Key Points
IVIg expands Tregs in vitro and in vivo via induction of COX-2–dependent PGE2 in DCs.
These functions of IVIg are mediated in part via interaction of IVIg and F(ab′)2 fragments of IVIg with DC-SIGN on DCs.
Abstract
CD4+CD25+FoxP3+ regulatory T cells (Tregs) play a critical role in the maintenance of immune tolerance. Intravenous immunoglobulin (IVIg), a therapeutic preparation of normal pooled human IgG, expands Tregs in various experimental models and in patients. However, the cellular and molecular mechanisms by which IVIg expands Tregs are relatively unknown. As Treg expansion in the periphery requires signaling by antigen-presenting cells such as dendritic cells (DCs) and IVIg has been demonstrated to modulate DC functions, we hypothesized that IVIg induces distinct signaling events in DCs that subsequently mediate Treg expansion. We demonstrate that IVIg expands Tregs via induction of cyclooxygenase (COX)-2–dependent prostaglandin E2 (PGE2) in human DCs. However, costimulatory molecules of DCs such as programmed death ligands, OX40 ligand, and inducible T-cell costimulator ligands were not implicated. Inhibition of PGE2 synthesis by COX-2 inhibitors prevented IVIg-mediated Treg expansion in vitro and significantly diminished IVIg-mediated Treg expansion in vivo and protection from disease in experimental autoimmune encephalomyelitis model. IVIg-mediated COX-2 expression, PGE2 production, and Treg expansion were mediated in part via interaction of IVIg and F(ab′)2 fragments of IVIg with DC-specific intercellular adhesion molecule-3-grabbing nonintegrin. Our results thus uncover novel cellular and molecular mechanism by which IVIg expands Tregs.
Introduction
Intravenous immunoglobulin G (IVIg) is a therapeutic preparation of normal human Ig purified from the pooled plasma of several thousand healthy donors. IVIg is used for the replacement therapy of primary immunodeficiencies and in the treatment of various pathological conditions, including immune thrombocytopenia (ITP), Kawasaki disease, anti-neutrophil cytoplasmic antibody-associated vasculitis, and others.1-5 Despite its therapeutic use for more than 3 decades, the mechanism by which IVIg benefits autoimmune patients with diverse pathogenesis is not completely understood. However, available data from patients and various experimental models indicate that IVIg can benefit patients with autoimmune and inflammatory diseases via several mutually nonexclusive mechanisms.1,5-13
CD4+CD25+FoxP3+ regulatory T cells (Tregs) play a critical role in the maintenance of immune tolerance and prevention of autoimmunity.14 Deficiency of Tregs or their defective functioning lead to autoimmune and inflammatory conditions, whereas expansion of Tregs and/or amelioration in their functions are associated with recovery from autoimmune diseases.14 Of note, several recent reports have demonstrated that IVIg expands Tregs.15-26 However, the cellular and molecular mechanisms by which IVIg expands Tregs are relatively unknown. As Treg expansion in the periphery requires signaling by antigen-presenting cells such as dendritic cells (DCs)27,28 and IVIg has been demonstrated to modulate DC functions,29-32 we hypothesized that IVIg induces distinct signaling events in DCs. These IVIg-educated DCs subsequently mediate the expansion of Tregs.
In the present report, we demonstrate that IVIg expands Tregs via induction of cyclooxygenase (COX)-2–dependent prostaglandin E2 (PGE2) in human DCs. Inhibition of PGE2 synthesis prevented DC-mediated Treg expansion by IVIg in vitro and significantly diminished IVIg-mediated Treg expansion in vivo in an experimental autoimmune encephalomyelitis (EAE) model. Modulation of IVIg-mediated Treg expansion by COX-2 inhibitor in vivo also had repercussions on the evolution of EAE. Further, we found that IVIg-mediated COX-2 expression and PGE2 production were mediated in part via interaction of IVIg with DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) on DCs. Our results thus uncover novel cellular and molecular mechanism by which IVIg expands human Tregs.
Methods
Cell culture reagents and antibodies
Fluorochrome-conjugated monoclonal antibodies (mAbs) to human CD4, CD25, DC-SIGN, HLA-DR, programmed death-1 (PD-1)-ligand 1 (PD-L1), PD-ligand 2 (PD-L2), OX-40L, and inducible T-cell costimulator (ICOS) ligand (ICOSL) and to mouse CD4 and CD25 were from BD Biosciences (Le Pont de Claix, France). Fluorochrome-conjugated mAb to FoxP3 and intracellular staining kit were from eBioscience (Paris, France). MAbs to COX-2 and β-actin were from Cell Signaling Technology (Ozyme, Saibt Quentin Yvelines, France). CD14 magnetic beads, CD4+ T cell isolation kit II, GM-CSF, and interleukin (IL)-4 were from Miltenyi Biotec (Paris, France). Blocking mAb to DC-SIGN and isotype control mAb were from R&D Systems (Lille, France).
Generation of human DCs
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy bags of healthy donors purchased from Hôpital Hôtel Dieu, Etablissement Français du Sang, Paris, France. Institutional Review Board (INSERM-EFS) approval was obtained for use of buffy bags of healthy donors. Informed consent was obtained in accordance with the Declaration of Helsinki. CD14+ monocytes were isolated from PBMC by using CD14 magnetic beads. The purity was >98%. Monocytes were cultured in RPMI-1640 medium containing 10% fetal calf serum for 6 days in the presence of cytokines GM-CSF (1000 IU/106 cells) and IL-4 (500 IU/106 cells) to obtain DCs and were used for subsequent experiments. DC purity was ≥98%.
Treatment of DCs and monocytes with IVIg
DCs (0.5 × 106/mL) were cultured with cytokines GM-CSF (1000 IU/106 cells) and IL-4 (500 IU/106 cells) alone or with IVIg (Privigen, CSL Behring AG, Bern, Switzerland) (10 or 15 mg/mL) for 24 hours. Circulating blood monocytes (0.5 × 106/mL) were cultured alone or with IVIg (15 mg/mL). The concentrations of IVIg used in these experiments were within the range of IVIg dose used in the patients and other experiments.19 IVIg was dialyzed before use and tested negative for endotoxins. F(ab′)2 fragments of IVIg were prepared by pepsin digestion (2% weight to weight ratio; Sigma-Aldrich, Saint-Quentin Fallavier, France) followed by dialysis and protein G chromatography to isolate F(ab′)2 fragments. Fc-fragments of IVIg were obtained from Dr M. C. Bonnet (Institut Mérieux, Lyon, France). F(ab′)2 fragments and Fc-fragments of IVIg were used at equimolar concentrations. The equimolar concentration of human serum albumin (HSA; Laboratoire Française de Biotechnologies, Les Ulis, France) was used as an irrelevant protein control.
Treatment of DCs with pharmacological inhibitor
Pharmacological inhibitors of COX-2 activity, NS-398 or celecoxib (both from Sigma-Aldrich), were reconstituted in dimethylsulfoxide (DMSO). DCs were treated with NS-398 at a concentration of 10 μM and celecoxib at 25 μM for 60 minutes followed by treatment with IVIg. The concentrations of NS-398 and celecoxib were chosen after careful titration experiments assessing the viability of the DCs.
Blocking antibody experiments
DCs (0.5 × 106) were preincubated with blocking mAb to DC-SIGN (5 μg) or isotype control mAb (5 μg) for 60 minutes followed by treatment with IVIg (15 mg/mL) for 24 hours.
Co-culture of DCs with CD4+ T cells
CD4+ T cells from PBMCs of healthy donors were isolated using CD4+ T cell isolation kit II. DCs following appropriate treatment were extensively washed and were cocultured with 0.2 × 106 CD4+ T at DC/T-cell ratios of 1:10 in 96-well U-bottom plates. The DC and CD4+ T cells were from unrelated donors and hence TCR and CD28 stimulations were not provided during coculture. However, effectively similar results were also obtained when autologous DC and CD4+ T cells were cocultured in the presence of anti-CD3 and anti-CD28 co-stimulation. After 4 days, Tregs were analyzed by flow cytometry (LSR II; BD Biosciences) by using a combination of CD4, CD25, and FoxP3 molecules.
Flow cytometry
Surface staining of DC and T cells was carried out by using fluorochrome-conjugated mAbs. For intracellular staining of Tregs, cells were fixed, permeabilized, and incubated at room temperature with fluorochrome-conjugated mAb to Foxp3. Five thousand cells were acquired for each sample and the data were processed by using FACS DIVA software (BD Biosciences).
Immunoblotting analysis of COX-2
DC pellets were lysed in RIPA buffer constituting 50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL each of aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF. Following protein concentration estimation, equal amounts of proteins from each sample were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Migrated proteins were then transferred onto polyvinylidene difluoride membranes by semidry western blotting method. Membranes were treated with 5% nonfat dry milk powder in Tris-buffered saline with Tween 20 (TBST; 20 mM Tris-HCl, pH 7.4, 137 mM NaCl, and 0.1% Tween 20) for 60 minutes to prevent nonspecific binding of detecting antibodies. The blots were incubated with COX-2 antibody in TBST with 5% bovine serum albumin (BSA). After washing with TBST, blots were incubated with horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody in TBST with 5% BSA for 2 hours. After further washing in TBST, the blots were developed with the ECL system (PerkinElmer) as per the manufacturer’s instructions. β-Actin served as a loading control.
EAE model and treatment with IVIg and NS-398
Animal studies were performed according to the guidelines of the Charles Darwin ethical committee for animal experimentation (Université Pierre et Marie Curie, Paris, France) at the pathogen-free animal facility of Centre de Recherche des Cordeliers, Paris. EAE was induced in 10-week-old female C57BL/6J mice (Janvier, Saint Berthevin, France) and evolution of disease was followed as previously reported.15,26 EAE was induced in 3 different groups and each group included 6 to 8 mice. Mice were immunized with 200 µg of MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK; PolyPeptide laboratory, Strasbourg, France) emulsified in complete Freund’s adjuvant (Sigma-Aldrich) containing 880 µg of nonviable Mycobacterium tuberculosis H37RA. A final volume of 200 µL was injected subcutaneously at 2 sites over the flanks. Additionally, 300 ng of pertussis toxin (List Biologic Laboratories, Meudon, France) was injected intravenously on the day of immunization and 48 hours later. IVIg was injected daily at 0.8 g/kg intraperitoneally from the day of the immunization until peak of the disease (day 16). NS-398 was intraperitoneally injected on every second day starting from the day of immunization until day 16 at 100 µg/mouse. An equivalent volume and concentration of DMSO was injected to control mice and mice treated with IVIg.
Isolation of mononuclear cells from the spleen and analysis of Tregs by flow cytometry
Mice were sacrificed during the onset of the EAE (day 12) by cervical dislocation and spleens were collected. Single cell suspensions were obtained by mechanical disaggregation and passing the cells through a 70-µm nylon membrane filter. Red blood cells were lysed using ACK lysis buffer.
Mononuclear cells from spleen were treated with anti-mouse CD16/32 antibody (BD Biosciences) to block Fc-receptors and then surface labeled with anti-mouse CD4 and CD25 mAbs. Cells were then washed, fixed, permeabilized, and incubated at room temperature with fluorochrome-conjugated mAb to Foxp3. Ten thousand events were acquired for each sample and analyzed using BD LSR II and FACS DIVA software.
Measurement of PGE2
PGE2 in cell-free culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA). ELISA plates were incubated with test samples overnight at 4°C, followed by 3 washes with phosphate-buffered saline (PBS)-Tween (PBST). After blocking with 1% BSA in PBST for 1 hour at 37°C, wells were incubated with anti-PGE2 antibodies (Sigma-Aldrich) for 6 hours at 37°C, followed by washing with PBST. The plates were incubated further with horseradish peroxidase–labeled anti-rabbit secondary antibody for 2 hours at 37°C, followed by development with 3, 3′, 5, 5′-tetra methylbenzidine (Sigma-Aldrich). The absorbance values were measured at 492 nm by using an ELISA reader.
Statistical analysis
The significance of differences between series of results with 3 or more groups was assessed using the 1-way ANOVA and comparison between sets of results was assessed using Tukey post-test. Data with 2 groups were analyzed by 2-way Student t test. Values of P < .05 were considered statistically correlated (*P < .05; **P < .01; ***P < .001). All statistical analyses were performed using Prism 5 software (GraphPad software).
Results
IVIg-treated human DCs expand Tregs
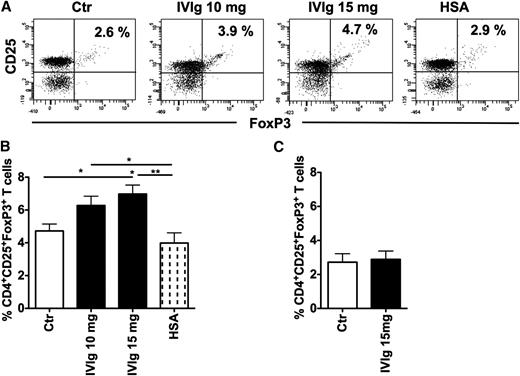
DCs have a unique ability to expand Tregs.27,28 We therefore first aimed at confirming whether IVIg-treated human DCs expand Tregs. Six-day-old monocyte-derived DCs were treated with IVIg at 10- and 15-mg concentrations for 48 hours. The DCs were washed extensively and then cocultured with CD4+ T cells for 4 days and Treg expansion was analyzed. As compared with untreated DCs, IVIg-treated DCs significantly expanded CD4+CD25+FoxP3+Tregs (Figure 1A-B). An equimolar concentration of HSA, used as an irrelevant protein control for IVIg, did not modify the ability of DCs to expand Tregs. Further, the effect of IVIg on Treg expansion was dose dependent. Nearly 20% more Tregs were expanded by 15 mg IVIg-treated DCs compared with 10 mg IVIg-treated DCs (Figure 1B). In contrast to DCs, IVIg-treated monocytes did not expand Tregs (Figure 1C), indicating that the effect of IVIg was specific for DCs. Together, these results imply that the Treg expansion observed in autoimmune patients following IVIg therapy is suggestive of the modulatory effect of IVIg on DCs.
IVIg-treated human DCs but not monocytes expand Tregs. (A) DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg (10 mg or 15 mg) or HSA for 24 hours. DCs were washed extensively and cocultured with CD4+ T cells for 4 days. Tregs (CD4+CD25+FoxP3+) were analyzed by flow cytometry. Representative dot blot of 8 independent experiments is shown. (B) Percentage (mean ± SEM, n = 8) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures, *P < .05; **P < .01. (C) Circulating monocytes were cultured alone or with IVIg (15 mg) for 24 hours. Cells were washed extensively and cocultured with CD4+ T cells for 4 days. Tregs (mean ± SEM, n = 8) were analyzed by flow cytometry.
IVIg-treated human DCs but not monocytes expand Tregs. (A) DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg (10 mg or 15 mg) or HSA for 24 hours. DCs were washed extensively and cocultured with CD4+ T cells for 4 days. Tregs (CD4+CD25+FoxP3+) were analyzed by flow cytometry. Representative dot blot of 8 independent experiments is shown. (B) Percentage (mean ± SEM, n = 8) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures, *P < .05; **P < .01. (C) Circulating monocytes were cultured alone or with IVIg (15 mg) for 24 hours. Cells were washed extensively and cocultured with CD4+ T cells for 4 days. Tregs (mean ± SEM, n = 8) were analyzed by flow cytometry.
IVIg does not alter the expression of costimulatory molecules of DCs implicated in Treg expansion
We then aimed at identifying the mechanisms by which IVIg-treated DCs expand Tregs. The interaction of several CD4+ T cell-DC costimulatory molecules, including PD-1 (CD279) receptor and its ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273); OX-40 and OX-40 ligand (OX-40L, CD252); and ICOS and ICOSL (CD275) could lead to Treg expansion.33-36 Therefore, to explore whether IVIg-mediated Treg expansion implicates these costimulatory molecules on DCs, we analyzed the effect of IVIg on the expression of the above molecules on DCs.
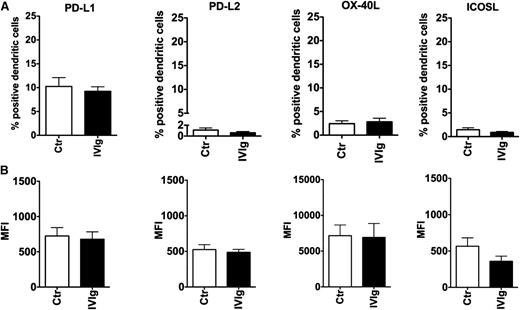
We found that IVIg at a 15-mg concentration did not alter the basal expression of PD-L1 and PD-L2 on DCs, the ligands for PD-1 (P > .05) (Figure 2A-B). The expression level of both the molecules was on par with untreated DCs. Similarly, IVIg treatment did not modify the expression of OX-40L and ICOSL on DCs (Figure 2). Together, these results indicated that costimulatory molecules on DCs are not implicated in IVIg-mediated Treg expansion.
Effect of IVIg on the expression of costimulatory molecules of DCs implicated in Treg expansion. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg for 24 hours. (A) The expression of PD-L1, PD-L2, OX-40L, and ICOSL depicted as percent positive cells (mean ± SEM, n = 7-8) and (B) mean fluorescence intensity (MFI). The difference in expression levels of costimulatory molecules between Ctr and IVIg groups was not statistically significant.
Effect of IVIg on the expression of costimulatory molecules of DCs implicated in Treg expansion. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg for 24 hours. (A) The expression of PD-L1, PD-L2, OX-40L, and ICOSL depicted as percent positive cells (mean ± SEM, n = 7-8) and (B) mean fluorescence intensity (MFI). The difference in expression levels of costimulatory molecules between Ctr and IVIg groups was not statistically significant.
IVIg induces COX-2 and PGE2 in DCs
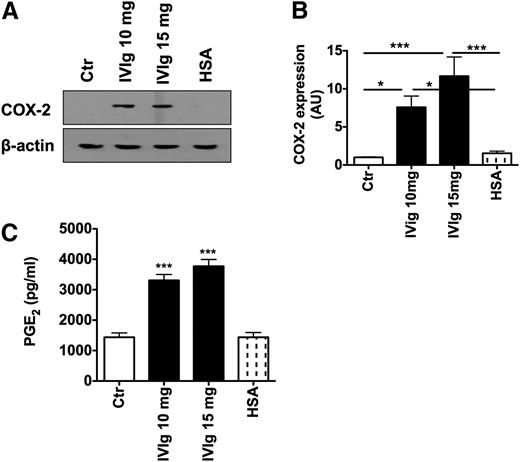
COX-2 and its product, PGE2, had been implicated in enhancing the attraction of Tregs and as a consequence for promoting the interaction of DCs and Tregs. In addition, COX-2 and PGE2 were also shown to augment DC-mediated Treg expansion.37 Therefore, to investigate whether expansion of Tregs by IVIg-treated DCs implicates the COX-2-PGE2 pathway, DC lysates were subjected to western blot for analyzing the effect of IVIg on COX-2 expression. Untreated DCs showed minimal expression of COX-2 and its expression was not altered by HSA (Figure 3A-B). However, treatment of DCs with IVIg lead to significantly enhanced expression of COX-2 (Figure 3A-B).
IVIg induces COX-2 and PGE2 in DCs. (A) DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg (10 mg or 15 mg) or HSA for 24 hours. Expression of COX-2 in DCs was analyzed by western blot. Representative blot of 7 independent experiments is shown. (B) The fold changes in COX-2 expression based on densitometry analysis of western blots (mean ± SEM, n = 7). AU, arbitrary units. (C) The level (mean ± SEM, n = 7) of production of PGE2 (pg/mL) by DCs under the experimental conditions as explained above, * P < .05; ***P < .001.
IVIg induces COX-2 and PGE2 in DCs. (A) DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg (10 mg or 15 mg) or HSA for 24 hours. Expression of COX-2 in DCs was analyzed by western blot. Representative blot of 7 independent experiments is shown. (B) The fold changes in COX-2 expression based on densitometry analysis of western blots (mean ± SEM, n = 7). AU, arbitrary units. (C) The level (mean ± SEM, n = 7) of production of PGE2 (pg/mL) by DCs under the experimental conditions as explained above, * P < .05; ***P < .001.
To confirm whether high expression of COX-2 in IVIg-treated DCs resulted in PGE2 production, the cell culture supernatants from IVIg-treated DCs were subjected to PGE2 analysis. We found that IVIg-treated DCs produced significantly higher quantities of PGE2 compared with untreated DCs and HSA-treated DCs (Figure 3C). The effect of IVIg on PGE2 production was dose dependent. Further, increased PGE2 observed in IVIg-treated DC cultures was a direct consequence of modulation of DCs and their intracellular signaling pathways and not due to soluble PGE2 present in the Ig preparations. In fact, IVIg preparation was negative for PGE2 as analyzed by ELISA.
Inhibition of COX-2 activity in DCs prevents IVIg-mediated Treg expansion
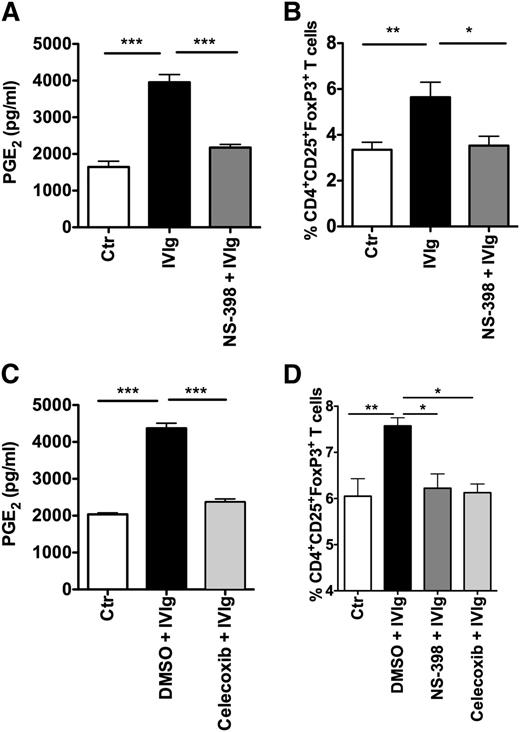
To validate the role of COX-2-induced PGE2 in IVIg-mediated Treg expansion, DCs were treated with COX-2 pharmacological inhibitors NS-398 or celecoxib, followed by culture with IVIg. Inhibition of COX-2 activity resulted in a significant reduction in IVIg-induced PGE2 production in DCs (Figure 4A,C), whereas DMSO, used as a solvent control, did not inhibit IVIg-mediated PGE2 production. Further, COX-2 inhibition also abolished the ability of IVIg-treated DCs to expand Tregs (Figure 4B,D). Together, these results demonstrated that the COX-2-PGE2 pathway is the critical player in IVIg-mediated expansion of Tregs.
Inhibition of COX-2 activity in DCs prevents IVIg-mediated Treg expansion. (A) Amount of secretion of PGE2 by DCs. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg for 24 hours. In parallel, DCs were treated with COX-2 inhibitor NS-398 followed by treatment with IVIg (NS-398+IVIg). PGE2 in cell-free culture supernatants was measured by ELISA (mean ± SEM, n = 7). (B) Percentage (mean ± SEM, n = 7) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures. DCs were treated as explained above. These DCs were washed extensively and cocultured with CD4+ T cells for 4 days. Tregs (CD4+CD25+FoxP3+) were analyzed by flow cytometry. (C) DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with DMSO (solvent control) followed by IVIg or with COX-2 inhibitor celecoxib followed by IVIg for 24 hours. The amount of secretion of PGE2 by DCs was measured (mean ± SEM, n = 7). (D) Percentage (mean ± SEM, n = 4) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures. DCs were pretreated with DMSO, NS-398, or celecoxib followed by IVIg and then cocultured with CD4+ T cells, *P < .05; **P < .01; ***P < .001.
Inhibition of COX-2 activity in DCs prevents IVIg-mediated Treg expansion. (A) Amount of secretion of PGE2 by DCs. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg for 24 hours. In parallel, DCs were treated with COX-2 inhibitor NS-398 followed by treatment with IVIg (NS-398+IVIg). PGE2 in cell-free culture supernatants was measured by ELISA (mean ± SEM, n = 7). (B) Percentage (mean ± SEM, n = 7) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures. DCs were treated as explained above. These DCs were washed extensively and cocultured with CD4+ T cells for 4 days. Tregs (CD4+CD25+FoxP3+) were analyzed by flow cytometry. (C) DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with DMSO (solvent control) followed by IVIg or with COX-2 inhibitor celecoxib followed by IVIg for 24 hours. The amount of secretion of PGE2 by DCs was measured (mean ± SEM, n = 7). (D) Percentage (mean ± SEM, n = 4) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures. DCs were pretreated with DMSO, NS-398, or celecoxib followed by IVIg and then cocultured with CD4+ T cells, *P < .05; **P < .01; ***P < .001.
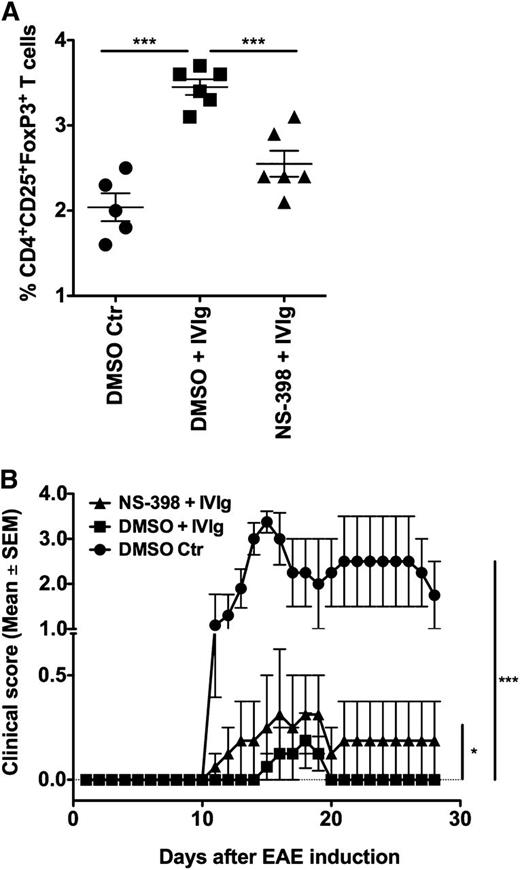
Inhibition of COX-2 prevents IVIg-mediated Treg expansion in vivo in the EAE model
To confirm the role of COX-2 in IVIg-mediated Treg expansion in vivo, we resorted to the EAE model. Previous studies have demonstrated that IVIg protects mice from EAE and was associated with an expansion of Tregs.15,26 Twelve days following the induction of EAE that corresponded to the onset of disease in the control group, mice were sacrificed and analyzed for Tregs in the spleen. In line with previous reports, we confirm that IVIg therapy in EAE is associated with significant expansion of Tregs (Figure 5A). Interestingly, COX-2 inhibitor NS-398 significantly prevented IVIg-mediated Treg expansion (Figure 5A), thus validating the in vivo relevance of COX-2-PGE2 pathway in the IVIg-mediated Treg expansion.
Inhibition of COX-2 prevents IVIg-mediated Treg expansion and protection in vivo in EAE model. EAE was induced in 10-week-old female C57BL/6J mice in 3 different groups. The first group received DMSO (solvent control for NS-398) on every alternative day until peak of the disease (day 16). The second group received IVIg (16 mg/mouse) every day and DMSO on every alternative day until day 16. The third group received IVIg every day and NS-398 (100 μg/mouse) on every alternative day until peak of the disease. (A) Mice were sacrificed on the day of onset of clinical signs (day 12) and splenic Tregs (CD4+CD25+FoxP3+) were analyzed by flow cytometry, ***P < .001. (B) Repercussion of COX-2 inhibition in vivo on IVIg-mediated protection from EAE. The development of clinical signs in all the 3 groups of mice was followed until day 28 following induction of EAE, *P < .05; ***P < .001.
Inhibition of COX-2 prevents IVIg-mediated Treg expansion and protection in vivo in EAE model. EAE was induced in 10-week-old female C57BL/6J mice in 3 different groups. The first group received DMSO (solvent control for NS-398) on every alternative day until peak of the disease (day 16). The second group received IVIg (16 mg/mouse) every day and DMSO on every alternative day until day 16. The third group received IVIg every day and NS-398 (100 μg/mouse) on every alternative day until peak of the disease. (A) Mice were sacrificed on the day of onset of clinical signs (day 12) and splenic Tregs (CD4+CD25+FoxP3+) were analyzed by flow cytometry, ***P < .001. (B) Repercussion of COX-2 inhibition in vivo on IVIg-mediated protection from EAE. The development of clinical signs in all the 3 groups of mice was followed until day 28 following induction of EAE, *P < .05; ***P < .001.
We further found that modulation of IVIg-mediated Treg expansion by COX-2 inhibitor in vivo also had repercussions on the evolution of EAE. As shown in Figure 5B, mice treated with combination of NS-398 and IVIg exhibited clear clinical signs of EAE in the early phase. In this group, clinical signs appeared as early as day 11 following immunization with MOG, whereas in the mice that received only IVIg, clinical signs began to appear on day 15. Thus, in mice that received NS-398 and IVIg, the clinical signs of EAE appeared 4 days earlier than in animals that received only IVIg. Also, the severity of disease was significantly higher in the group that received the NS-398 and IVIg combination compared with the group that was treated with only IVIg. Essentially, the pattern of evolution of the disease in mice that were treated with NS-398 and IVIg was similar to that of the control group. Further, the course of EAE was shorter in the mice that received only IVIg and after 5 days, these mice entered complete remission. On the contrary, mice that received the NS-398 and IVIg combination displayed a prolonged period of disease, ie, 18 days and diseased mice did not enter into remission (Figure 5B).
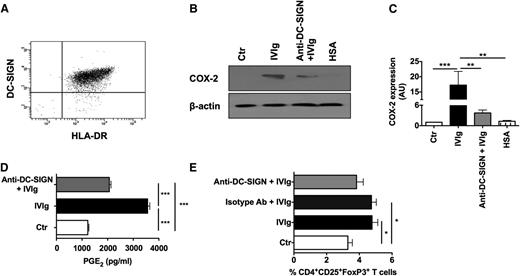
DC-SIGN is partially implicated in IVIg-mediated PGE2 production in DCs
Recently, it was demonstrated that the antiinflammatory functions of IVIg are mediated via interaction of terminal α2,6-sialic acid linkages at Asp297 of IVIg with C-type lectin receptor SIGN-R1 (in mice) or DC-SIGN (in humans),38-40 although antibody-mediated experimental models of ITP show that the protective effect of IVIg is independent of sialylation of IgG.41 Therefore, we investigated whether the interaction of DC-SIGN and IVIg is indispensable for the expression of COX-2 and production of PGE2 by DCs. We confirm that the monocyte-derived human DCs express high levels of DC-SIGN (Figure 6A). Further, blockade of DC-SIGN on DCs before IVIg treatment lead to significant inhibition of IVIg-mediated COX-2 expression (Figure 6B-C) and of PGE2 production (Figure 6D). However, DC-SIGN blockade leads only to partial (P > .05) inhibition of Treg expansion by IVIg (Figure 6E). These results thus indicated that IVIg-mediated activation of COX-2-PGE2 pathway is mediated at least in part by signaling via DC-SIGN. The nonsignificant inhibition of Treg expansion by IVIg-treated DCs upon DC-SIGN blockade despite significant inhibition of COX-2 and PGE2 could be due to either receptor recycling on DCs or due to lack of total abolition of PGE2 production in DCs upon DC-SIGN blockade.
DC-SIGN is partially implicated in IVIg-mediated COX-2 expression and PGE2production in DCs and Treg expansion. (A) Expression of DC-SIGN in DCs as analyzed by flow cytometry. (B) Western-blot analysis of COX-2 expression in DCs. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg or HSA for 24 hours. In parallel, DCs were preincubated with blocking antibodies to DC-SIGN followed by treatment with IVIg (Anti-DC-SIGN+IVIg). Representative blot of 6 experiments is shown. (C) The fold changes in the COX-2 expression based on densitometry analysis of western blots (mean ± SEM, n = 6). AU, arbitrary units. (D) Secretion (mean ± SEM, n = 6) of PGE2 by DCs that were treated as explained above. (E) Percentage (mean ± SEM, n = 7) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures. DCs were pretreated with DC-SIGN blocking antibodies or isotype control antibodies followed by treatment with IVIg and then cocultured with CD4+ T cells, *P < .05; **P < .01; ***P < .001.
DC-SIGN is partially implicated in IVIg-mediated COX-2 expression and PGE2production in DCs and Treg expansion. (A) Expression of DC-SIGN in DCs as analyzed by flow cytometry. (B) Western-blot analysis of COX-2 expression in DCs. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg or HSA for 24 hours. In parallel, DCs were preincubated with blocking antibodies to DC-SIGN followed by treatment with IVIg (Anti-DC-SIGN+IVIg). Representative blot of 6 experiments is shown. (C) The fold changes in the COX-2 expression based on densitometry analysis of western blots (mean ± SEM, n = 6). AU, arbitrary units. (D) Secretion (mean ± SEM, n = 6) of PGE2 by DCs that were treated as explained above. (E) Percentage (mean ± SEM, n = 7) of CD4+CD25+FoxP3+ cells in the DC-CD4+ T cell cocultures. DCs were pretreated with DC-SIGN blocking antibodies or isotype control antibodies followed by treatment with IVIg and then cocultured with CD4+ T cells, *P < .05; **P < .01; ***P < .001.
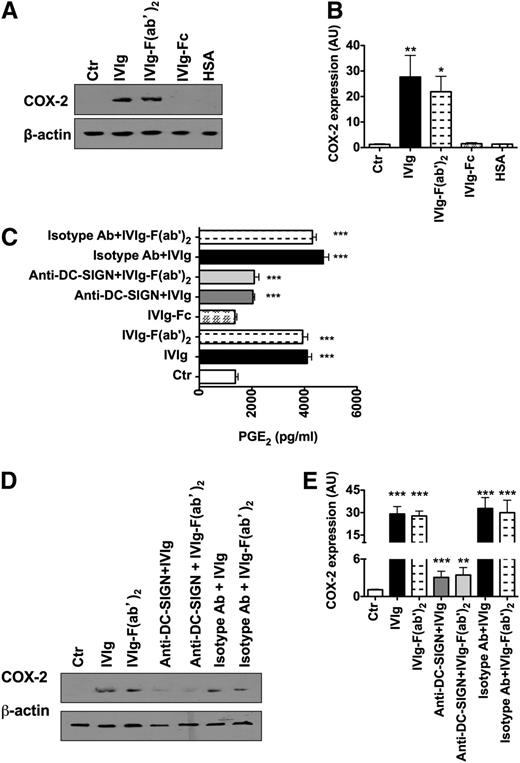
IVIg-mediated COX-2 induction and PGE2 production in DCs are F(ab′)2 dependent
By using Sambucus nigra agglutinin lectin fractionation, 2 recent reports demonstrated that a significant amount of sialylation is found in the Fab region of IVIg and not in the Fc region.42,43 Accordingly, the antiinflammatory activity was observed with Fab sialylation and not with Fc. Interestingly, F(ab′)2 Fragments of IVIg exerted an antiinflammatory effect on DCs similar to that of intact IgG molecules of IVIg.29,42,44 Therefore, we explored the role of F(ab′)2 and Fc fragments of IVIg on COX-2 activation and PGE2 synthesis by DCs. We found that IVIg-mediated COX-2 induction and PGE2 production in DCs were mediated mainly via F(ab′)2 fragments (Figure 7A-C), whereas Fc fragments had a minimal effect. Similar to results with IVIg, blockade of DC-SIGN on DCs lead to significant inhibition of COX-2 expression and PGE2 production by F(ab′)2 fragments of IVIg (Figure 7C-E).
IVIg-mediated COX-2 induction and PGE2production in DCs are F(ab′)2dependent. (A,D) Western-blot analysis of COX-2 expression in DCs. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg or equimolar concentrations of either F(ab′)2 fragments or Fc fragments of IVIg or HSA for 24 hours (A). In some experiments, DCs were preincubated with blocking antibodies to DC-SIGN or isotype control antibodies followed by treatment with equimolar concentrations of either IVIg or F(ab′)2 fragment of IVIg (D). Representative blot of 6 to 7 experiments is shown. (B,E) The fold changes in the COX-2 expression (mean ± SEM, n = 6-7) based on densitometry analysis of western blots in the above experiments. AU, arbitrary units. (C) Secretion (mean ± SEM, n = 7) of PGE2 by DC that were treated as explained above, *P < .05; **P < .01; ***P < .001.
IVIg-mediated COX-2 induction and PGE2production in DCs are F(ab′)2dependent. (A,D) Western-blot analysis of COX-2 expression in DCs. DCs were cultured in GM-CSF and IL-4 alone (Ctr) or with IVIg or equimolar concentrations of either F(ab′)2 fragments or Fc fragments of IVIg or HSA for 24 hours (A). In some experiments, DCs were preincubated with blocking antibodies to DC-SIGN or isotype control antibodies followed by treatment with equimolar concentrations of either IVIg or F(ab′)2 fragment of IVIg (D). Representative blot of 6 to 7 experiments is shown. (B,E) The fold changes in the COX-2 expression (mean ± SEM, n = 6-7) based on densitometry analysis of western blots in the above experiments. AU, arbitrary units. (C) Secretion (mean ± SEM, n = 7) of PGE2 by DC that were treated as explained above, *P < .05; **P < .01; ***P < .001.
Discussion
CD4+CD25+FoxP3+ Tregs play a vital role in preventing inflammation and autoimmunity.14 The deficiency of Tregs in humans as a consequence of mutations in the FoxP3 gene causes a severe autoimmune inflammatory disorder called immune dysregulation polyendocrinopathy enteropathy X-linked syndrome. Similarly, in experimental models, deficiency of Tregs either due to genetic knockdown of FoxP3 gene or depletion leads to either appearance of autoimmune disease or exacerbation of ongoing disease.14 Therefore, therapeutic approaches that enhance Treg numbers and/or amelioration of their function have drawn considerable interest in recent years.
Several reports have demonstrated that IVIg therapy is associated with an expansion of Tregs in the periphery.15,17,18,21-23 In the present study, we aimed at identifying the cellular and molecular mechanism by which IVIg expands Tregs. Among professional antigen-presenting cells, myeloid DCs were considered as potent immune cells that could efficiently expand Tregs.45 In addition, ours and other reports have demonstrated that IVIg exerts modulatory effect on DCs,29,30,32 thus indicating that Treg expansion by IVIg observed in autoimmune patients and experimental models might be a repercussion of DC education by IVIg.
Available data indicate that DCs can expand Tregs via co-stimulatory molecules such as PD-L1 and 2, ICOSL, and OX-40L.33-36,46 The interaction of PD-L1 and 2 with PD-1, the members of CD28/B7 family, has a critical role in balancing the T-cell tolerance through inhibiting the activation of effector cells while promoting the generation and expansion of Tregs.33,34 Pathogens such as Mycobacterium can hijack the immune system by activating the PD-L-PD-1 pathway and thus enhancing the Tregs and preventing the protective immune responses.34,46 Although ICOS, another member of CD28/B7 family of costimulatory molecules, and OX-40, a member of tumor necrosis factor receptor superfamily, are constitutively expressed on Tregs, their expression on other T cell subsets is restricted to activated cells.35,36 In contrast to PD-1, these costimulatory molecules provide positive stimuli to activated CD4+ T cells and depending on the context of the inflammatory responses, they can promote Th1, Th2, or Th17 responses.35,36 Thus, the ability of ICOSL and OX-40L to induce Treg activation and expansion depends on the cytokine milieu.35,36 We found that IVIg did not enhance or modify the expression of any of the costimulatory molecules on DCs that are implicated in Treg expansion, including Notch ligands,47 thus indicating that costimulatory pathway does not play an important role in IVIg-mediated Treg expansion.
Next, we turned our attention toward DC-derived soluble factors such as TGF-β and PGE2 that are known to provide stimulation for Treg induction and expansion.45,48,49 However, we previously observed that in the presence of TGF-β, there was no induction of human Tregs by IVIg20 and TGF-β was not increased in the splenocytes of IVIg-treated mice.15 This data suggested that TGF-β is not implicated in IVIg-mediated Treg expansion.
PGE2 is an arachidonic acid metabolite product. COX enzymes play a critical role in converting arachidonic acid released from the cell membrane into prostaglandins. Among 3 COX isoenzymes, COX-1 is constitutively expressed in variety of cells and act as a housekeeping enzyme to maintain the basal levels of PGE2. On the contrary, COX-2 is an inducible enzyme.37 As PGE2 can bind to 4 different receptors, PGE2 exerts diverse biological functions on the immune cells. These functions include regulation of T-cell differentiation and proliferation, inhibition of cytokines and chemokines by innate immune cells such as DCs and macrophages and suppression of effector functions of NK cells and granulocytes. Thus, PGE2 can regulate the inflammation and immune responses.37
Several lines of evidence also indicate that PGE2 enhances the generation of Tregs and their expansion both in humans and mice.37 In addition, PGE2 also mediate suppressive functions of Tregs.49 Vaccination with PGE2-educated DCs has been shown to induce Treg expansion in cancer patients.45 Elevated levels of PGE2 and COX-2 activity leading to induction of Tregs and FoxP3, expansion of Tregs, and enhanced suppressive functions have been described in tumor conditions.50 Thus, COX2-PGE2-Treg represents an immunosuppressive network. As COX-2 inhibition in DCs lead to downregulation of PGE2 and IVIg-mediated Treg expansion and inhibition of COX-2 in vivo has been shown to reduce the frequency of Tregs and their activity,50 we might conclude that Treg expansion in vivo in autoimmune patients following IVIg therapy might be due to enhancement of PGE2. In fact, inhibition of COX-2 activity by systemic injection of NS-398 leads to significant downregulation of IVIg-mediated Treg expansion in the EAE model. Also, COX-2 inhibitor significantly decreased IVIg-mediated protection from EAE. In line with these results, our preliminary data also suggest that beneficial effects of IVIg in autoimmune patients are associated with an enhancement of PGE2 in the circulation.
Our results thus provide a novel cellular and molecular mechanism for IVIg in that it links innate and adaptive immune compartment to mediate immune tolerance via Tregs. As IVIg-treated circulating monocytes did not expand Tregs imply that IVIg-mediated Treg expansion could be specific for DCs. Further work is necessary to identify DC subset(s) implicated in COX-2-dependent IVIg-mediated Treg expansion. Our results also indicate that Treg expansion by IVIg in autoimmune patients could involve a 2-step process: recognition and signaling in DCs by IVIg leading to COX-2 activation and PGE2 synthesis and action of PGE2 on Tregs leading to their expansion.
Various animal models implicate a role for sialylation of Fc fragments in the beneficial effect of IVIg.38-40 However, recent publications also demonstrate that Fc-sialylation is dispensable for antiinflammatory effects of IVIg.29,41,42,44 We have observed that IVIg-mediated COX-2 induction is F(ab′)2 dependent in part via DC-SIGN, indicating that sialylation in F(ab′)2 fragments does have a role in the observed phenomenon. That DC-SIGN blockade did not completely abolish PGE2 production and Treg expansion by IVIg also points toward a sialylation/DC-SIGN-independent mechanism for IVIg on DCs to mediate Treg expansion. Although certain diseases such as ITP have been demonstrated to benefit from Fc-fragments of IVIg, we believe that bifurcation of IVIg functions as Fc- or F(ab′)2-dependent is artificial; no single mechanism of IVIg appears to be solely responsible for the beneficial effects of IVIg observed in diverse autoimmune diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chaitrali Saha for the help.
The work was supported by Institut National de la Santé et de la Recherche Médicale, Université Pierre et Marie Curie, Université Paris Descartes (J.B. and S.V.K.); Centre National de la Recherche Scientifique (S.V.K.); European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement HEALTH-2010.2.4.5-2 ALLFUN (J.B.); Indian Institute of Science, Department of Biotechnology, Department of Science and Technology, Council for Scientific and Industrial Research, India (K.N.B.); Coopération INSERM-ICMR-AO 2009/2010 (J.B. and K.N.B.); fellowships from INSERM-ICMR, Council for Scientific and Industrial Research (J.T.); and Journées de Neurologie de Langue Française (M.R.).
Authorship
Contribution: J.T., P.H., M.S., and M.S.M. performed the experiments and analyzed the data; M.R. performed the experiments; J.-M.V. and L.M. provided advice; K.N.B. and S.V.K. provided advice and analyzed the data; and J.B. conceived the project, designed the experiments, analyzed the data and wrote the paper
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jagadeesh Bayry, INSERM U 872, Equipe 16-Centre de Recherche des Cordeliers, 15 rue de l’Ecole de Médicine, Paris, F-75006, France; e-mail: jagadeesh.bayry@crc.jussieu.fr; and Srini V. Kaveri, INSERM U 872, Equipe 16-Centre de Recherche des Cordeliers, 15 rue de l’Ecole de Médicine, Paris, F-75006, France; e-mail: srini.kaveri@crc.jussieu.fr.
References
Author notes
J.T., P.H., and M.S. contributed equally to this study.