value of the same E/T was set in relation to this mean by the equation (1 − value/mean) × 100. Outliers were determined and neglected. For assessing mouse NK cell self-destruction, cells were coincubated with RMA/S target cells as indicated for 4 hours. The percentage of a specific increase in Annexin-V–positive cells was calculated as follows: The mean of the percentage of Annexin-V–positive cells in the absence of target cells was determined for each experiment. Subsequently, normalization was conducted by dividing the percentage of Annexin-V–positive cells of a given experiment at a given E/T by the the mean percentage of Annexin-V–positive cells without targets × 100 – 100.
Statistical analysis
Outliers were calculated by the Fourth-Spread (fs) method. The median and the 75th and 25th quartiles of the data set were determined. The fs was calculated as 75th to 25th quartile. The upper and lower outlier boundaries (OBs) were determined as median + (1.5*fs) and Median − (1.5*fs), respectively. Values lying out of each OB were regarded as outlier. Statistical analysis was performed using GraphPad’s Prism software. Equal variances were determined using D’Agostino’s and Pearson’s omnibus test, α = .05).
For a description of antibodies, reagents, cDNA constructs, siRNA transfection, purification of CGs, and deglycosylation of cells, see the supplemental Material and methods (available on the Blood Web site).
Results
Surface CD107a protects target cells from NK cell–mediated lysis
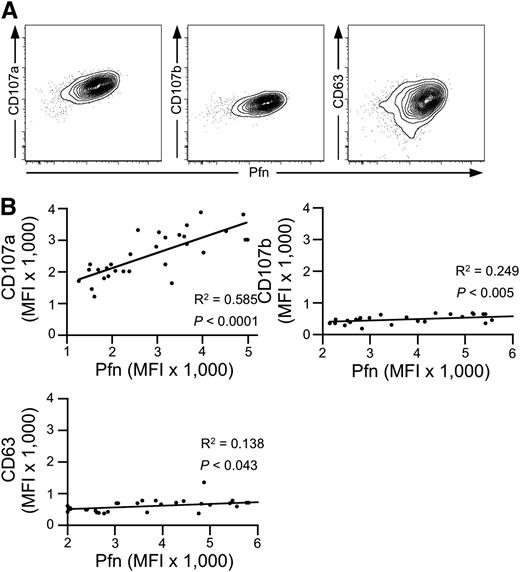
We first investigated the expression of CD107a/LAMP-1, CD107b/LAMP-2, and CD63/LAMP-3, as well as perforin in cytotoxic NK and T-cell subsets by intracellular staining followed by flow cytometry. Among the LAMPs, CD107a displayed the highest levels of expression in cytotoxic, perforin-expressing subsets of NK (CD3–CD56dim) and T (CD3+CD8+CD27–) cells (supplemental Figure 1A). Intracellular expression of both CD107a and CD107b was enriched in cytotoxic lymphocyte subsets, whereas expression of CD63 was not. Upon stimulation, surface expression of CD107a and CD107b was induced on NK and T cells, as previously described.22,23 In contrast, CD63 was expressed constitutively on the cell surface and increased upon stimulation6 (supplemental Figure 1B-C). At the single-cell level, intracellular CD107a expression positively correlated with perforin expression (Figure 1A). Similarly, albeit weaker, intracellular CD107b expression correlated with perforin expression, whereas intracellular CD63 expression did not clearly correlate with perforin expression. Comparing intracellular mean expression of granule constituents in the CD3–CD56dim NK cell subset of 30 healthy donors, perforin expression correlated strongly with that of CD107a but less so with CD107b or CD63 (Figure 1B). Thus, expression of CD107a, followed by that of CD107b, correlates most closely with the expression of perforin in cytotoxic lymphocytes, when comparing both intracellular expression levels among cytotoxic lymphocytes in an individual as well as mean expression in cytotoxic lymphocyte populations among different individuals.
Correlation of intracellular CD107a and perforin expression. (A) Intracellular FACS staining of CD107a, CD107b, and CD63 in combination with perforin (Pfn) in primary human NK cells (gated on CD3–CD56dim lymphocytes). Shown is one representative donor of 30. (B) Correlation among CD107a, CD107b, CD63, and Pfn on the population level. The Pfn mean fluorescence intensity (MFI) of cell populations acquired as in (A) was determined and plotted against the MFI of CD107a, CD107b, and CD63 for each healthy adult donor (N = 30). Linear regression was calculated according to Pearson. R2 and P values are indicated.
Correlation of intracellular CD107a and perforin expression. (A) Intracellular FACS staining of CD107a, CD107b, and CD63 in combination with perforin (Pfn) in primary human NK cells (gated on CD3–CD56dim lymphocytes). Shown is one representative donor of 30. (B) Correlation among CD107a, CD107b, CD63, and Pfn on the population level. The Pfn mean fluorescence intensity (MFI) of cell populations acquired as in (A) was determined and plotted against the MFI of CD107a, CD107b, and CD63 for each healthy adult donor (N = 30). Linear regression was calculated according to Pearson. R2 and P values are indicated.
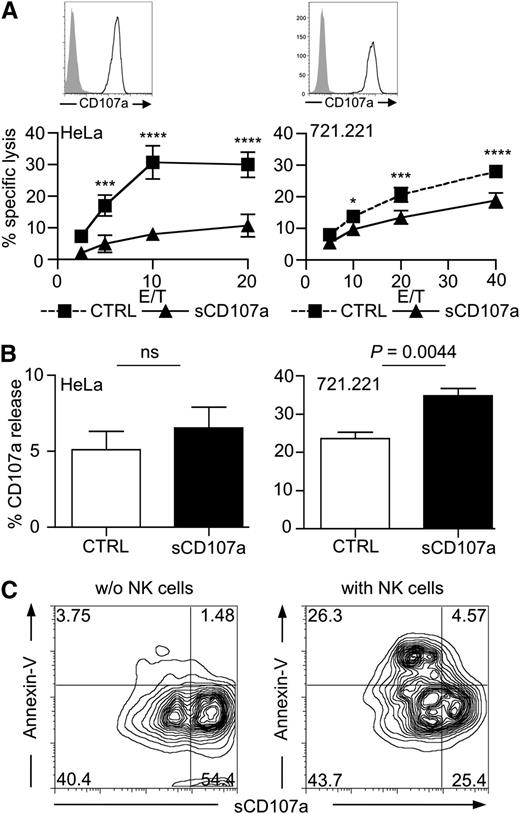
In light of these correlations, we hypothesized that CD107a might be involved in transiently protecting cytotoxic cells from perforin-mediated self-destruction during target cell lysis. If this were true, overexpression of CD107a on the membrane of target cells should reduce their sensitivity toward lysis by cytotoxic lymphocytes. To test this, we generated HeLa and 721.221 cells stably overexpressing CD107a on their surface (sCD107a). To facilitate cell surface expression, the cytoplasmic domain of CD107a was truncated by introducing a stop codon, thereby removing the cytoplasmic HAGY (single amino acid code) motif implicated in lysosomal targeting of newly synthesized CD107a molecules31 (supplemental Figure 2). Surface CD107a expression was verified by flow cytometry (Figure 2A) and microscopy (supplemental Figures 2B and 3). Next, we investigated whether sCD107a expression could affect the lysis of transfected cells by cytotoxic lymphocytes. Because of cytotoxic NK cells’ broad specificity for tumor cells, we used them as a model system. Remarkably, sCD107a expression reduced the NK cell–mediated killing of HeLa and 721.221 cells compared with the killing of control-transfected cells (Figure 2A).
Surface CD107a renders cells less sensitive to NK cell–mediated lysis. (A) Surface CD107a reduces NK cell lysis of target cells. Top: Surface expression of CD107a on the indicated transfected cells was tested by FACS analysis using anti-CD107a antibody (black) or isotype control (shaded). Bottom: 4-hour 51Cr release assay using IL-2–activated human NK cells with HeLa (left) or 721.221 (right) target cells, stably expressing surface CD107a (triangles) or Clec12B as a control (squares), at the indicated effector to target (E/T) ratios. Values represent triplicates ± SD; statistical analysis was performed using 2-way analysis of variance (ANOVA); n = 5. From low to high E/T, the P values are: nonsignificant .0009; < .0001; and < .0001 (for HeLa cells); and nonsignificant .0315; .0002; and < .0001 (for 721.221 cells). (B) Degranulation induced by sCD107a-expressing target cells. IL-2–activated human NK cells were incubated for 3 hours with HeLa (left) or 721.221 (right) cells expressing either Clec12B (CTRL) or sCD107a in the presence of anti-CD107a antibody. CD107a externalization on CD56+ events was assessed; means ± SEM statistical analysis was performed using the unpaired 2-tailed Student t test; n = 6. (C) 721.221 cells expressing low levels and high levels of sCD107a were incubated for 4 hours without (left) or with (right) IL-2–activated human NK cells. Cells were stained for CD56 and CD107a, followed by Annexin-V staining. Shown are the events of the CD56– gate; n = 3 independent experiments.
Surface CD107a renders cells less sensitive to NK cell–mediated lysis. (A) Surface CD107a reduces NK cell lysis of target cells. Top: Surface expression of CD107a on the indicated transfected cells was tested by FACS analysis using anti-CD107a antibody (black) or isotype control (shaded). Bottom: 4-hour 51Cr release assay using IL-2–activated human NK cells with HeLa (left) or 721.221 (right) target cells, stably expressing surface CD107a (triangles) or Clec12B as a control (squares), at the indicated effector to target (E/T) ratios. Values represent triplicates ± SD; statistical analysis was performed using 2-way analysis of variance (ANOVA); n = 5. From low to high E/T, the P values are: nonsignificant .0009; < .0001; and < .0001 (for HeLa cells); and nonsignificant .0315; .0002; and < .0001 (for 721.221 cells). (B) Degranulation induced by sCD107a-expressing target cells. IL-2–activated human NK cells were incubated for 3 hours with HeLa (left) or 721.221 (right) cells expressing either Clec12B (CTRL) or sCD107a in the presence of anti-CD107a antibody. CD107a externalization on CD56+ events was assessed; means ± SEM statistical analysis was performed using the unpaired 2-tailed Student t test; n = 6. (C) 721.221 cells expressing low levels and high levels of sCD107a were incubated for 4 hours without (left) or with (right) IL-2–activated human NK cells. Cells were stained for CD56 and CD107a, followed by Annexin-V staining. Shown are the events of the CD56– gate; n = 3 independent experiments.
The reduced killing sCD107a-expressing targets cells could have been the result of reduced NK cell adhesion caused by repulsion mediated by the highly glycosylated CD107a molecules. However, NK cell adherence to target cells was not affected by sCD107a expression (supplemental Figure 4A). In addition, sCD107a was clearly detectable in the contact area between the NK cells and the sCD107a-expressing HeLa cells (supplemental Figure 3). Moreover, NK-cell degranulation upon incubation with target cells was, if anything, slightly enhanced by sCD107a expression (Figure 2B). The notion that sCD107a-expressing cells are more resistant to lysis was further substantiated by the observation that, in a 721.221 cell line with mixed expression levels of sCD107a, the cells with lower surface expression of CD107a were preferentially killed by NK cells (Figure 2C). Together, the data indicate that surface CD107a expression on target cells can specifically inhibit NK cell–mediated lysis without impairing recognition and degranulation. Therefore, CD107a surface expression may protect cells from lysis by inhibiting the function of perforin or granzymes.
Surface CD107a reduces perforin binding
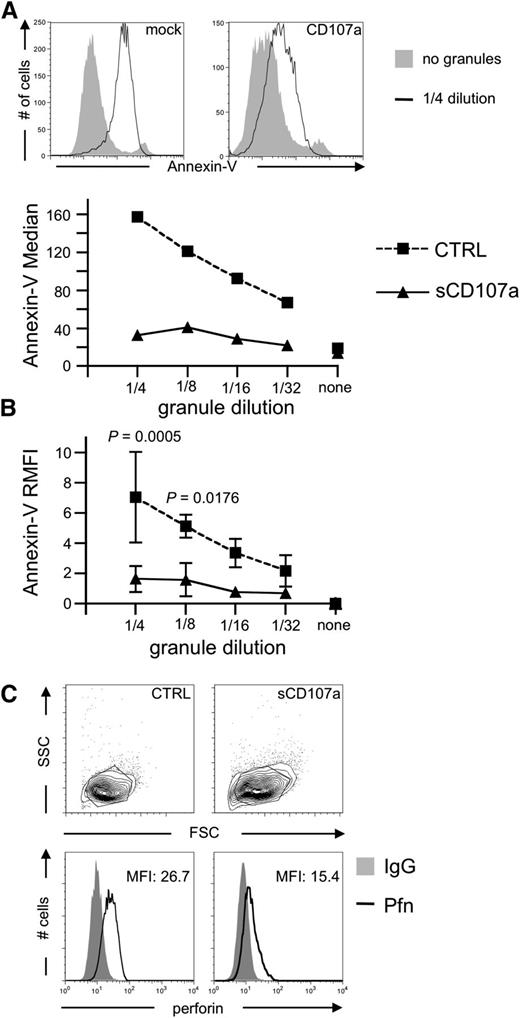
To test whether sCD107a can directly inhibit the effect of perforin or granzymes, CGs were purified from the human NK cell line YTS9 and incubated with sCD107a or control-transfected HeLa cells. CGs induced apoptosis in control-transfected HeLa cells in a dose-dependent manner (Figure 3A-B). However, sCD107a-expressing HeLa cells were significantly less sensitive to apoptosis. Similar results were obtained for CGs purified from the human NK cell line NKL (data not shown), demonstrating that surface CD107a expression can directly protect cells from lysis by CG content. Upon incubation of HeLa cells with purified CGs, perforin bound to the surface of the cell (Figure 3C). Interestingly, such binding was significantly less prominent in the case of sCD107a-expressing cells (Figures 3C and 4E), which might explain their reduced sensitivity toward the content of the purified CGs.
CD107a-expressing target cells are less sensitive to Pfn-mediated apoptosis induction. (A) HeLa cells were coincubated with sonicated CGs purified from YTS cells. After 4 hours, cells were stained with Annexin-V to assess apoptotic cells. Top: Annexin-V staining of the indicated cells with or without granules. Bottom: Median Annexin-V fluorescence intensities at different granule dilutions. (B) Relative median fluorescence of 3 independent experiments as depicted in (A). Statistical analysis was performed using 2-way ANOVA; n = 6. (C) Representative surface staining of Pfn on the indicated HeLa cells after 4 hours of coincubation with sonicated CGs; n = 8.
CD107a-expressing target cells are less sensitive to Pfn-mediated apoptosis induction. (A) HeLa cells were coincubated with sonicated CGs purified from YTS cells. After 4 hours, cells were stained with Annexin-V to assess apoptotic cells. Top: Annexin-V staining of the indicated cells with or without granules. Bottom: Median Annexin-V fluorescence intensities at different granule dilutions. (B) Relative median fluorescence of 3 independent experiments as depicted in (A). Statistical analysis was performed using 2-way ANOVA; n = 6. (C) Representative surface staining of Pfn on the indicated HeLa cells after 4 hours of coincubation with sonicated CGs; n = 8.
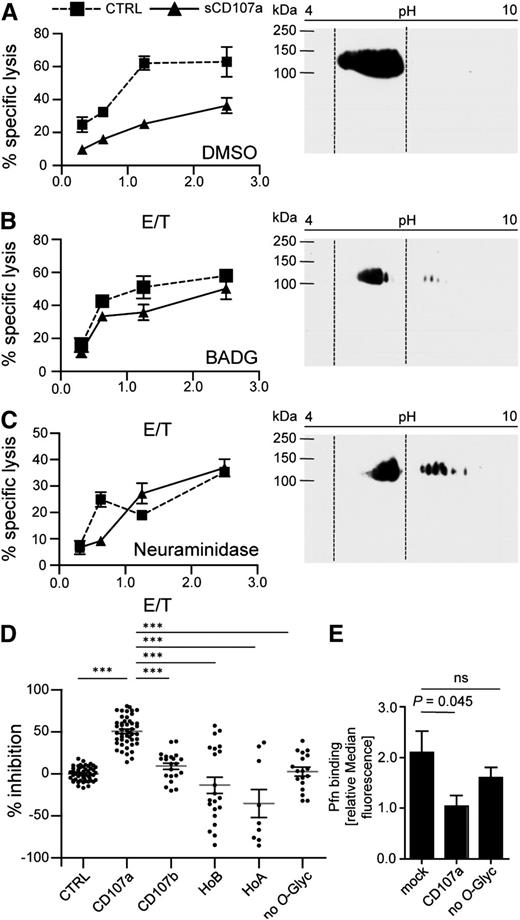
O-linked glycosylation of CD107a is necessary for reduced killing of surface CD107a-expressing cells. sCD107a (triangle) or control (squares) expressing 721.221 cells were incubated for 48 hours in the presence of DMSO (A) or BADG (B), or for 2 hours with NA (C). Left panels: Cells were used in a 4-hour 51Cr release assay with IL-2–activated human NK cells at different E/T ratios. Values represent triplicates ± SD. Right panels: Cells were lysed and analyzed by 2-dimensional anti-CD107a Western blotting analysis. Dotted lines indicate localization of CD107a in DMSO-treated control samples; n = 3. (D) IL-2–activated human NK cells were incubated with HeLa cells stably expressing the indicated sCD107a mutants. Specific lysis was assessed by 4-hour 51Cr release assay. Percentage of inhibition was calculated as stated in Materials and methods. Shown is the mean ± SEM. Each dot represents a single value of triplicates from 4 to 13 experiments of an E/T of 10/1. Significances were analyzed using 1-way ANOVA (P < .0001) followed by Bonferroni’s multiple comparison; ***P < .001. (E) Statistics for Pfn binding ± SEM as analyzed in Figure 3C. Statistical analysis was performed by 1-way ANOVA; n = 8. ns, not significant.
O-linked glycosylation of CD107a is necessary for reduced killing of surface CD107a-expressing cells. sCD107a (triangle) or control (squares) expressing 721.221 cells were incubated for 48 hours in the presence of DMSO (A) or BADG (B), or for 2 hours with NA (C). Left panels: Cells were used in a 4-hour 51Cr release assay with IL-2–activated human NK cells at different E/T ratios. Values represent triplicates ± SD. Right panels: Cells were lysed and analyzed by 2-dimensional anti-CD107a Western blotting analysis. Dotted lines indicate localization of CD107a in DMSO-treated control samples; n = 3. (D) IL-2–activated human NK cells were incubated with HeLa cells stably expressing the indicated sCD107a mutants. Specific lysis was assessed by 4-hour 51Cr release assay. Percentage of inhibition was calculated as stated in Materials and methods. Shown is the mean ± SEM. Each dot represents a single value of triplicates from 4 to 13 experiments of an E/T of 10/1. Significances were analyzed using 1-way ANOVA (P < .0001) followed by Bonferroni’s multiple comparison; ***P < .001. (E) Statistics for Pfn binding ± SEM as analyzed in Figure 3C. Statistical analysis was performed by 1-way ANOVA; n = 8. ns, not significant.
Glycosylation is involved in the protective function of sCD107a
To test whether the related molecule CD107b can also protect cells from granule-mediated lysis, we similarly expressed CD107b on the surface of HeLa cells. Interestingly, sCD107b expression did not protect HeLa cells from NK cell–mediated lysis (Figure 4D; supplemental Figure 4B). However, because CD107a and CD107b are detected by different antibodies, we cannot directly compare their absolute expression levels. Therefore, it may be possible that higher expression levels of sCD107b could also protect from NK cell–mediated lysis. CD107a and CD107b differ substantially in their hinge region (supplemental Figure 2C), which is probably their only site for O-linked glycans, but does not contain N-linked glycans. We speculated that the glycosylation of CD107a might be important for the lysis-reducing effect. To further explore this, we interfered with O-glycosylation by treating sCD107a or control-transfected 721.221 cells with the inhibitor benzyl 2-acetamido-2-deoxy-α-d-galactopyranoside (BADG) or dimethyl sulfoxide (DMSO) as control. After BADG treatment, CD107a shifted to a more basic pH in a 2-dimensional Western blot analysis, indicating reduced O-glycosylation (Figure 4A-B). Although the difference between sCD107a and control-transfected cells in the susceptibility against NK cell–mediated killing was still evident in DMSO-treated cells, BADG-treated sCD107a-expressing cells were equally prone to NK cell lysis compared with control-transfected cells (Figure 4A-B). We also assessed the importance of sialic acids for the effect of CD107a by using α2-3,6,8,8-neuraminidase (NA) that cleaves these moieties from newly synthesized proteins. Also after NA treatment, sCD107a-expressing cells were equally prone to NK cell lysis compared with control-transfected cells (Figure 4C). In addition to sCD107a, BADG and NA treatment affects the glycosylation of many other proteins. We therefore always compared treatment of sCD107a and control-transfected cells, as off-target effects should affect both cells equally. However, to specifically study the importance of CD107a glycosylation, a series of mutants were created (supplemental Figure 5) and expressed in HeLa cells at levels comparable with the sCD107a wild-type (WT) (supplemental Figure 6). A mutant sCD107a protein lacking all putative O-glycosylated serines and threonines within the hinge failed to protect target cells from NK cell–mediated lysis, underscoring the essential nature of O-linked glycans in the hinge for the function of CD107a (Figure 4D). We also mutated the different O-linked glycosylation sites in CD107a, either in isolation or in different combinations (supplemental Figures 5-7), but could not find any single glycosylation site solely responsible for the effect of CD107a. To specifically address the different glycosylation of the hinge between CD107a and CD107b, we generated a recombinant sCD107a protein comprising the hinge of CD107b (HoB). This mutant also failed to protect transfected cells from lysis, suggesting a special role of the CD107a hinge. However, cells expressing the HoA mutant (CD107b carrying the CD107a hinge) were also not protected from lysis. This indicates that the hinge region of CD107a is necessary, but not sufficient, for its protective effect. To study the possible effects of the domains N- and C-terminal of the hinge, chimeras were created by exchanging either region with that of the homologous protein (supplemental Figure 5). Our data show that a chimera of CD107a expressing the C-terminal region of CD107b (AAB) does not protect HeLa cells from NK cell–mediated lysis (supplemental Figure 7). This indicates that this C-terminal region, in addition to the hinge of CD107a, is involved in mediating its protective effect.
We also correlated perforin binding with the function of the different mutants. Perforin binding to sCD107a-expressing cells was significantly reduced, but this reduction was abolished upon mutation of all the hinge O-glycosylation sites (Figure 4E). Together these data suggest that hinge-associated sialic acids and O-glycosylation are important for inhibiting perforin binding to the membrane and thereby mediating the resistance of sCD107a-expressing cells to NK cell–mediated lysis.
Surface CD107a protects primary human NK cells from degranulation-associated damage
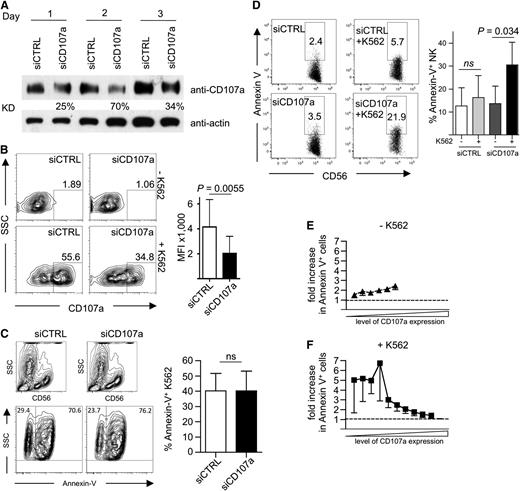
Next, we wanted to test the role of CD107a in protecting degranulating NK cells from damage induced by their CG content. CD107a expression was reduced by siRNA-mediated knockdown in primary human NK cells. Knockdown efficiency was highest on day 2 after siRNA delivery, with a 70% reduction of CD107a protein levels (Figure 5A). This resulted in a reduction of CD107a externalization on NK cells after target cell–induced degranulation (Figure 5B), whereas the killing of K562 cells was unaffected (Figure 5C and data not shown). More strikingly, in CD107a knockdown cells, the MFI of CD107a after degranulation was reduced by ∼50% when compared with control siRNA-treated NK cells (Figure 5B).
siRNA-mediated knockdown of CD107a in primary NK cells increases their apoptosis after target cell encounter. (A) Kinetic of CD107a knockdown. Human NK cells transfected with siRNA against CD107a or control siRNA (CTRL) were analyzed at the indicated day by anti-CD107a and anti-actin Western blotting analysis. KD, knockdown efficiency in relation to siCTRL-treated cells normalized to actin. (B) Left: Degranulation on day 2 after siRNA transfection in response to K562 cells. Cells were coincubated at an E/T of 0.5 for 3 hours in the presence of anti-CD107a antibodies and analyzed by FACS. Right: MFI of CD107a within the CD56+ gate was determined for siCTRL- (–) and siCD107a- (+) transfected NK cells. Statistics were performed using the paired 2-tailed Student t test; n = 5 ± SD. (C-F) Human NK cells transfected with siRNA against CD107a or control siRNA were incubated for 4 hours in the presence of anti-CD107a antibodies without (E) or with (C,F) K562. Cells were stained for CD56, followed by Annexin-V staining, and then analyzed by FACS. (C) K562 were identified by gating on CD56-negative events (top) and analyzed for Annexin-V staining. Data were plotted as mean ± SEM (right). Statistics were performed using the paired 2-tailed Student t test (C-D). (D) CD56+ NK cells were analyzed for Annexin-V staining in the absence or presence of K562 target cells. A representative staining is shown on the left. Statistical summary of data from 5 independent experiments are shown on the right. (E-F) The data shown in (D) were analyzed in relation to CD107a staining. See “Material and methods” for details on normalization. Dotted lines indicate equal ratios.
siRNA-mediated knockdown of CD107a in primary NK cells increases their apoptosis after target cell encounter. (A) Kinetic of CD107a knockdown. Human NK cells transfected with siRNA against CD107a or control siRNA (CTRL) were analyzed at the indicated day by anti-CD107a and anti-actin Western blotting analysis. KD, knockdown efficiency in relation to siCTRL-treated cells normalized to actin. (B) Left: Degranulation on day 2 after siRNA transfection in response to K562 cells. Cells were coincubated at an E/T of 0.5 for 3 hours in the presence of anti-CD107a antibodies and analyzed by FACS. Right: MFI of CD107a within the CD56+ gate was determined for siCTRL- (–) and siCD107a- (+) transfected NK cells. Statistics were performed using the paired 2-tailed Student t test; n = 5 ± SD. (C-F) Human NK cells transfected with siRNA against CD107a or control siRNA were incubated for 4 hours in the presence of anti-CD107a antibodies without (E) or with (C,F) K562. Cells were stained for CD56, followed by Annexin-V staining, and then analyzed by FACS. (C) K562 were identified by gating on CD56-negative events (top) and analyzed for Annexin-V staining. Data were plotted as mean ± SEM (right). Statistics were performed using the paired 2-tailed Student t test (C-D). (D) CD56+ NK cells were analyzed for Annexin-V staining in the absence or presence of K562 target cells. A representative staining is shown on the left. Statistical summary of data from 5 independent experiments are shown on the right. (E-F) The data shown in (D) were analyzed in relation to CD107a staining. See “Material and methods” for details on normalization. Dotted lines indicate equal ratios.
To investigate functional consequences of CD107a reduction for NK cell viability, cells were analyzed by Annexin-V staining. In this assay, we directly compared CD107a knockdown NK cells with control siRNA-treated cells and also measured the level of CD107a externalization. Without target cell incubation, the NK cells showed very little difference in Annexin-V staining when comparing CD107a knockdown with control cells (Figure 5D-E), demonstrating that the reduction of CD107a had no intrinsic toxic effect on the NK cells. Upon target cell–induced degranulation, however, we observed an increase of apoptotic NK cells in the CD107a knockdown sample (Figure 5D,F). Interestingly, this increase was only evident in the population of NK cells with reduced CD107a externalization, whereas cells with high CD107a externalization showed no increased apoptosis (Figure 5F). Because knockdown of CD107a reduces the amount of CD107a, which is externalized at the IS during NK cell degranulation, our results suggest that this reduced amount of CD107a is no longer able to effectively protect the NK cell membrane from damage induced by the content of its own CGs.
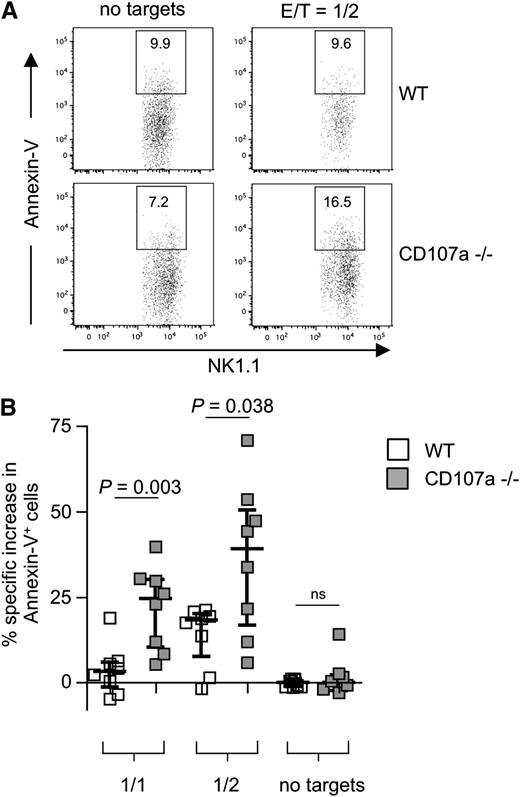
To study the effect of the complete absence of CD107a, NK cells from CD107a knockout mice were investigated.26 A slight but significant reduction in the overall frequency of NK cells was observed in the spleen of CD107a−/− mice (supplemental Figure 8A). We also analyzed the expression of several NK cell receptors and found minor variations in fresh NK cells, but not after IL-2 culturing (supplemental Figure 8B). When testing the ability of NK cells to kill RMA/S cells, a cell line susceptible to NK cell–mediated lysis, no difference between WT and CD107a−/− NK cells was found (supplemental Figure 8C). To test the propensity for NK cell suicide, IL-2–expanded NK cells were coincubated with RMA/S cells at different E/T ratios for 4 hours. In the absence of target cells, no significant difference in apoptosis was observed between CD107a−/− and WT NK cells, demonstrating no inherent toxic effect caused by the absence of CD107a. However, after target cell incubation, a significantly higher rate of CD107a−/− NK cells underwent apoptosis compared with WT NK cells (Figure 6). The effect was dependent on the amount of target cell exposure (24.6% more apoptotic CD107a−/− NK cells at an E/T of 1 and 39.1% more apoptotic CD107a−/− NK cells above WT control cells at an E/T of 0.5). This directly demonstrates that the absence of CD107a is associated with an increase in NK cell apoptosis, specifically after target cell encounter, and supports our hypothesis that CD107a is involved in protecting NK cells from degranulation-associated damage.
CD107a-deficient NK cells are more susceptible to apoptosis after target cell encounter. (A) Purified splenic IL-2–expanded NK cells from WT (top) or CD107a−/− (bottom) mice were incubated for 4 hours in the absence or presence of RMA/S cells. NK cells were identified by gating on CD3–NK1.1+ cells, and Annexin-V–positive cells were quantified. (B) Statistical summary of the data. Percentage of specific increase in Annexin-V–positive cells was calculated as described in “Material and methods”. NK cells conjugated to target cells were ignored to avoid false-positive events through apoptotic targets. Statistical analysis was performed using the 2-tailed Mann-Whitney U test. ns, not significant.
CD107a-deficient NK cells are more susceptible to apoptosis after target cell encounter. (A) Purified splenic IL-2–expanded NK cells from WT (top) or CD107a−/− (bottom) mice were incubated for 4 hours in the absence or presence of RMA/S cells. NK cells were identified by gating on CD3–NK1.1+ cells, and Annexin-V–positive cells were quantified. (B) Statistical summary of the data. Percentage of specific increase in Annexin-V–positive cells was calculated as described in “Material and methods”. NK cells conjugated to target cells were ignored to avoid false-positive events through apoptotic targets. Statistical analysis was performed using the 2-tailed Mann-Whitney U test. ns, not significant.
Discussion
Although CGs contain inactive forms of perforin and granzymes as a result of acidic pH, low concentrations of Ca2+, and complex formation with proteoglycans, the active forms of those proteins accumulate in the synaptic cleft upon degranulation. Thus, mechanisms are needed to protect cytotoxic lymphocytes from lysis by their own CGs. Here we have provided evidence that CD107a is involved in this protection. Reduction of CD107a by siRNA-mediated knockdown and knockout of CD107a caused an increase in apoptotic NK cells upon target cell encounter. This was likely caused by self-destruction of the degranulating NK cells, no longer protected by CD107a, and would explain the reason we saw this effect only in NK cells with low levels of sCD107a under knockdown conditions. However, not all CD107a−/− NK cells died upon degranulation. This may be explained by the fact that cytotoxic lymphocytes are protected from degranulation-mediated suicide by redundant systems including serpinB9,18,19 possibly cathepsin B,20 and, according to our data, CD107a.
Target cell lysis was not altered significantly in the absence of CD107a. Although the reduction of CD107a affects NK cell survival upon degranulation, these cells could still efficiently degranulate and thereby kill one locally attached target. In addition, only a fraction of NK cells die upon degranulation in the absence of CD107a. Although serial killing would be impaired, the time frame for our assays was probably too short to see a significant contribution by serial killing.32
Our data suggest that CD107a mediates its protection at least in part by reducing the binding of perforin to the NK cell membrane in the IS. Interestingly, in 1990, Jiang and colleagues predicted a molecule on the membrane of cytotoxic lymphocytes that interferes with perforin binding.33 They performed deglycosylation and desialylation experiments and suggested that sialic acids and glycans play an important role in this activity. In view of our results, it is tempting to speculate that CD107a could be the molecule they were investigating.
Recent studies have demonstrated that the C-terminal Ca2+-binding C2 domain mediates initial contact of perforin to the membrane.34 Subsequently, conformational changes and membrane attack together with perforin polymerization induce the formation of a membrane pore.34,35 The C2 domain of perforin is rich in negatively-charged amino acids. Therefore, the contact of the C2 domain to the plasma membrane would be hindered by negatively charged membrane proteins. Our data show that the O-glycan moieties within the hinge of CD107a are important for its activity. The negative charges of these residues could prevent the binding of perforin to the membrane.
Our careful analysis using single, double, and triple mutants of O-glycosylated Ser/Thr within the hinge of CD107a did not reveal any hot spot responsible for protection. We cannot exclude that N-linked glycosylation outside of the hinge region may contribute to CD107a function, because we could not interfere with N-linked glycosylation in living cells. However, it is likely that the overall structure of CD107a is important. The membrane-proximal C terminus of the structurally related CD63/LAMP-3 was found to adopt a globular β-fold that is in direct proximity to the membrane and structurally less erected from the bilayer as previously suggested. Depending on the conformation of the O-glycosylated N-terminal part of the molecule, the N terminus could also be in close proximity to the membrane.36 Thus, it is likely that CD107a also occupies more membrane area than has been appreciated previously and thus could efficiently control access to the bilayer. It is possible that the glycans function to adjust the molecule in an orderly structure to occupy a maximal membrane area. We therefore propose that the hinge of CD107a and its glycosylation is involved in orienting the protein in a way that mediates protection from lysis. However, we can currently not explain why the ABB chimera was still protective, although it comprises neither the CD107a hinge nor its C-terminal region.
LFA-1 on NK cells interacting with ICAM-1 on target cells controls focused degranulation, and therefore CD107a accumulation, in a large, stable cluster.37 This cluster is bordered by LFA-1, which may function to reduce diffusion of CD107a and to increase CD107a concentrations in this region. In addition, CD107a surface expression peaks within 1 hour of target cell engagement24 and CD107a is actively recycled from the cell surface,37 underscoring that not only the localization but also the timing of CD107a surface exposure is regulated. CD107a would therefore confer a locally confined and transient resistance to apoptosis for cytotoxic lymphocytes. This agrees with the observation that cytotoxic lymphocytes are not inherently resistant to lysis because they can unidirectionally kill each other.3 A transient resistance to apoptosis would also prevent pathogens from hijacking cytotoxic lymphocytes as lysis-resistant hosts to establish a persistent infection.
On the other hand, reports have described perforin-dependent induction of membrane repair in target cells.38 Membrane repair entails recruitment of lysosomal membranes to the plasma membrane and may thus also induce CD107a expression on target cells.39 Therefore, target cells may also protect themselves from by surface expression of CD107a, and upregulation of CD107a surface expression may represent a mechanism of immune escape for tumors. In fact, CD107a has been detected on the surface of some tumor cells such as the myelomonocytic leukemia cell lines U937 and HL60.25,40 This could be caused by deregulated CD107a expression or lysosomal trafficking.41
In summary, we provide evidence for a novel mechanism that can transiently protect cytotoxic lymphocytes from suicide, enabling unidirectional killing of target cells. In regards to the possible physiological consequences of CD107a deficiency, CD107a−/− mice are viable and fertile,26 with an upregulation of CD107b observed in certain organs.42 We did not find any major defects in NK cell development and maturation in CD107a−/− mice, but splenic NK cell numbers were reduced. This could be the result of increased degranulation-induced NK cell apoptosis in these mice. However, no severe immunologic phenotype has been described for CD107a−/− mice. This may be because the system to protect cytotoxic cells from degranulation-induced damage is redundant. It not only relies on CD107a, but also on the expression of serpinB918,19 and cathepsin B.20 In addition, the CD107a−/− mice have only been kept under pathogen-free conditions. Therefore, further studies of the immune system and infectious challenges of these mice are warranted to determine to what degree the absence of CD107a may negatively affect cytotoxic lymphocyte survival and the capacity to clear infections and tumors. Interestingly, a study by De Carvalho Bittencourt et al described an antibody against murine CD107a, which could reduce the occurrence of diabetes when injected into NOD mice.43 This was associated with a reduction in IFNγ-producing pancreatic infiltrating T cells. In light of our data, it is interesting to speculate that antibody-mediated blocking of CD107a may interfere with its protective effect and therefore result in the selective depletion of autoreactive lymphocytes during degranulation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christian Schütz for cloning of CD107a/b, Dieter Stefan for cell sorting, Janine Kästner and Sabine Wingert for technical support, Birgitta Messmer for isolation and culture of NK cells, and Peter Bröde for help with statistical analysis.
A. Cohnen was a fellow of the postgraduate program “Differential activation and integration of signaling modules within the immune system,” funded by the postgraduate education program of Baden-Württemberg.
This work was supported by the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology/SBCancer by ForSys (BMBF), by the BioFuture funding of the BMBF, and grants from the DFG (TRR83 [C.W.] and SFB877 [O.J., P.S.]).
Authorship
Contribution: C.W. and A. Cohnen designed the study; A. Cohnen, S.C.C., A.S., H.S., and M.C. performed the experiments; C.W., A. Cohnen, A. Cerwenka, and Y.T.B. collected and analyzed the data; P.S. and O.J. provided the mice and reagents; C.W., A. Cohnen, and Y.T.B. wrote the manuscript; and all authors gave conceptual advice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carsten Watzl, Leibniz Research Center for Working Environment and Human Factors–IfADo, Ardeystrasse 67, 44139 Dortmund, Germany; e-mail: watzl@ifado.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal