Key Points
TTT-motif in beta2-integrin binds kindlin-3.
Mutation of TTT-motif affects T-cell homing not activation.
Abstract
Kindlin-3 is mutated in the rare genetic disorder, leukocyte adhesion deficiency type III, which is characterized by deficient integrin-mediated adhesion of leukocytes and platelets. However, the specific roles of kindlin-3–β2-integrin interactions in T-cell adhesion and homing and immune responses in vivo remain unclear. Here, we show that the TTT motif in β2 integrins controls kindlin-3 binding. Mutation of the kindlin-3 binding site in β2 integrins caused a loss of firm adhesion of T cells under both static and shear flow conditions and a reduction of T-cell homing to lymph nodes in vivo. However, atomic force microscopy studies of integrin-ligand bonds revealed that initial ligand binding could still occur, and 2-dimensional T-cell migration was reduced but not abolished by the TTT/AAA mutation in the β2 integrin. Importantly, dendritic cell–mediated T-cell activation in vivo was normal in TTT/AAA β2 integrin knock-in mice. Our results reveal a selective role of the kindlin-3–integrin association for lymphocyte functions in vivo; the integrin–kindlin-3 interaction is particularly important in adhesion strengthening under shear flow, and for T-cell homing to lymph nodes, but dispensable for T cell activation which occurs in a shear-free environment.
Introduction
Integrin-mediated cell adhesion is vital for leukocyte function and thus for host defense against pathogens. The β2 integrins interact with intercellular adhesion molecules (ICAM) on endothelial cells surrounding blood vessels, mediating firm adhesion necessary for leukocyte migration into lymph nodes and sites of inflammation.1 LFA-1 (αLβ2) is also a component of the immunologic synapse that forms between CD4 T cells and antigen-presenting cells, and can provide costimulation of T cells, thereby reducing the threshold for T-cell activation.2-5 The fundamental importance of β2 integrins is highlighted by leukocyte adhesion deficiency type-I (LAD-I), where expression of these integrins is low or absent.6 Patients with this disease have recurrent bacterial infections because of a deficiency in leukocyte extravasation.
Integrins are maintained in a low-affinity state in resting cells until, after stimulation of the cell through surface receptors (eg, T-cell receptor [TCR] or chemokine receptors), “inside-out” signals result in conformational changes in the integrin, allowing binding to ligands. Thereafter, integrin “outside-in” signals initiate downstream effects.7 Integrin function is regulated by the binding of cytoplasmic proteins, such as talin, kindlin-3, filamin, and 14-3-3 proteins, to the β2 integrin intracellular domain.8-12 The integrin activator talin plays an essential role both in lymphocyte homing and in T-cell activation in vivo.13
The integrin regulator kindlin-3 is essential for β2 integrin–mediated neutrophil trafficking and β3 integrin–mediated platelet aggregation in vivo.10,14 In addition, kindlin-3 mutations have been identified in patients with leukocyte adhesion deficiency type-III (LAD-III), a rare genetic disorder characterized by recurrent bacterial infections and severe bleeding.15,16 Kindlin-3 null animals die shortly after birth because of uncontrolled bleeding, and they also display severely impaired lymphocyte development, with reduced cellularity of the spleen and thymus, and a lack of mesenteric lymph nodes.10 Therefore, the role of kindlin-3 in mature lymphocytes in vivo has not been reported. In addition, the specific role of the β2 integrin–kindlin-3 interaction (rather than the presence of kindlin-3) in leukocytes is undetermined.
We have previously shown that a TTT motif in the β2 integrin cytoplasmic domain is essential for integrin-mediated cell adhesion, actin reorganization, and cell spreading in vitro.8,9,17,18 However, the role of this motif in regulating β2 integrin functions in vivo is currently unknown.
Here, we show that the TTT site in the β2 integrin mediates the interaction with kindlin-3. To investigate the role of the kindlin-3–integrin interaction in vivo, we have generated a knock-in mouse containing a TTT/AAA substitution in the β2 integrin cytoplasmic domain. In CD4 T cells, the loss of kindlin-3 binding resulted in impaired firm adhesion to ICAM-1, and reduced homing to lymph nodes, whereas initial integrin-ligand bonds and 2-dimensional migration on ligand were relatively unaffected. In addition, CD4 T-cell activation in the spleen after intravenous transfer of peptide-loaded wild-type (WT) dendritic cells (DCs) was unaffected by the TTT/AAA mutation in the β2 integrin. Our data reveal a selective role for the integrin-kindlin-3 interaction in T-cell biology in vivo.
Methods
Mice
Mice were maintained in the Wellcome Trust Biocentre, University of Dundee, in compliance with UK Home Office Animals (Scientific Procedures) Act 1986 guidelines. The constitutive Itgb2 knock-in mice were made on a C57Bl/6 background by TaconicArtemis. The C57BL/6N Tac Es cell line was used, and T759A, T760A, and T761A mutations were introduced into exon 16 of the Itgb2 gene. The positive selection marker (puromycin resistance) was flanked by F3 sites and inserted into intron 14. The remaining F3 recombination site after Flp removal of the positive selection marker is located in a nonconserved region of the genome. The TTT/AAA mutation in the gene of the knock-in mice was verified by polymerase chain reaction (PCR) and sequencing. Genotyping of the knock-in mice was routinely performed by PCR for the F3 site (forward: CGTATCCTGCTCAACACAAGG; reverse: GTCACCACCTACTCGTGTTCC). In all experiments, homozygous mice were used, with WT littermates as controls. C57/Bl6 mice were obtained from Charles River.
Flow cytometry and tetramer staining
Single-cell suspensions of lymphoid tissues were prepared. The following conjugated antibodies were used (from BD Bioscience unless otherwise stated, clones given in brackets): CD4 (RM4-5), CD8a (53-6.7), CD11a (αL, 2D7), CD18 (β2 integrin, C71/16), CD25 (PC61), CD29 (β1 integrin, Ha2/5), CD43 (S7), CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), PSGL-1 (2PH1), B220 (RA3-6B2; eBioscience), F4/80 (BM8; eBioscience), Gr-1 (RB6-8C5; eBioscience). Fc block (clone 2.4G2; BD Bioscience) was included in all stains. Intracellular staining for Foxp3 (FJK-16s) was performed according to the manufacturer’s instructions (eBioscience). PE-labeled MHC class II tetramers (NIH tetramer core facility) containing either the H19env peptide or an irrelevant peptide-negative control were used at 1/100 dilution in RPMI plus 10% fetal bovine serum for 3 hours at 37°C. Propidium iodide (Sigma-Aldrich) was added immediately before acquisition on an LSR Fortessa flow cytometer (Becton Dickinson). Data were analyzed using FlowJo software (TreeStar).
T-cell isolation and culture
CD4 T cells were isolated from spleens and lymph nodes by positive selection using CD4 microbeads and sorting over LS columns (both Miltenyi Biotec), according to the manufacturer’s instructions. Effector T cells were generated by culturing T cells with 0.5 μg/mL anti-CD3 (clone 2C11; R&D Systems) with 20 ng/mL interleukin (IL)-2 (Novartis) for 2 days. Cells were then washed and maintained in IL-2 for a further 5 days. For in vitro T-cell activation, purified CD4 T cells were stimulated with anti-CD3 or PdBu (Sigma-Aldrich).
FRET analysis
HEK293 cells were seeded in 35-mm dishes containing glass coverslips. Cells were transfected with constructs for human monomeric YFP-kindlin-3 and CFP-CD18 (pmEYFP-kindlin-3 and either pmECFP-CD18 or pmECFP-CD18TTT/AAA), using Fugene6 (Roche) according to the manufacturer’s protocol. Forty-eight hours after transfections, cells were fixed in −20°C methanol for 15 minutes, washed in phosphate-buffered saline, and mounted in Mowiol on glass slides. Slides of human YFP-kindlin-3– and human CFP-CD18–transfected HEK293 cells were imaged on a DeltaVision Deconvolution microscope (Applied Precision) mounted on an Olympus IX71 with a 63 × 1.42 NA plan apo oil immersion lens (Olympus) and acquired with a 12-bit Coolsnap HQ camera (Roper). Fluorescence excitation was provided by a 300-W Xenon lamp. Exposure time was 500 ms, which gave a similar intensity value of 400 to 700 for both fluorophores. YFP was bleached using a 532-nm diode laser (Coherent) at 100% laser power for 0.5 second to achieve 50% bleach. Data analysis was performed using SoftWorx software (Applied Precision). FRET efficiency (E) was calculated as E=1/(FCFP(pre)/FCFP(post)), where FCFP(pre) and FCFP(post) are the mean CFP emission intensities before and after photo-bleaching.
GST pulldowns
GST, GST-wt-β2-integrin membrane, and cytoplasmic domain, or GST-TTT/AAA-β2-integrin membrane and cytoplasmic domain were expressed, and equal amounts of fusion proteins linked to glutathione Sepharose using standard methods. GST pulldowns were performed using CD4 effector T-cell lysates. Bound talin was detected with immunoblotting with the 8d4 antibody (Sigma-Aldrich).
Immunoblotting
T cells were lysed in 1% Tx-100, 150 mM NaCl, 50 mM Tris pH 7.4, 10 mM ethylenediamine tetraacetic acid (EDTA), 50 mM NaF, and 1 mM sodium orthovanadate containing protease inhibitor cocktail (Roche). Primary antibodies p-PLC-γ (phosphorylation site Tyr 783), kindlin-3, β2 integrin, 14-3-3 proteins, and Src were acquired from Cell Signaling. After incubation in horseradish peroxidase–conjugated secondary antibodies, blots were developed by standard chemiluminescence techniques.
Real-time quantitative PCR
Naïve and effector CD4 T cells from WT and knock-in mice were lysed, and RNA was purified with a NucleoSpin RNA II Total RNA isolation kit (Macherey-Nagel). cDNA was synthesized from 0.35 µg of total RNA with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies). Taqman gene expression assay for CD18 was performed with Taqman Fast Advanced Master Mix and quantitative real-time PCR with StepOnePlus System (Applied Biosystems) and StepOnePlus Software v2.3 (Applied Biosystems). Gene expression was normalized with 18S rRNA, and the target gene expression was calculated by the comparative CT method (Applied Biosystems).
Static adhesion assays
ICAM-1 (6 μg/mL; R&D Systems) was coated onto flat-bottomed 96-well Maxisorp plates (Nunc) overnight at 4°C. Wells were blocked with 1% milk for 1 hour 15 minutes at 37°C. T cells were resuspended at 2 × 106 cells/mL in binding medium (RPMI + 0.1% bovine serum albumin, 40 mM HEPES, and 2 mM MgCl2). Where indicated, cells were stimulated with 200 nM PdBu (Sigma-Aldrich) or 10 μg/mL anti-CD3 (clone 2C11; R&D Systems) immediately before addition to the plate for 30 minutes at 37°C, before gentle washing and detection as previously described.19
Adhesion under shear flow
Ibidi VI0.4 μ-slides were coated with 6 μg/mL ICAM-1 overnight at 4°C. Alternatively, mouse endothelial cells (bEnd.3 cells) were grown on plates and stimulated for 24 hours with 10 ng/mL TNFα. Where indicated, plates were treated with mouse SDF-1 (CXCL12, 10 μg/mL) for 30 minutes at 37°C. Shear flow adhesion assay was performed as in reference 19. Rolling rates were determined using Imaris software cell tracking (Bitplane AG).
Atomic force microscopy
Atomic force microscopy was performed as previously described.20 In brief, Arrow TL2 cantilevers (NanoSensors) were functionalized with 0.5 μg/mL purified anti-CD43 (eBioscience). Cantilevers were calibrated for sensitivity and spring constants before experiments. Tissue culture plates (TPP) were coated with 3 μg/mL ICAM-1. Single effector T cells were attached to cantilever using a force of 1 nN for 10 seconds of contact time. The cantilever was then maneuvered over the ICAM-1 and lowered until a force of 0.5 nN was reached for the indicated contact time. All measurements were taken with a closed-loop and constant force mode using JPK Instruments CellHesion 200 module. As controls, adhesion to bare plastic and to ICAM-1 in the presence of EDTA was measured.
Migration on ICAM-1
Eight-well μ-slides (Ibidi) were coated with 3 μg/mL ICAM-1 overnight at 4°C. After washing in phosphate-buffered saline, 70 000 effector T cells were added to the plate in 400 μL of binding medium. Time-lapse microscopy was performed on a Nikon Eclipse Ti microscope with perfect focus and multipoint site visiting stage, using a CFI Plan Fluor ELWD 40× phase objective and a Photometrics Cascade II 1024 black illuminated EMCCD camera. Fifteen-minute sequences of videos were analyzed using Imaris software (Bitplane AG). Cell spreading was analyzed using Volocity software (PerkinElmer).
In vivo homing assays
CD4 T cells from WT and knock-in mice were labeled with CFSE and CellTrace Violet (Life Technologies), respectively, according to the manufacturer’s instructions. WT and knock-in cells were mixed at a 1:1 ratio, and 1 × 107 cells were injected intravenously into recipient WT mice. Sixteen hours later, donor cells in tissues were identified by flow cytometry.
In vivo T-cell activation assay
DCs were generated by culturing bone marrow for 10 days in 10 ng/mL GM-CSF (Peprotech) in nontreated TPP, with feeding on days 3, 6, and 8. DCs were activated with 100 ng/mL lipopolysaccharide (LPS) (Sigma-Aldrich) overnight and loaded with 50 μg/mL Moloney murine leukemia virus H19env (123-141) peptide for 90 minutes. After washing, 1-2 × 106 LPS-activated peptide-loaded DCs were injected intravenously into WT or knock-in mice. The spleens of recipient mice were isolated 7 days post immunization and antigen-specific CD4 T cells identified by MHC class II tetramer staining.
Statistical analysis
The Student 2-tailed t test and 2-way analysis of variance test (Graphpad Prism) were used to calculate statistical significance.
Results
The TTT-motif in the β2 integrin tail mediates kindlin-3 binding
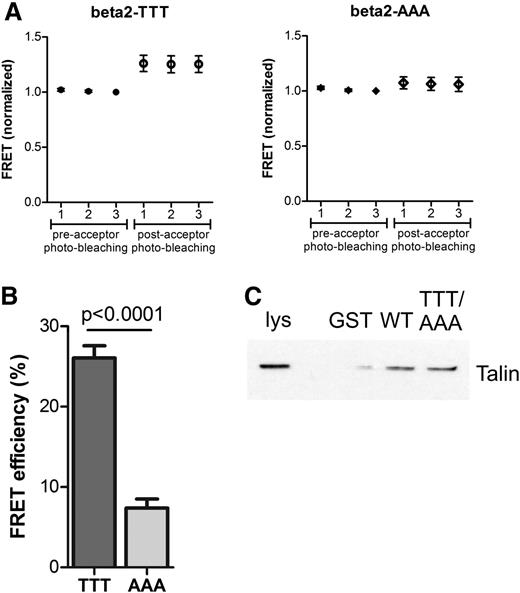
Kindlin-3 is important for β2 integrin–mediated adhesion in leukocytes.10 Conserved threonine residues in β1 and β3 integrin cytoplasmic domains are involved in kindlin binding in vitro.21 Therefore, to investigate the role of the threonine triplet (Thr758-760 in human β2 integrin; Thr759-761 in murine β2 integrin) in kindlin-3–β2 integrin binding, we used fluorescence resonance energy transfer (FRET) with WT and TTT/AAA-mutated β2 integrins, together with kindlin-3, in transfected cells. In FRET, excitation of the donor fluorophore leads to energy transfer to the acceptor fluorophore only if the donor and acceptor are in close proximity to each other (eg, when 2 proteins attached to the fluorophores are interacting in a cell). In acceptor photo-bleaching FRET, the acceptor of the FRET pair is bleached, leading to an increase in fluorescence from the donor. The results show that the WT form of the β2 integrin (TTT) shows an increase in FRET after acceptor photo-bleaching, indicating an interaction between the β2 integrin and kindlin-3 (Figure 1A-B). In cells transfected with the mutated β2-AAA integrin, there is only a minor increase in FRET after acceptor photo-bleaching, indicating a loss of the interaction when the TTT-motif is mutated (Figure 1A-B). Thus, the TTT motif is essential for the integrin-kindlin interaction in cells (Figure 1A-B). In contrast (and as expected as talin binds upstream of this site in integrins8,9,22,23 ), full-length talin from cell lysates can still bind to the TTT/AAA-mutated β2 integrin cytoplasmic domain (Figure 1C), as shown by GST pulldown assays. Together with published data describing talin/kindlin-integrin interactions,24 these results confirm that the TTT site in the β2 integrin is required for the kindlin-3–β2-integrin interaction but not for talin binding to the integrin.
The TTT motif in the β2 integrin tail mediates kindlin-3 binding. (A) The interaction between human WT β2 integrin (TTT, left) or mutant β2 integrin (AAA, right) with human kindlin-3 was measured in transfected HEK293 cells using FRET. In each case, representative graphs are shown with 3 measurements before and 3 measurements after acceptor photo-bleaching. Error bars indicate standard deviation. (B) FRET results of the β2–kindlin-3 interaction are summarized as percentage FRET efficiency (N = 23 cells, mean ± SEM). (C) The interaction of talin with WT-β2-integrin or TTT/AAA β2-integrin was assessed by GST pulldowns with GST alone, GST-WT-β2-integrin (WT), and GST-TTT/AAA-β2-integrin (TTT/AAA) from T-cell lysates followed by immunoblotting with an antitalin antibody. Lys, T cell lysate. Experiment is representative of N = 2.
The TTT motif in the β2 integrin tail mediates kindlin-3 binding. (A) The interaction between human WT β2 integrin (TTT, left) or mutant β2 integrin (AAA, right) with human kindlin-3 was measured in transfected HEK293 cells using FRET. In each case, representative graphs are shown with 3 measurements before and 3 measurements after acceptor photo-bleaching. Error bars indicate standard deviation. (B) FRET results of the β2–kindlin-3 interaction are summarized as percentage FRET efficiency (N = 23 cells, mean ± SEM). (C) The interaction of talin with WT-β2-integrin or TTT/AAA β2-integrin was assessed by GST pulldowns with GST alone, GST-WT-β2-integrin (WT), and GST-TTT/AAA-β2-integrin (TTT/AAA) from T-cell lysates followed by immunoblotting with an antitalin antibody. Lys, T cell lysate. Experiment is representative of N = 2.
Generation of β2TTT/AAA integrin knock-in mice
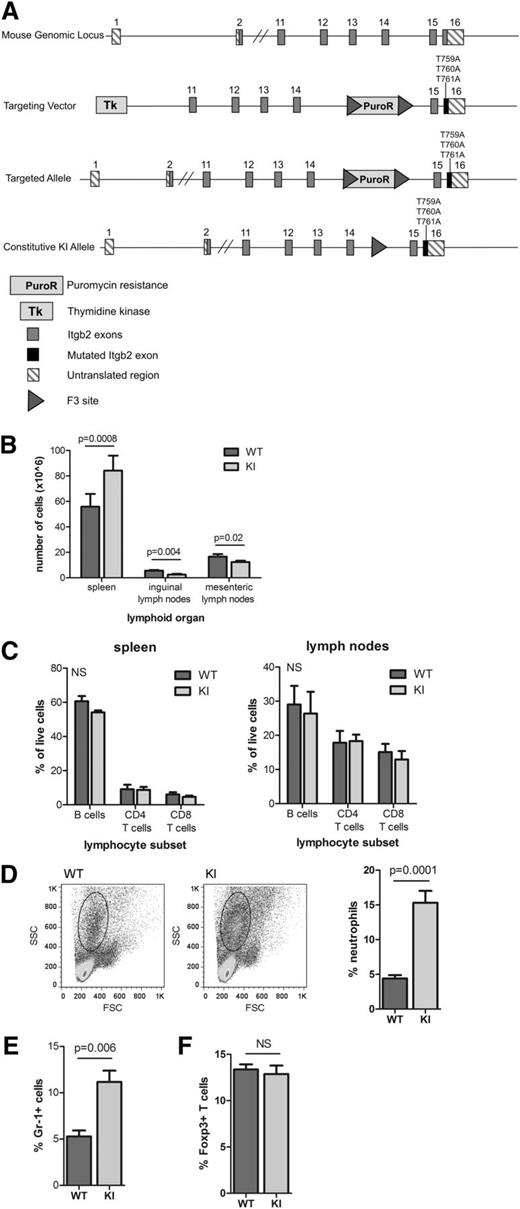
To assess the roles of the TTT motif and β2–kindlin-3 interactions in leukocyte function in vivo, we generated a constitutive β2TTT/AAA integrin knock-in mouse by gene targeting (Figure 2A). This mouse model contains a TTT/AAA substitution in the β2 integrin subunit, thereby abolishing the integrin-kindlin-3 association. Mutant mice were born at Mendelian ratios, were fertile, and were healthy (not shown). β2TTT/AAA integrin knock-in mice displayed splenomegaly and significantly reduced lymph node cellularity compared with WT littermates (Figure 2B). Lymphocyte subset proportions (B cells, CD4 T cells, and CD8 T cells) in secondary lymphoid tissues were normal (Figure 2C), whereas absolute numbers of leukocytes in the spleen (including B cells, T cells, and DCs) were increased (data not shown). These results show that the TTT/AAA mutation does not affect lymphocyte development, but elevated numbers of leukocytes in the spleen suggest altered homing because of a loss of integrin function. In addition, the numbers of circulating neutrophils were significantly increased in β2TTT/AAA integrin knock-in mice (Figure 2D-E). Numbers of splenic regulatory Foxp3+ T cells were normal in knock-in mice (Figure 2F), in contrast to reports from talin and β2 integrin null mice.13,25 These results suggest that the TTT/AAA mutation leads to a selective loss of β2 integrin function, affecting lymphocyte recirculation and neutrophil migration, as seen in β2 integrin and LFA-1 null mice,26,27 but not lymphocyte development, in contrast to kindlin-3 null mice.10
Generation and phenotype of β2TTT/AAA integrin knock-in mice. (A) Arrangement of the murine Itgb2 genomic locus (top), targeting vector (second from top), targeted Itgb2 allele after homologous recombination (second from bottom), and the constitutively expressed Itgb2 knock-in allele after Flp recombination (bottom). F3–Flp recombination target site. (B) The size of secondary lymphoid tissues (spleen, inguinal lymph nodes, and mesenteric lymph nodes) in 6- to 10-week-old WT and β2TTT/AAA integrin knock-in (KI) homozygote mice, presented as cell number (N = 5 mice). (C) Proportions of B cells and CD4 and CD8 T cells in the spleen and inguinal lymph nodes of 6- to 10-week-old WT and KI mice (N = 3). (D) Circulating neutrophil numbers were identified by forward/side scatter profiles, with representative plots (left) and pooled data from 6 mice (right). (E) Gr-1 staining of blood samples to identify circulating neutrophils (N = 6 mice). (F) Splenic regulatory T cells were identified by intracellular Foxp3 staining (N = 6 mice). In all cases, Student t test was used to calculate significance values. NS, not significant. In all cases, mean ± SEM are presented.
Generation and phenotype of β2TTT/AAA integrin knock-in mice. (A) Arrangement of the murine Itgb2 genomic locus (top), targeting vector (second from top), targeted Itgb2 allele after homologous recombination (second from bottom), and the constitutively expressed Itgb2 knock-in allele after Flp recombination (bottom). F3–Flp recombination target site. (B) The size of secondary lymphoid tissues (spleen, inguinal lymph nodes, and mesenteric lymph nodes) in 6- to 10-week-old WT and β2TTT/AAA integrin knock-in (KI) homozygote mice, presented as cell number (N = 5 mice). (C) Proportions of B cells and CD4 and CD8 T cells in the spleen and inguinal lymph nodes of 6- to 10-week-old WT and KI mice (N = 3). (D) Circulating neutrophil numbers were identified by forward/side scatter profiles, with representative plots (left) and pooled data from 6 mice (right). (E) Gr-1 staining of blood samples to identify circulating neutrophils (N = 6 mice). (F) Splenic regulatory T cells were identified by intracellular Foxp3 staining (N = 6 mice). In all cases, Student t test was used to calculate significance values. NS, not significant. In all cases, mean ± SEM are presented.
β2TTT/AAA integrin knock-in T cells display impaired adhesion
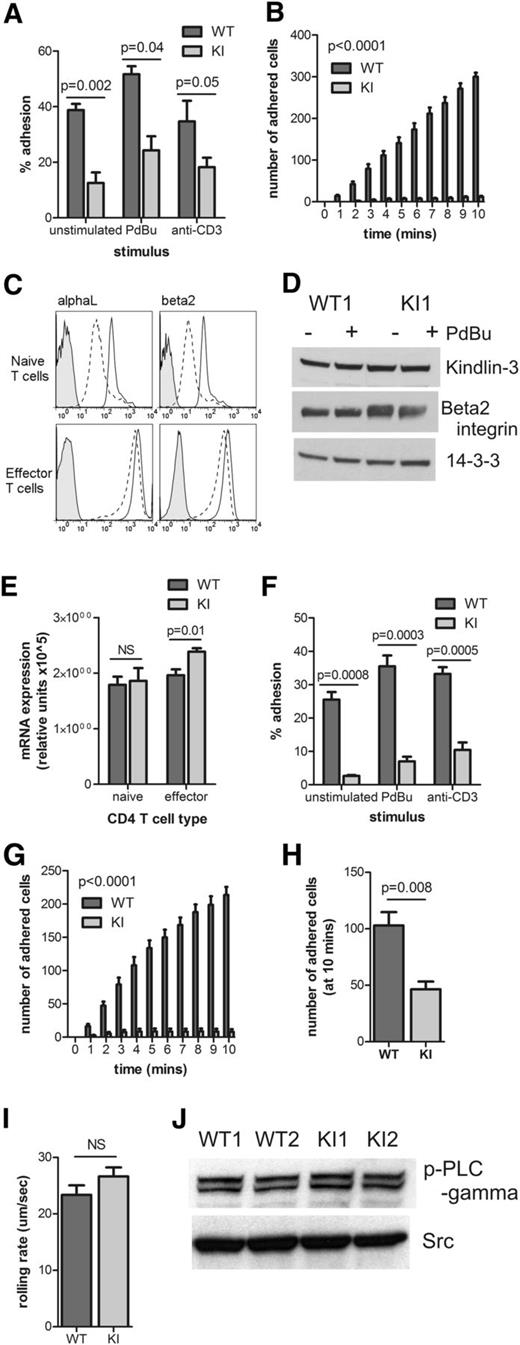
To determine the effects of the loss of the TTT motif and β2–kindlin-3 interactions on cellular adhesion, naïve CD4 T cells were isolated from WT and knock-in mice and compared in their binding to the β2 integrin ligand, ICAM-1. Under static conditions, which mimic T-cell adhesion in tissues where shear flow is absent, β2TTT/AAA integrin knock-in T cells showed reduced adhesion to ligand (Figure 3A). More importantly, under conditions of shear flow (mimicking conditions in blood vessels20 ), SDF-1–induced adhesion of naïve CD4 T cells to ICAM-1 was almost completely abolished by the TTT/AAA mutation (Figure 3B). However, LFA-1 surface expression in knock-in naïve T cells was lower than in WT cells (Figure 3C top). LFA-1 expression is upregulated in effector T cells.28 Therefore, effector CD4 T cells (CD44high CD62Llow, data not shown) were generated by in vitro culture.29 As expected, effector CD4 T cells upregulated LFA-1, and the LFA-1 expression profiles of WT and knock-in cells were similar (Figure 3C, bottom). Importantly, protein levels of kindlin-3 and β2 integrin were similar in WT and knock-in effector T cells (Figure 3D), and β2 integrin mRNA levels were similar in WT and knock-in cells (Figure 3E). However, β2TTT/AAA integrin knock-in effector T cells still displayed severely reduced adhesion to ICAM-1 compared with WT cells (Figure 3F), demonstrating that the deficiency in adhesion is not caused by differences in LFA-1 expression levels.
β2TTT/AAA integrin knock-in T cells display impaired adhesion. (A) Adhesion of WT and KI-naïve CD4 T cells to ICAM-1 under static conditions. Cells were unstimulated, or stimulated with 200 nM PDBu or 10 μg/mL anti-CD3 (N = 4). (B) SDF-1–induced adhesion of naïve CD4 T cells to ICAM-1 under shear flow conditions (N = 4). (C) The expression of αL (left) and β2 (right) integrin subunits in WT (solid line) and KI (dashed line)-naïve CD4 T cells ex vivo (top) and cultured effector CD4 T cells (bottom). Plots are representative of N = 3 to 5. Shaded line represents the isotype control. (D) Kindlin-3, β2 integrin and 14-3-3 protein levels in WT and KI effector CD4 T cells, with and without stimulation with 200 nM PdBu. N = 1 is shown as representative of N = 2. (E) β2 integrin mRNA levels in WT and KI-naïve and effector CD4 T cells were determined by reverse-transciptase quantitative PCR (N = 4). (F) Effector CD4 T-cell adhesion to ICAM-1 under static conditions, using unstimulated, PDBu– or anti-CD3–stimulated cells (N = 5). (G) Effector CD4 T-cell adhesion to ICAM-1 under shear flow conditions (N = 4). (H) Effector CD4 T-cell adhesion to bEnd.3 cells under shear flow, after 10 minutes of adhesion (N = 3). (I) Rolling rates of effector CD4 T cells on ICAM-1 under shear flow (N = 3). (J) p-PLCγ and Src levels in effector CD4 T cells were determined by Western blot analysis. N = 2 is shown and is representative of N = 4. Student t test was used to calculate significance values in panels A,E-F,H-I. Two-way analysis of variance was performed in panels B,G. NS, not significant. In all cases, mean ± SEM is shown.
β2TTT/AAA integrin knock-in T cells display impaired adhesion. (A) Adhesion of WT and KI-naïve CD4 T cells to ICAM-1 under static conditions. Cells were unstimulated, or stimulated with 200 nM PDBu or 10 μg/mL anti-CD3 (N = 4). (B) SDF-1–induced adhesion of naïve CD4 T cells to ICAM-1 under shear flow conditions (N = 4). (C) The expression of αL (left) and β2 (right) integrin subunits in WT (solid line) and KI (dashed line)-naïve CD4 T cells ex vivo (top) and cultured effector CD4 T cells (bottom). Plots are representative of N = 3 to 5. Shaded line represents the isotype control. (D) Kindlin-3, β2 integrin and 14-3-3 protein levels in WT and KI effector CD4 T cells, with and without stimulation with 200 nM PdBu. N = 1 is shown as representative of N = 2. (E) β2 integrin mRNA levels in WT and KI-naïve and effector CD4 T cells were determined by reverse-transciptase quantitative PCR (N = 4). (F) Effector CD4 T-cell adhesion to ICAM-1 under static conditions, using unstimulated, PDBu– or anti-CD3–stimulated cells (N = 5). (G) Effector CD4 T-cell adhesion to ICAM-1 under shear flow conditions (N = 4). (H) Effector CD4 T-cell adhesion to bEnd.3 cells under shear flow, after 10 minutes of adhesion (N = 3). (I) Rolling rates of effector CD4 T cells on ICAM-1 under shear flow (N = 3). (J) p-PLCγ and Src levels in effector CD4 T cells were determined by Western blot analysis. N = 2 is shown and is representative of N = 4. Student t test was used to calculate significance values in panels A,E-F,H-I. Two-way analysis of variance was performed in panels B,G. NS, not significant. In all cases, mean ± SEM is shown.
Effector T cells adhere to ICAM-1 and endothelial cells without integrin affinity upregulation by chemokine stimulation.20,28 This chemokine-independent adhesion of effector T cells is dependent on high integrin expression and PLC-γ activity.20,28 Interestingly, firm adhesion of effector T cells to both ICAM-1 (Figure 3G) and endothelial cells (Figure 3H) under shear flow conditions was severely reduced by the TTT/AAA mutation. However, TTT/AAA-mutated integrins were able to mediate normal cell rolling on ICAM-1 under shear flow (Figure 3I), a process that requires integrin-ligand interactions but not firm adhesion. This result indicates that, although the integrin-kindlin-3 linkage is abolished, initial integrin-ligand bonds can still form. In addition, normal PLC-γ phosphorylation levels were observed in effector CD4 T cells (Figure 3J). Together, these results reveal the fundamental requirement for the integrin-kindlin interaction in T cell firm adhesion and adhesion strengthening under shear stress.
Adhesion strengthening is compromised in cells where integrin-kindlin-3 interactions are disrupted
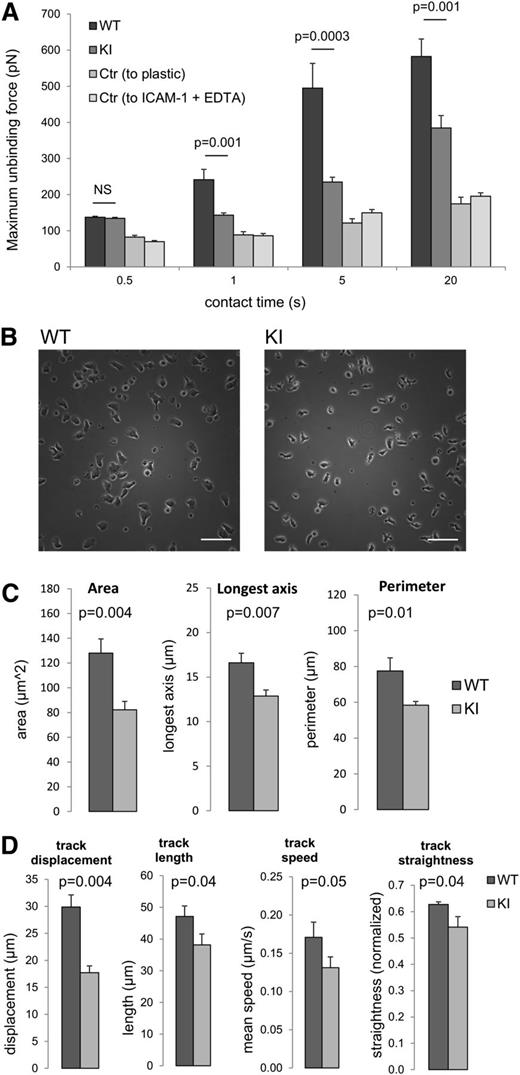
To further investigate the effects of the loss of kindlin-3–β2 integrin interactions on ligand binding, we used force spectroscopy to directly measure the forces required to disrupt integrin-ligand contacts. Individual effector CD4 T cells were attached to a cantilever and allowed to contact an ICAM-1–coated surface for defined times. The force required to separate the effector T cell from the integrin ligand was then measured (maximum unbinding force, Figure 4A) by determining cantilever deflection using a laser beam reflected onto a quadrant photodiode. At the shortest contact time of 0.5 seconds, the WT and knock-in cells bound equivalently, indicating that the initial LFA-1–ICAM-1 interaction is normal in knock-in cells. However, at longer contact times of 1 to 20 seconds, the force required to disrupt WT T-cell integrin–ligand interactions was significantly greater than that for β2TTT/AAA integrin knock-in T cells (Figure 4A). As controls, we measured effector CD4 T-cell adhesion to bare plastic and to ICAM-1 in the presence of EDTA (Figure 4A). These results suggest that adhesion maturation leading to firm adhesion to ligand was deficient in β2TTT/AAA knock-in cells.
Loss of β2–kindlin-3 interactions in CD4 T cells results in impaired adhesion strengthening and impaired migration on ICAM-1 in vitro. (A) AFM measurements of the forces required to detach WT and KI effector CD4 T cells from an ICAM-1–coated surface, presented as maximum unbinding force. The control readings (Ctr) indicate WT cell adhesion to bare plastic, and WT cell adhesion to ICAM-1 in the presence of EDTA. At all contact times, adhesion of both WT and knock-in cells to ICAM-1 was significantly above binding to bare plastic or ICAM-1 in the presence of EDTA. N = 50 individual T cells, with mean ± SEM. (B) Still images of WT and KI effector CD4 T cells plated onto ICAM-1–coated surfaces. Scale bars represent 50 μm. Images are representative of cells from N = 8 mice. (C) WT and KI effector CD4 T-cell spreading on ICAM-1 was quantified in terms of area, longest axis, and perimeter. Cells are from N = 3 mice. (D) Effector CD4 T-cell 2-dimensional migration on ICAM-1 was viewed using time-lapse microscopy over a 15-minute period and videos analyzed to quantify the parameters indicated. Cells are from N = 8 mice, with mean ± SEM. Student t test was used to calculate significance values. NS, not significant.
Loss of β2–kindlin-3 interactions in CD4 T cells results in impaired adhesion strengthening and impaired migration on ICAM-1 in vitro. (A) AFM measurements of the forces required to detach WT and KI effector CD4 T cells from an ICAM-1–coated surface, presented as maximum unbinding force. The control readings (Ctr) indicate WT cell adhesion to bare plastic, and WT cell adhesion to ICAM-1 in the presence of EDTA. At all contact times, adhesion of both WT and knock-in cells to ICAM-1 was significantly above binding to bare plastic or ICAM-1 in the presence of EDTA. N = 50 individual T cells, with mean ± SEM. (B) Still images of WT and KI effector CD4 T cells plated onto ICAM-1–coated surfaces. Scale bars represent 50 μm. Images are representative of cells from N = 8 mice. (C) WT and KI effector CD4 T-cell spreading on ICAM-1 was quantified in terms of area, longest axis, and perimeter. Cells are from N = 3 mice. (D) Effector CD4 T-cell 2-dimensional migration on ICAM-1 was viewed using time-lapse microscopy over a 15-minute period and videos analyzed to quantify the parameters indicated. Cells are from N = 8 mice, with mean ± SEM. Student t test was used to calculate significance values. NS, not significant.
Two-dimensional T-cell migration is impaired but not abolished by the TTT/AAA mutation
We next analyzed the morphology and migration of WT and β2TTT/AAA knock-in effector CD4 T cells placed on ICAM-1. Both WT and β2TTT/AAA integrin knock-in effector T cells were polarized and formed contacts with the integrin ligand (Figure 4B), although WT cells were significantly more spread than knock-in cells (Figure 4C). These results implicate differences in actin reorganization in cells where the kindlin-3-integrin interaction is disrupted (Figure 4B-C). Using time-lapse microscopy of effector T-cell migration on ICAM-1, we observed that β2TTT/AAA integrin knock-in effector T cells were less motile, migrated at slower speeds, and had impaired directed migration when compared with WT cells, although the cells could still interact with ICAM-1 and mediate cell migration (Figure 4D). Together, our results show that the kindlin-3-integrin interaction is not absolutely necessary for integrin-mediated 2-dimensional migration but is necessary when adhesion strengthening is required under shear flow conditions.
Loss of integrin-kindlin-3 interactions result in impaired homing in vivo
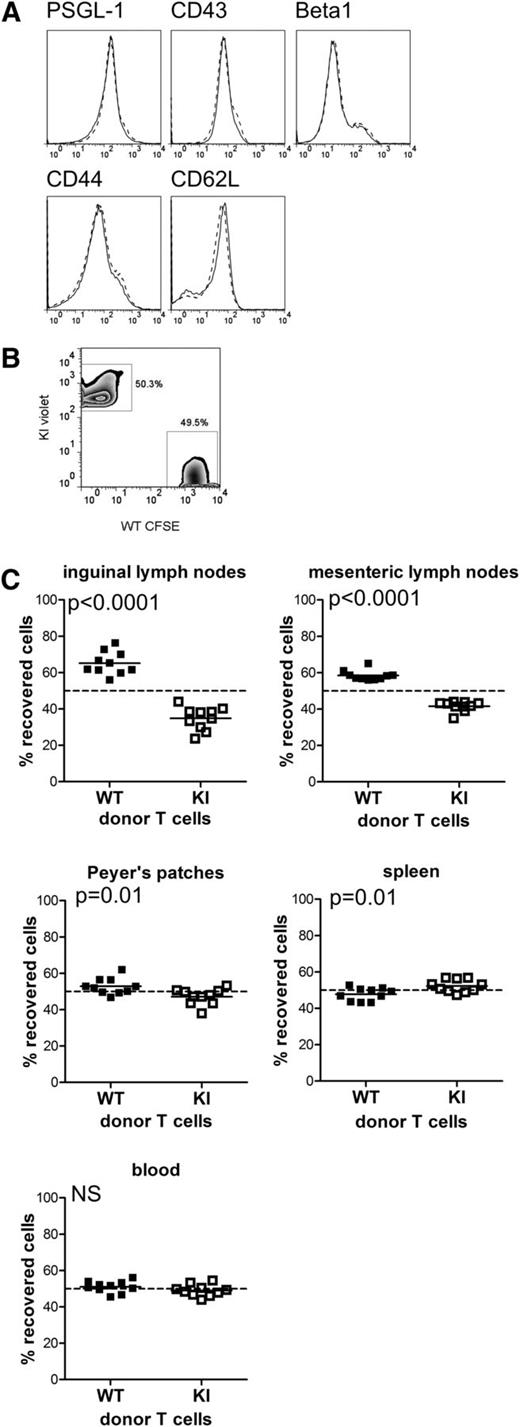
LFA-1 is essential for homing of lymphocytes to lymph nodes.1 Therefore, we next studied the effect of the integrin TTT/AAA mutation on T-cell homing in vivo. CD4 T cells were isolated from WT and knock-in mice. Expression of other adhesion molecules (PSGL-1, CD43, β1 integrin, CD44, and CD62L) was equivalent in WT and knock-in cells (Figure 5A). After intravenous adoptive transfer of fluorescently-labeled naïve CD4 T cells (Figure 5B), β2TTT/AAA integrin knock-in CD4 T cells were found in significantly reduced numbers in the lymph nodes of recipient mice compared with WT cells (Figure 5C). Conversely, greater numbers of knock-in T cells were found in the spleen, whereas numbers of WT and knock-in T cells in the blood were equal (Figure 5C). This result is consistent with T-cell distribution in the knock-in mice, which display elevated T cell numbers in the spleen and normal circulating T cell numbers (data not shown), and also with results from LFA-1–deficient T cells,27 suggesting that T cell homing to the spleen occurs independently of LFA-1. These data confirm that the binding of kindlin-3 to the TTT-region of the β2 cytoplasmic tail is essential for the normal function of LFA-1 during in vivo homing to lymph nodes, most likely by affecting the firm attachment of lymphocytes to endothelial cells under conditions of shear flow.
Loss of β2–kindlin-3 interactions in CD4 T cells results in altered homing in vivo. (A) Expression levels of the adhesion molecules PSGL-1, CD43, β1 integrin, CD44, and CD62L in WT (solid line) and KI (dashed line)-naïve CD4 T cells. (B) Naïve CD4 T cells were isolated from WT and KI mice, labeled with CFSE and CellTrace Violet, respectively, and mixed at a 1:1 ratio. (C) After adoptive transfer into recipient WT mice, the localization of WT and KI donor cells in the organs indicated was analyzed 16 hours post transfer. Plotted values indicate the recovery of WT or knock-in donor cells as a percentage of total recovered cells. Data are pooled from 2 independent experiments: 5 mice per group per experiment. Each data point represents an individual mouse, with the mean indicated. The dashed line shows 1:1 recovery of WT and KI T cells. Deviation from the dashed line indicates differential homing of WT and KI cells. The minimal, but significant, defect in KI T-cell homing to the Peyer’s patches likely reflects the involvement of other integrins in access to gut-associated lymphoid tissue. Student t test was used to calculate P values. NS, not significant.
Loss of β2–kindlin-3 interactions in CD4 T cells results in altered homing in vivo. (A) Expression levels of the adhesion molecules PSGL-1, CD43, β1 integrin, CD44, and CD62L in WT (solid line) and KI (dashed line)-naïve CD4 T cells. (B) Naïve CD4 T cells were isolated from WT and KI mice, labeled with CFSE and CellTrace Violet, respectively, and mixed at a 1:1 ratio. (C) After adoptive transfer into recipient WT mice, the localization of WT and KI donor cells in the organs indicated was analyzed 16 hours post transfer. Plotted values indicate the recovery of WT or knock-in donor cells as a percentage of total recovered cells. Data are pooled from 2 independent experiments: 5 mice per group per experiment. Each data point represents an individual mouse, with the mean indicated. The dashed line shows 1:1 recovery of WT and KI T cells. Deviation from the dashed line indicates differential homing of WT and KI cells. The minimal, but significant, defect in KI T-cell homing to the Peyer’s patches likely reflects the involvement of other integrins in access to gut-associated lymphoid tissue. Student t test was used to calculate P values. NS, not significant.
β2TTT/AAA integrin knock-in CD4 T-cell activation in vitro and in vivo is normal
In addition to its role in cellular adhesion and migration, LFA-1 is thought to provide a costimulatory signal to T cells during activation.3-5 We therefore investigated the requirement for β2 integrin–kindlin-3 interactions in CD4 T-cell activation in vitro and in vivo.
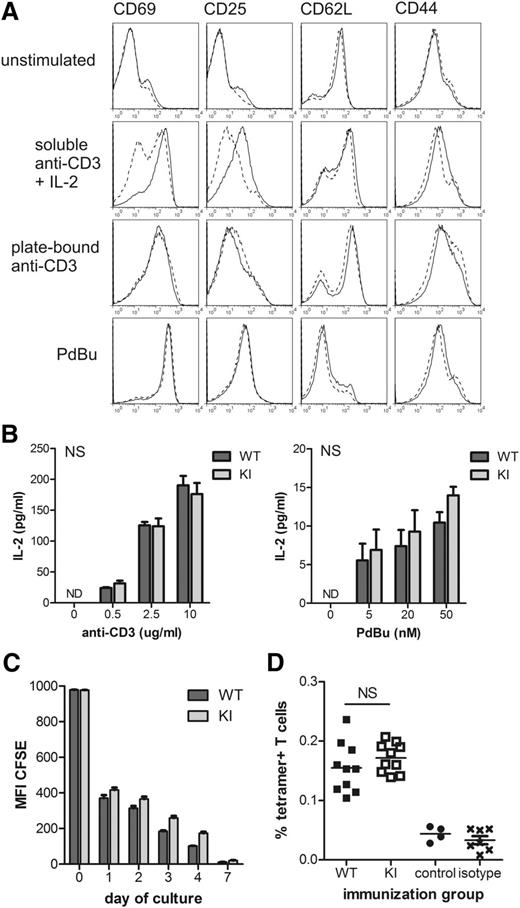
After polyclonal stimulation with plate-bound anti-CD3 or phorbol ester, β2TTT/AAA integrin knock-in CD4 T-cell activation was normal, as measured by expression of the activation markers CD69, CD25 (IL-2 receptor), and CD44, which are upregulated upon activation, and CD62L (l-selectin), which is shed from the T-cell surface during activation (Figure 6A). Production of IL-2, the cytokine made by activated T cells to signal in an autocrine manner and promote proliferation, was also normal in the knock-in T-cell cultures after stimulation (Figure 6B). In contrast, stimulation with soluble anti-CD3 in conjunction with IL-2 resulted in reduced levels of activation of β2TTT/AAA integrin knock-in T cells (Figure 6A) and delayed, but not abolished, T-cell proliferation (Figure 6C), suggesting a differential requirement for β2–kindlin-3 interactions depending on the stimuli used in vitro.
β2TTT/AAA integrin knock-in CD4 T-cell activation in vitro and in vivo is normal. (A) Purified naïve CD4 T cells were activated in vitro using soluble anti-CD3 (2.5 μg/mL) plus IL-2 (20 ng/mL), plate-bound anti-CD3 (2.5 μg/mL at 37°C for 4 hours), or phorbol ester (PdBu, 50 nM). WT (solid line) and KI (dashed line) T-cell activation was measured 24 hours later by expression of the typical activation markers: CD69, CD44, CD25, and CD62L. Histograms are representative of N = 4. (B) IL-2 production by WT and KI T cells after 24-hour stimulation with plate-bound anti-CD3 or PdBu. Data are pooled from N = 4, with mean ± SEM displayed. (C) WT and KI CD4 T cells were labeled with CFSE before activation with soluble anti-CD3 plus IL-2. Proliferation was measured by flow cytometry. Data are pooled from N = 2 and are representative of N = 4. (D) WT and KI mice received an intravenous adoptive transfer of LPS-matured, peptide-loaded WT DCs, and the antigen-specific CD4 T-cell response in the spleen was measured using MHC class II tetramers 7 days later. Data are pooled from 2 independent experiments, with 5 mice per group per experiment. Each data point represents an individual mouse, with mean indicated. The control group received no adoptive transfer. Isotype shows staining of the WT immunization group with an irrelevant peptide-tetramer. Student t test was used to calculate P values. NS, not significant.
β2TTT/AAA integrin knock-in CD4 T-cell activation in vitro and in vivo is normal. (A) Purified naïve CD4 T cells were activated in vitro using soluble anti-CD3 (2.5 μg/mL) plus IL-2 (20 ng/mL), plate-bound anti-CD3 (2.5 μg/mL at 37°C for 4 hours), or phorbol ester (PdBu, 50 nM). WT (solid line) and KI (dashed line) T-cell activation was measured 24 hours later by expression of the typical activation markers: CD69, CD44, CD25, and CD62L. Histograms are representative of N = 4. (B) IL-2 production by WT and KI T cells after 24-hour stimulation with plate-bound anti-CD3 or PdBu. Data are pooled from N = 4, with mean ± SEM displayed. (C) WT and KI CD4 T cells were labeled with CFSE before activation with soluble anti-CD3 plus IL-2. Proliferation was measured by flow cytometry. Data are pooled from N = 2 and are representative of N = 4. (D) WT and KI mice received an intravenous adoptive transfer of LPS-matured, peptide-loaded WT DCs, and the antigen-specific CD4 T-cell response in the spleen was measured using MHC class II tetramers 7 days later. Data are pooled from 2 independent experiments, with 5 mice per group per experiment. Each data point represents an individual mouse, with mean indicated. The control group received no adoptive transfer. Isotype shows staining of the WT immunization group with an irrelevant peptide-tetramer. Student t test was used to calculate P values. NS, not significant.
T-cell activation in vivo requires contacts to form between antigen-presenting cells, such as DCs, and T cells, and occurs in the shear-free environment of secondary lymphoid organs such as the spleen and lymph nodes. Therefore, to investigate whether T-cell activation in vivo was affected by abolishing the integrin-kindlin interaction, we transferred peptide-loaded LPS-matured WT DCs intravenously into recipient WT or knock-in mice and analyzed the antigen-specific T-cell response in the spleen using MHC class II tetramers. We specifically looked at splenic T-cell activation because the knock-in mutation did not impair migration of T cells into this organ (Figure 5B). We used WT DCs as antigen-presenting cells in this experiment because the β2 integrin activation state in DCs has been shown to modify T-cell activation.30,31 Importantly, antigen-specific CD4 T-cell activation was found to be equivalent in WT and beta2TTT/AAA integrin knock-in mice (Figure 6D), implying that the kindlin-3 interaction with LFA-1 in T cells is not required for T cell activation in the spleen in vivo under these experimental conditions. Thus, in contrast to the essential role of the kindlin-3-integrin interaction in mediating adhesion strengthening under shear flow, it appears that kindlin-3–mediated adhesion strengthening is not essential for DC–T cell interactions leading to T-cell activation in the shear-free environment of the spleen in vivo.
Discussion
β2 integrins are important adhesion and signaling receptors with pivotal roles in immune responses in vivo, as shown by studies in integrin null mice1,26,27 and in the human syndromes LAD-I and LAD-III.6 Kindlin-3 is mutated in LAD-III and has been described to be important for β2 integrin–mediated adhesion in vitro.32,33 Here, we have shown that the TTT-domain in the β2 integrin tail is essential for kindlin-3 binding. Our finding complements a previous study showing that kindlin-3 binding to the β2 integrin cytoplasmic domain is dependent on the distal NXXF motif.10 Thus, both the TTT-domain and the downstream NXXF motif mediate kindlin-3 binding to the β2 integrin tail, as has also been described for the corresponding β1 integrin–kindlin-2 interaction.24
Although it is apparent that kindlin-3 is important for neutrophil trafficking into sites of inflammation, the specific roles of integrin-kindlin-3 interactions in T cells in vivo have not been described. Here, we have generated a β2TTT/AAA integrin knock-in mouse, which has allowed us to study the role of this interaction domain in T-cell development, activation, and function. In contrast to kindlin-3 null animals,10 T-cell development was normal in β2TTT/AAA integrin knock-in mice, allowing us to dissect the role of the kindlin-3–β2 integrin interaction in mature lymphocytes. This result also indicates that kindlin-3 has β2 integrin-independent functions in lymphocytes. We have now shown that ablating the kindlin-3–β2 integrin interaction results in impaired T-cell homing but, surprisingly, does not affect T-cell activation in vivo.
The constitutive TTT/AAA substitution in mice resulted in splenomegaly and reduced lymph node size, reflecting the impairment in lymphocyte adhesion to ICAM-1 and endothelial cells under conditions of shear flow, and the reduced homing to lymph nodes in vivo. We have shown that initial contacts between β2 integrins and ICAM-1 could still occur when the TTT-motif was mutated. For example, integrins could still mediate rolling on ICAM-1 under shear flow, 2-dimensional T-cell migration was found to be reduced but not abolished, and the forces required for disruption of early integrin-ligand bonds were found to be equivalent for WT and knock-in T cells. Thus, impaired adhesion and homing of β2TTT/AAA integrin knock-in T cells is likely caused by the loss of kindlin-3–mediated stabilization of LFA-1–ICAM-1 firm adhesion. It is known that the integrin-kindlin interaction links the integrin to the actin cytoskeleton,33 and the integrin TTT/AAA mutation abolishes integrin-mediated actin reorganization in cells.8 Therefore, it is likely that an impairment of the integrin-kindlin-actin linkage in TTT/AAA-mutated cells results in an inability of cells to resist shear flow forces and mediate firm adhesion under these conditions.
In addition to binding kindlin-3, the TTT region of the β2 integrin cytoplasmic domain is also a binding site for 14-3-3 proteins.8,17 However, this interaction only occurs when Thr758 is phosphorylated,8,17 and in effector T cells, where firm adhesion under shear flow conditions is abolished, this residue is not phosphorylated.28 Therefore, the effect of the mutation on firm adhesion is likely not the result of a loss of 14-3-3 protein interaction.
Talin, another important integrin regulator, binds to the membrane-proximal NPXY site in integrins, which is upstream of the TTT site.22,23 We and others have shown previously and here that binding of talin to the integrin β-chain is not affected by mutations in the TTT site,9,24 and furthermore, talin can still activate β2 integrins carrying mutations in this region.8 Therefore, the TTT/AAA mutation is unlikely to affect talin-mediated integrin activation directly.
One clear outcome of the TTT/AAA mutation was reduced surface expression of β2 integrins in leukocytes, despite normal levels of β2 integrin mRNA and total cellular β2 integrin protein in knock-in cells. A recent study showed that threonine substitution (TT/AA) in the β1 integrin cytoplasmic domain results in reduced binding of SNX17, a protein involved in receptor recycling from endosomal compartments, thus preventing their degradation.24 A similar mechanism might account for the reduced integrin expression seen in β2TTT/AAA integrin knock-in cells. However, effector T cells did not display a significant decrease in integrin expression but still showed a profound defect in adhesion, demonstrating that reduced integrin surface expression in the TTT/AAA mice does not account for the adhesion defect in this model.
Further to its established role in lymphocyte recirculation, LFA-1 is thought to provide a costimulatory signal to T cells.3-5,18 Kindlin-3 has been postulated to play a role in T cell–DC interactions in vitro,33 but the significance of this for in vivo T-cell immune responses, and the specific involvement of kindlin-3–β2 interaction in T-cell activation, is unclear. Here, we found that in vivo T-cell activation in the spleen was equivalent in WT and β2TTT/AAA integrin knock-in mice, suggesting that T-cell activation does not necessarily require kindlin-3 association with LFA-1. DC–T cell interactions leading to antigen-specific T-cell activation occur in the shear-free environment of the spleen or lymph nodes, and our data suggest that integrin-kindlin-3 interactions are not absolutely required to stabilize cell-cell contacts under these circumstances. In contrast, antigen-induced splenic CD4 T-cell proliferation in both β2 and talin null mice has been reported to be reduced,13,34 indicating that LFA-1 itself and talin-mediated LFA-1 activation is indispensable for splenic T-cell activation in vivo. Together, these data implicate that talin and kindlin-3 play different roles in LFA-1 regulation of T-cell activation in vivo, although further studies are required to investigate this more thoroughly.
In conclusion, we have now described that the TTT-motif in the β2 integrin cytoplasmic domain, which mediates kindlin-3 binding, is essential for adhesion strengthening of T cells under shear flow conditions but not for T-cell activation in vivo in a shear-free environment. These results implicate a selective role for the integrin-kindlin-3 interaction in certain β2 integrin–mediated events but not others.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank JPK Instruments (Germany) for the loan of the CellHesion200 platform, Sam Swift (Electron Microscopy Facility, University of Dundee) for FRET microscopy, and David McGloin and Paul Campbell for AFM support.
This study was supported by BBSRC, Cancer Research UK, Arthritis Research UK, Tenovus Scotland, The Anonymous Trust, and the Academy of Finland (all to S.C.F).
Authorship
Contribution: S.C.F. and V.L.M. planned the study; V.L.M., M.M., T.S., H.S.L., and S.C.F. performed the experiments; A.P. provided essential technical help; and S.C.F. and V.L.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susanna C. Fagerholm, Medical Research Institute, Jacqui Wood Cancer Centre, Ninewells Hospital & Medical School, University of Dundee, Dundee, DD1 9SY, Scotland, United Kingdom; or Institute of Biotechnology, PO Box 56, 00014 University of Helsinki, Finland; e-mail: s.c.fagerholm@dundee.ac.uk or susanna.fagerholm@helsinki.fi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal