Key Points
RARα2 activates Wnt and hedgehog pathways in maintaining myeloma stem cell features and drug resistance.
Abstract
We previously demonstrated that RARα2 expression is increased in CD138 selected plasma cells of relapsed multiple myelomas (MMs), and increased expression was linked to poor prognosis in newly diagnosed MM patients. In the present study, we demonstrate that increased RARα2 confers myeloma stem cell features. Higher expression of RARα2 was identified in the multiple myeloma stem cell (MMSC) fraction. Overexpression of RARα2 in bulk MM cell lines resulted in: 1) increased drug resistance; 2) increased clonogenic potential; 3) activation of both Wnt and Hedgehog (Hh) pathways; 4) increased side population and aldehyde dehydrogenase levels; and 5) increased expression of embryonic stem cell genes. The opposite effects were seen with RARα2 knockdown. We demonstrate that RARα2 induces drug resistance by activating the drug efflux pump gene ABCC3 and anti-apoptotic Bcl-2 family members. Inhibition of Wnt signaling or ABCC3 function could overcome drug resistance in RARα2 overexpressing MM cells. We also showed that in the 5TGM1 mouse model, targeting of the Wnt and Hh pathways using CAY10404, cyclopamine, or itraconazole significantly reduced the myeloma tumor burden and increased survival. Targeting RARα2 or its downstream signaling pathways provides a potential strategy to eliminate MMSC.
Introduction
Cancer stem cells (CSCs) have been identified in multiple malignancies,1,2 including multiple myelomas (MM).3 Besides the distinctive properties of constituting a small fraction of tumor cells with self-renewal capacity, able to propagate the disease, CSCs are thought to be, just like hematopoietic stem cells, much more resistant to chemo- and radiotherapy and to have better DNA repair mechanisms and increased antiapoptotic activity.1,2,4 Evidence of the existence of a MM stem cell has been provided by Matsui et al3 showing that the CD138−/CD19+ fraction has a greater clonogenic potential and has the phenotype of a memory B-cell (CD19+, CD27+). The CD138− cell fraction contains significantly higher levels of aldehyde dehydrogenase (ALDH), a marker for stem cells.3,5 CD138− cells are resistant to cyclophosphamide, dexamethasone, bortezomib, and lenalidomide, whereas the CD138+ fraction is sensitive to these drugs.3,5 The CD138−/CD19+ cells in the MM bone marrow are surface and cytoplasmic light chain-restricted.6 However, not all researchers agree on the multiple myeloma stem cell (MMSC) phenotype. The Weissman group7 considers the CD19−/CD45low/−/CD38high/CD138+ cells to be the tumor-initiating cells in myeloma. Also, the Dana-Farber group found no correlation between the side population (SP) cells, which are enriched for CSCs and CD138 expression.8
We previously reported that the 30% of newly diagnosed myeloma patients, who expressed the retinoic acid receptor alpha2 (RARα2) in their CD138 selected plasma cells, had a significantly inferior outcome.9 RARα2 expression was also highly significantly increased in myelomas rapidly relapsing after transplantation compared with paired baseline samples.9 These findings strongly suggest the existence at diagnosis of a RARα2 expressing drug-resistant subclone, which can be CD138+. Retinoic acid is a nonhormonal ligand for the nuclear receptor, and it is a biologically active form of vitamin A. There are 2 major isoforms for RARα (α1 and α2) performing unique and different functions from other RAR or retinoid X receptor types and isoforms. Previous investigations have shown the distinct expression patterns of RARα1 and RARα2 in normal tissues, with RARα1 ubiquitously expressed in all stages of embryos and adult tissues, whereas RARα2 was present in a limited number of tissues such as intestine, lung, and liver.10 Furthermore, RARα2 is a more potent inhibitor of cell differentiation than RARα1,11-13 suggesting that RARα2 may play an important role in maintaining cells in an undifferentiated stem cell state.
Very little is known about the genetic make-up of CSCs, which makes it difficult to target such cells. However, the Hedgehog (Hh) pathway, Wnt signaling, Notch, and BMI-1 are typically active in CSCs.1,14-19 The Matsui group has demonstrated that Hh signaling maintains the tumor stem cell compartment in myeloma.20 MM cells have also been reported to depend on an active Wnt signaling; epigenetic dysregulation of Wnt signaling pathways resulted in promoting MM cell proliferation, migration, invasion, and drug resistance.21-23 In the present work, we find increased RARα2 expression in MMSC and explore its function in inducing drug resistance and maintaining MM stem cell features. The association of RARα2 and its downstream targets with drug resistance is assessed using in vivo and in vitro myeloma models.
Methods
Cell lines, patient samples, and cell culture
Human MM cell lines were cultured in RPMI 1640 containing 10% heat-inactivated fetal calf serum at 37°C in humidified 5% CO2.9,24-26 The details were described in the supplemental Data (available on the Blood Web site).
Clinical bone marrow samples were obtained from MM patients in Huntsman Cancer Institute, University of Utah according to the ARUP protocol 25009. Studies were approved by the Institutional Review Board of the University of Utah. Informed consent was obtained in accordance with the Declaration of Helsinki.
CD138+ or CD138− subsets were isolated from MM cell lines using autoMACS and CD138 microbeads. Subsequent flow cytometric analysis demonstrated >5% contamination.
Gene expression profiling (GEP)
Specific gene silencing or overexpressing RARα2 in MM cells by lentivirus expression vector system
Co-immunoprecipitation (Co-IP)
Co-IP was performed as previously described.30 Briefly, HEK-293 cells were transiently cotransfected with plasmids containing a 3× FLAG tag (RARα2) or a V5 tag (Nanog) at the C terminus of the protein using Lipofectamine. Endogenous protein interaction was verified in RARα2 overexpressing myeloma cell line OCI-MY5. Benzonase (150 U/mL; Millipore, Billerica, MA) was added to the OCI-MY5 cell lysate to exclude the possibility of chromatin-mediated protein interaction. Specific antibodies or control immunoglobulin (IgG) (Bethyl Laboratories) were added and incubated overnight with cell lysate followed by protein dynabeads for 2 hours at 4°C. The pulled-down proteins were extracted and examined by western blots as described before.
Flow cytometry analysis
ALDH activity was tested by the Aldefluor reagent (Stem Cell Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. Diethylaminobenzaldehyde was used as control staining. The SP analysis was conducted as previously described.8 Briefly, Hoechst (10 μg/mL) was incubated with cells (106 cells/mL) in the 37°C water bath for 90 minutes. Verapamil (100 μM) was used as negative control.
For the apoptosis and multidrug-resistance assays, all the procedures were performed as described in the protocols (Annexin V Apoptosis Detection Kit APC; eBioscience; eFluxx-ID Multidrug resistance assay kits; Enzo Life Sciences) as previously reported.24
Clonogenic formation assay
Colony formation was performed as previously reported.24 Briefly, 10 000 MM cells were seeded in 0.33% agar cultures. The colonies were fed with medium in the presence or absence of drugs; after 3 weeks, the plates were imaged and colony numbers were counted by ImageJ.
Cell growth and viability
CD138+/− cells of ARK and KMS11 were sorted and cultured in RPMI-1640 complete medium with the addition of different concentration of drugs all-trans retinoic acid (ATRA) and cyclopamine from Sigma-Aldrich (St. Louis, MO) and CAY10404 from Cayman Chemical (Ann Arbor, MI) for 5 days. To determine the role of the Wnt pathway in RARα2 signaling, KSM11 and ARK cells were treated with Wnt3a (20 ng/mL) and Shh (200 ng/mL) for 72 hours in the 1640 medium containing ATRA. Cell viability was determined as described in the previous report.31
Quantitative reverse-transcription polymerase chain reaction (RT-PCR) assays
RNeasy Mini kit (Qiagen, Valencia, CA) was used to isolate total RNA from myeloma cells, and first-strand cDNA was synthesized using the SuperScript III RT kit (Invitrogen, Carlsbad, CA). The relative quantitation of each gene is calculated using ΔΔCT. Each sample is standardized with the endogenous control gene (ie, β-actin).
Western blot
Total or nuclear protein was isolated with the Mammalian Cell Extraction Kit or Nuclear/Cytosol Fractionation Kit, respectively (BioVision, Mountain View, CA). All primary antibodies were purchased from Cell Signaling Technology (Danvers, MA). β-Actin and histone H2b were used to normalize the amount of protein in each sample.
Treatments with Wnt and Hh inhibitors in the 5TGM1 murine myeloma model
A total of 0.5 × 106 5TGM1 CD138+ cells or CD138− cells were injected into 6-week-old C57BL/KaLwRij mice through the tail vein. Mice were divided into 5 experimental groups (5TGM1 CD138+, 5TGM1 CD138−, 5TGM1 CD138− treated with itraconazole, 5TGM1 CD138− treated with CAY10404, and 5TGM1 CD138− treated with cyclopamine). CAY10404 treatment (20 mg/kg, intraperitoneally [I.P.]), cyclopamine (20 mg/kg, I.P.), and itraconazole (20 mg/kg, I.P.) were given 3 times/week starting 7 days after the injection of 5TGM1 cells. Blood from each mouse was taken once each week. An enzyme-linked immunosorbent assay kit (Bethyl Laboratories, Montgomery, TX) was used to measure titers of IgG2b secreted by 5TGM1 myeloma tumor cells according to protocol.
Results
RARα2 expression is significantly higher in myeloma stem cells than in bulk of myeloma cells
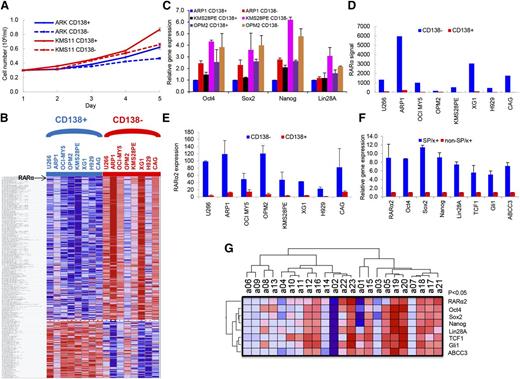
To clarify the characteristics of the CD138+ and CD138− cells, these fractions were separated from ARK and KMS11 MM cell lines, and the differences in growth were evaluated in short-term cultures for 5 days. The CD138+ cells showed significantly higher cell growth compared with CD138− cells (P < .05) (Figure 1A). GEP analyses were performed on the CD138+ and CD138− cells isolated from 8 MM cell lines. A total of 291 genes were significantly differentially expressed in the CD138− cell population compared with CD138+ control cells (P < .05), including 209 upregulated genes and 82 downregulated genes (Figure 1B). The order of differentially expressed genes between CD138+ with CD138− fraction in 8 MM Cells is provided in supplemental Table 1. Consistent with increased cell proliferation characteristics of CD138+ myeloma cells, many genes, significantly downregulated in CD138− cells, were related to DNA replication, cell cycle progression, and chromosomal stability, suggesting that the CD138+ cells are more proliferative, whereas the CD138− cells are more quiescent. In addition, the CD138+ and CD138− fractions from ARP1, KMS28PE, and OPM2 cells were separated, and real-time PCR results showed that the induced pluripotent stem cell (iPS) genes, Oct4, Sox2, Nanog, and Lin28A, were expressed significantly higher in CD138− than in CD138+ cells (P < .05) (Figure 1C). This suggests that iPS genes were activated in the CD138− fraction in MM. Of the 209 significantly upregulated genes in the CD138− cells from 8 MM cell lines, RARα was ranked number 3 (Figure 1D; supplemental Figure 1). Because GEP does not differentiate between RARα1 and RARα2, we performed real-time PCR and detected significantly higher expression of RARα2 in CD138− MM stem cells than in CD138+ MM tumor cells (Figure 1E). We also isolated MMSCs by selecting SP cells plus the MM-specific marker κ light chain (SP/κ+) from an apheresis product of primary MM patients with 8% circulating plasma cells. The mRNA expression levels of RARα2, iPS genes, Wnt (TCF1), Hh (Gli1), and drug efflux pump (ABCC3) in SP/κ+ MM cells were also significantly higher in MMSCs (SP/κ+) than in non-SP/κ+ MM cells (Figure 1F). We further correlated RARα2 expression with these same genes in 23 CD138-selected primary myeloma samples by RT-PCR. As shown in Figure 1G, RARα2 was highly correlated with the expression of Oct4, Sox2, Nanog, Lin28A, TCF1, CCND1, Gli1, and ABCC3 (P < .05).
RARα2 expression increases in myeloma stem cells. (A) Cell growth between CD138+ and CD138− cells from ARK and KMS11 was compared with a hemocytometer for 5 days. Cells from the CD138+ fraction exhibited higher proliferation than CD138− cells. All results were expressed as means ± SD of 3 independent experiments. (B) A supervised hierarchical cluster showed 291 significant differentially expressed genes between CD138+ and CD138− cells from 8 myeloma cell lines. Red for a gene indicates expression above the median and blue indicates expression below the median. Myeloma cell lines were plotted on the vertical axis and the gene probe sets are listed on top along the horizontal axis. (C) The expression of Oct4, Sox2, Nanog, and Lin28A was examined in CD138− and CD138+ cells of ARP1, KMS28PE, and OPM2 myeloma lines by real-time PCR. (D) RARα expression (Y-axis) was compared between CD138− cells with CD138+ cells in 8 myeloma cell lines using GEP analysis. (E) RARα2 expression was compared in the CD138− fraction vs the CD138+ fraction of 8 MM cell lines using real-time-PCR. (F) Real-time PCR showed that expression of RARα2, Oct4, Sox2, Nanog, Lin28A, TCF1, Gli1, and ABCC3 was increased in MMSCs (SP/κ+) cells compared with non-SP/κ+cells in a primary MM patient sample. (G) A Heatmap showed the expression of RARα2, Oct4, Sox2, Nanog, Lin28A, TCF1, Gli1, and ABCC3 genes in 23 primary MM samples as detected by RT-PCR.
RARα2 expression increases in myeloma stem cells. (A) Cell growth between CD138+ and CD138− cells from ARK and KMS11 was compared with a hemocytometer for 5 days. Cells from the CD138+ fraction exhibited higher proliferation than CD138− cells. All results were expressed as means ± SD of 3 independent experiments. (B) A supervised hierarchical cluster showed 291 significant differentially expressed genes between CD138+ and CD138− cells from 8 myeloma cell lines. Red for a gene indicates expression above the median and blue indicates expression below the median. Myeloma cell lines were plotted on the vertical axis and the gene probe sets are listed on top along the horizontal axis. (C) The expression of Oct4, Sox2, Nanog, and Lin28A was examined in CD138− and CD138+ cells of ARP1, KMS28PE, and OPM2 myeloma lines by real-time PCR. (D) RARα expression (Y-axis) was compared between CD138− cells with CD138+ cells in 8 myeloma cell lines using GEP analysis. (E) RARα2 expression was compared in the CD138− fraction vs the CD138+ fraction of 8 MM cell lines using real-time-PCR. (F) Real-time PCR showed that expression of RARα2, Oct4, Sox2, Nanog, Lin28A, TCF1, Gli1, and ABCC3 was increased in MMSCs (SP/κ+) cells compared with non-SP/κ+cells in a primary MM patient sample. (G) A Heatmap showed the expression of RARα2, Oct4, Sox2, Nanog, Lin28A, TCF1, Gli1, and ABCC3 genes in 23 primary MM samples as detected by RT-PCR.
Increased RARα2 expression induces stem cell characteristics in MM
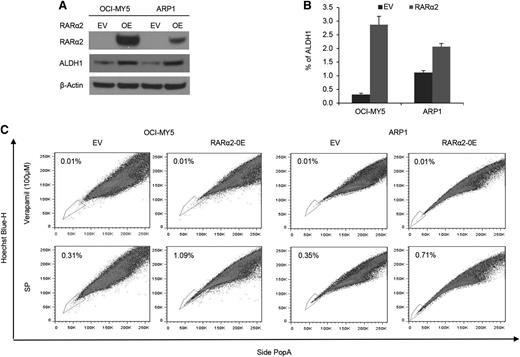
To assess whether RARα2 was indeed the driver rather than an associated phenomenon of drug resistance and stem cell features, we overexpressed RARα2 in bulk cells of low-expressing MM cell lines of ARP1 and OCI-MY5 (Figure 2A). ALDH is a marker of CSCs and a predictor of drug resistance in multiple cancers.32,33 We examined ALDH activity in RARα2 overexpressing ARP1 and OCI-MY5 cells using the fluorescent ALDH substrate Aldefluor and western-blot analysis. ALDH expression and activity significantly increased in RARα2 overexpressing ARP1 and OCI-MY5 compared with the control cells (Figure 2A-B). The frequency of SP cells in ARP1 and OCI-MY5 MM cell lines also significantly increased to 1.09% in OCI-MY5 cells and 0.71% in ARP1 cells compared with the empty vector-transfected control cells (P < .05) (Figure 2C).
Increased RARα2 expression induces stem cell characteristics in MM. (A) Western blots showed ALDH protein levels in OCI-MY5 and ARP1 cells transfected with either RARα2 or empty vector. (B) ALDH activity was evaluated in RARα2 overexpressing cells OCI-MY5 and ARP1 by flow cytometry analysis. (C) SP fractions of OCI-MY5 and ARP1 cells were examined by flow cytometry and the results show the percentages of SP in RARα2 overexpressing cells (1.09% in OCI-MY5 and 0.71% in ARP1) and EV control cells (0.31% in OCI-MY5 and 0.35% in ARP1).
Increased RARα2 expression induces stem cell characteristics in MM. (A) Western blots showed ALDH protein levels in OCI-MY5 and ARP1 cells transfected with either RARα2 or empty vector. (B) ALDH activity was evaluated in RARα2 overexpressing cells OCI-MY5 and ARP1 by flow cytometry analysis. (C) SP fractions of OCI-MY5 and ARP1 cells were examined by flow cytometry and the results show the percentages of SP in RARα2 overexpressing cells (1.09% in OCI-MY5 and 0.71% in ARP1) and EV control cells (0.31% in OCI-MY5 and 0.35% in ARP1).
RARα2 upregulates and physically binds with reprogramming genes essential for iPS and promotes colony formation in MM cell lines
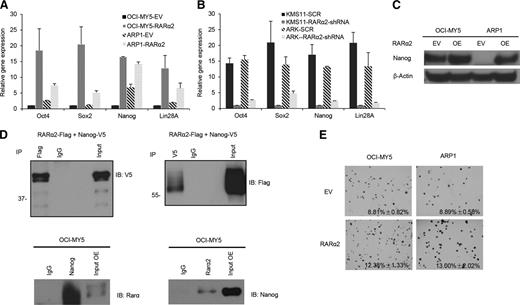
The property of self-renewal is shared by iPS and CSCs. We found that overexpression of RARα2 in ARP1 and OCI-MY5 increased the transcription of Nanog, Sox2, Oct4, and Lin28A (Figure 3A), whereas RARα2 knockdown in KMS11 and ARK MM (high baseline expression of RARα2) decreased the expression of those genes as tested by real-time PCR (Figure 3B). Western-blot results showed that in ARP1 and OCI-MY5 MM cell lines, overexpressing RARα2 upregulated Nanog expression, whereas the protein level of Sox2 and Oct4 were undetectable by western blot in these 2 cell lines (data not shown) (Figure 3C). To determine whether RARα2 directly interacts with members of the core pluripotency network, we employed Co-IP assays in HEK cells, which have no baseline expression of Nanog, and RARα2-overexpressing OCI-MY5 cells. RARα2 interacted robustly with Nanog when either protein was used as bait for Co-IP (Figure 3D) in HEK cells. To verify the interaction of endogenous RARα2 with Nanog and exclude the possibility of chromatin-mediated protein interaction, we performed the Co-IP with lysates prepared from OCI-MY5 cells in the presence of Benzonase. Our results show that these 2 proteins physically interact endogenously in OCI-MY5 cells (Figure 3D).
RARα2 upregulates and physically binds with iPS reprogramming genes and promotes colony formation in MM cell lines. (A-B) The expression of Oct4, Sox2, Nanog, and Lin28A genes was examined in OCI-MY5, ARP1, KMS11, and ARK MM cells over- or underexpressed RARα2 by RT-PCR. (C) Western blots exhibited Nanog expression in RARα2 OE and EV OCI-MY5 and ARP1 cells. (D) Total lysates were prepared from HEK-293 cells transiently transfected with the constructs indicated. Co-IP was performed with anti-Flag antibody (left) or anti-V5 antibody (right), followed by western blotting with the antibodies indicated. Control IgG was used as a negative control for the Co-IP. A total of 1% of the lysate used for Co-IP was used as input. Co-IP was repeated in both directions using OCI-MY5 cells to demonstrate interaction of RARα2 with Nanog in the presence of benzonase. (E) The clonogenic capacity was compared between RARα2-OE and EV cells of OCI-MY5 and ARP1 lines (magnification ×40).
RARα2 upregulates and physically binds with iPS reprogramming genes and promotes colony formation in MM cell lines. (A-B) The expression of Oct4, Sox2, Nanog, and Lin28A genes was examined in OCI-MY5, ARP1, KMS11, and ARK MM cells over- or underexpressed RARα2 by RT-PCR. (C) Western blots exhibited Nanog expression in RARα2 OE and EV OCI-MY5 and ARP1 cells. (D) Total lysates were prepared from HEK-293 cells transiently transfected with the constructs indicated. Co-IP was performed with anti-Flag antibody (left) or anti-V5 antibody (right), followed by western blotting with the antibodies indicated. Control IgG was used as a negative control for the Co-IP. A total of 1% of the lysate used for Co-IP was used as input. Co-IP was repeated in both directions using OCI-MY5 cells to demonstrate interaction of RARα2 with Nanog in the presence of benzonase. (E) The clonogenic capacity was compared between RARα2-OE and EV cells of OCI-MY5 and ARP1 lines (magnification ×40).
The clonogenic capacity of ARP1 and OCI-MY5 cells was significantly increased by RARα2 overexpression compared with the empty-vector (EV) controls (Figure 3E). The colony formation rate of ARP1 cells increased from 9% to 13% after RARα2 was overexpressed, and it rose from 9% to 12% in OCI-MY5 cells (P < .05).
RARα2 overexpression activates both the Wnt and Hh signaling pathways
CSC activates specific pathways such as Wnt, Hh, Notch, and BMI. Increased nuclear (functional) β-catenin and Gli1 levels were detected in RARα2 overexpressing MM cells compared with empty-vector (EV)-transfected controls (Figure 4A). In addition, silencing RARα2 in ARK and KMS11 cells showed decreased expression of nuclear β-catenin and Gli1 after transfection with RARα2-shRNA (Figure 4B). We also detected significantly higher expression of other downstream targets of Wnt and Hh signaling, including TCF1, TCF4, LEF1, CD44, CCND1, SMO, and Gli1 by using real-time PCR in ARP1 and OCI-MY5 overexpressing RARα2 (Figure 4C). Because we had previously shown that ATRA induced cell apoptosis in RARα2-positive myeloma cells, we subsequently tested whether activation of Wnt and Hh pathways could rescue myeloma cells from apoptosis induced by ATRA. Activators of Wnt or Hh signaling were combined with ATRA to treat ARK and KMS11 cells with high expression of RARα2. Wnt3a (20 ng/mL) or Shh (200 ng/mL) partially rescued ARK and KMS11 cells from apoptosis (Figure 4D) induced by ATRA. Western blots showed that Wnt and Hh activators were able to only very partially restore levels of β-catenin and Gli1expression, which were decreased after treatment with RARα2-shRNA or ATRA (Figure 4E).
RARα2 activates both Wnt and Hh signaling. (A) Western blots show the nuclear expression of β-catenin and Gli1 in OCI-MY5 and ARP1 cells overexpressed RARα2. (B) Western blots show the nuclear expression of β-catenin and Gli1 in ARK and KMS11 cells transfected with either RARα2 shRNA or scrambled oligonucleotide (SCR). (C) Real time-PCR revealed the expression of TCF1, TCF4, LEF1, CD44, CCND1, SMO, and Gli1 in RARα2-overexpressing myeloma cells and the EV cells. (D) Cell viability was evaluated in ARK and KMS11 cells treated with ATRA, Wnt3a, and Shh or combinations. All results are expressed as means ± SD of 3 independent experiments. (E) Western blots show the expression of β-catenin and Gli1 in ARK and KMS11 cells treated with ATRA, Wnt3a, and Shh or combinations.
RARα2 activates both Wnt and Hh signaling. (A) Western blots show the nuclear expression of β-catenin and Gli1 in OCI-MY5 and ARP1 cells overexpressed RARα2. (B) Western blots show the nuclear expression of β-catenin and Gli1 in ARK and KMS11 cells transfected with either RARα2 shRNA or scrambled oligonucleotide (SCR). (C) Real time-PCR revealed the expression of TCF1, TCF4, LEF1, CD44, CCND1, SMO, and Gli1 in RARα2-overexpressing myeloma cells and the EV cells. (D) Cell viability was evaluated in ARK and KMS11 cells treated with ATRA, Wnt3a, and Shh or combinations. All results are expressed as means ± SD of 3 independent experiments. (E) Western blots show the expression of β-catenin and Gli1 in ARK and KMS11 cells treated with ATRA, Wnt3a, and Shh or combinations.
High expression of RARα2 is associated with drug resistance
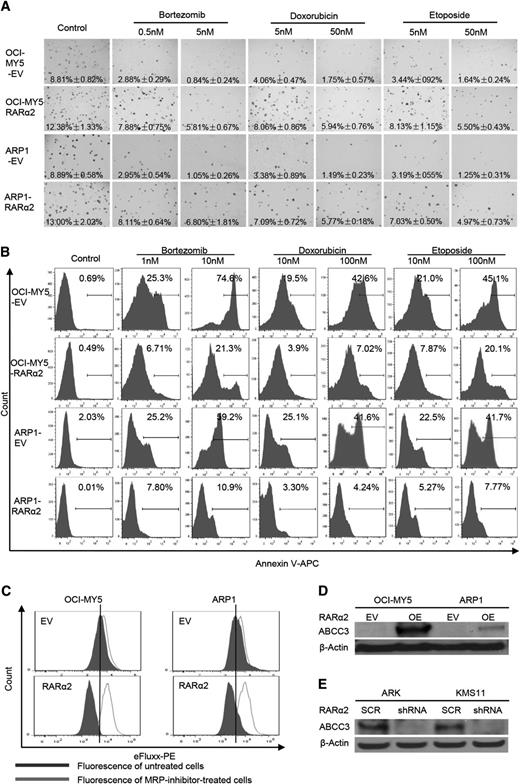
OCI-MY5 and ARP1 cells transfected with RARα2 or EV were seeded on 0.3% soft-agar to assess colony formation. Multiple drugs, including bortezomib (0.5 and 5 nm), doxorubicin (5 and 50 nm), and etoposide (5 and 50 nm) were added to the plates. EV cells with or without these drugs were used as controls. As shown in Figure 5A, RARα2 overexpressing cells showed increased colony formation compared with control cells. The number of colonies in the control cells decreased dramatically in the presence of the 3 drugs tested, whereas MM cells overexpressing RARα2 showed much less decrease in their colony formation capacity, indicating a clear correlation between RARα2 and drug resistance. The Apoptosis Detection Kit was used to detect whether the drug resistance induced by overexpression of RARα2 was associated with a decrease in apoptosis induced by the antimyeloma drugs. After the cells were treated with the drugs for 48 hours, flow cytometry showed that both RARα2-overexpressing ARP1 and OCI-MY5 cells contained much less apoptosis than control cells (Figure 5B) (P < .05).
High expression of RARα2 induces drug-resistance in myeloma cells. (A) The clonogenic capacity was compared between RARα2 OE and EV cells of OCI-MY5 and ARP1 lines treated with bortezomib, doxorubicin, and etoposide (magnification ×40). (B) Cell apoptosis was compared between RARΑ2 OE and EV cells of OCI-MY5 and ARP1 lines treated with bortezomib, doxorubicin, and etoposide by flow cytometry. (C) Flow cytometry shows the activity of drug efflux pump between RARα2 OE and EV cells in OCI-MY5 and ARP1 lines. (D-E) Western blots show the expression of ABCC3 in RARα2-OE OCI-MY5 and ARP1 cells (D) as well as in RARα2-shRNA KMS11 and ARK cells (E).
High expression of RARα2 induces drug-resistance in myeloma cells. (A) The clonogenic capacity was compared between RARα2 OE and EV cells of OCI-MY5 and ARP1 lines treated with bortezomib, doxorubicin, and etoposide (magnification ×40). (B) Cell apoptosis was compared between RARΑ2 OE and EV cells of OCI-MY5 and ARP1 lines treated with bortezomib, doxorubicin, and etoposide by flow cytometry. (C) Flow cytometry shows the activity of drug efflux pump between RARα2 OE and EV cells in OCI-MY5 and ARP1 lines. (D-E) Western blots show the expression of ABCC3 in RARα2-OE OCI-MY5 and ARP1 cells (D) as well as in RARα2-shRNA KMS11 and ARK cells (E).
To explain this observed drug resistance, the eFluxx-ID Multidrug resistance assay was performed and showed that overexpression of RARα2 increased drug resistance by increasing MRP expression in both ARP1 and OCI-MY5 cells compared with the control cells (Figure 5C). To further confirm this finding, we examined the expression of the multi-drug resistance genes ABCG2, ABCB1, ABCC1, and ABCC3 using western blots. As shown in Figure 5D, the expression of ABCC3 (MRP3) was significantly increased in ARP1 and OCI-MY5 overexpressing RARα2, whereas the expression of ABCG2, ABCB1, an ABCC1 remained unchanged (data not shown). In addition, the expression of ABCC3 decreased in the RARα2-silenced ARK and KMS11cells (Figure 5E).
We subsequently examined the expression of Bcl-2 family genes Bcl-2, Bcl-xl, Mcl-1, Bad, Bax, and Puma, which have been associated with drug resistance in CSCs. As shown in Figure 6A, the expression of Bcl-2 and Mcl-1 was significantly increased in ARP1 and OCI-MY5 overexpressing RARα2, whereas the expression of the other Bcl-2 family members mentioned above remained unchanged (data not shown). In addition, the expression of Bcl-2 and Mcl-1 decreased in the RARα2-silenced ARK and KMS11 cells (Figure 6B). RARα2-overexpressing cells ARP1 and OCI-MY5 were treated for 24 hours with the Cox-2 inhibitor (CAY10404, 10 μM), which downregulates the Wnt pathway in several tumors.34-36 Using the eFluxx-ID Multidrug resistance assay, flow cytometry showed that CAY10404 decreased the activity of the efflux pump induced by RARα2 (Figure 6C). Interestingly, an inhibitor of the MRP3 transporter (MK571, 20 nM) could not alter the RARα2-induced drug resistance by itself; however, the combination of MK571 and bortezomib (5 nM) significantly inhibited colony formation in the RARα2-overexpressing cells (Figure 6D).
Inhibition of RARα2 and its downstream signaling pathways decreases drug resistance induced by overexpression of RARα2. (A-B) Western blots show the expression of Bcl-2, Bcl-xl, and Mcl-1 in RARα2-OE OCI-MY5 and ARP1 cells (A) and in RARα2-shRNA ARK1 and KMS11 cells (B). (C) Flow cytometry shows the effect of COX-2 inhibitor, CAY10404, in cell membrane pump efflux induced by overexpression of RARα2 in OCI-MY5 and ARP1 cells. (D) The ABC transporter inhibitor MK-571 blocks RARα2-induced drug resistance in ARP1 cells. Clonogenic assay shows the effect of bortezomib or combination of MK571 and bortezomib on the clonogenic formation in RARα2-overexpressing ARP1 cells compared with the control (magnification ×40).
Inhibition of RARα2 and its downstream signaling pathways decreases drug resistance induced by overexpression of RARα2. (A-B) Western blots show the expression of Bcl-2, Bcl-xl, and Mcl-1 in RARα2-OE OCI-MY5 and ARP1 cells (A) and in RARα2-shRNA ARK1 and KMS11 cells (B). (C) Flow cytometry shows the effect of COX-2 inhibitor, CAY10404, in cell membrane pump efflux induced by overexpression of RARα2 in OCI-MY5 and ARP1 cells. (D) The ABC transporter inhibitor MK-571 blocks RARα2-induced drug resistance in ARP1 cells. Clonogenic assay shows the effect of bortezomib or combination of MK571 and bortezomib on the clonogenic formation in RARα2-overexpressing ARP1 cells compared with the control (magnification ×40).
Targeting MMSCs through inhibition of Wnt and Hh signaling in vitro in human MM cell lines and in the 5TGM1 myeloma mouse model
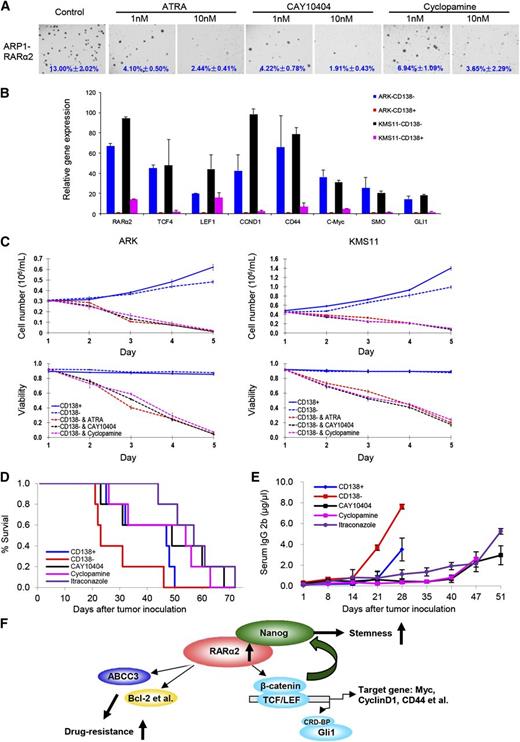
Because overexpression of RARα2 in RARα2 low-expressing cell lines activates both the Wnt and Hh signaling pathway activity, we subsequently evaluated the effects of ATRA, CAY10404, and the Hh inhibitor (cyclopamine) on ARP1 and OCI-MY5 cells overexpressing RARα2 by using the colony formation assay. ARP1 and OCI-MY5 cells overexpressing RARα2 were treated with or without ATRA (1 nM, 10 nM), CAY10404 (1 nM, 10 nM), or cyclopamine (1 nM, 10 nM) for 2 weeks. As shown in Figure 7A, all 3 drugs significantly inhibited the colony formation of RARα2-overexpressing MM cells even at low concentrations. To further demonstrate that RARα2 plays an important role in maintaining MMSC “stemness” through activating Wnt and Hh signaling, real-time PCR was performed and showed that the expression levels of RARα2 and levels of other down-stream targets of Wnt and Hh signaling, such as TCF4, LEF1, CCND1, CD44, c-Myc, SMO, and Gli1, were higher in CD138− compared with CD138+ MM cells of ARK and KMS11 (Figure 7B). We hypothesized that a strategy that targets not only RARα2, but also the Wnt and Hh pathways would be additive or synergistic in eliminating MMSC. We thus evaluated the effects of ATRA, CAY10404, and cyclopamine on the growth of CD138− and CD138+ MM cells. Purified CD138− and CD138+ cells from ARK and KMS11 were treated with ATRA, CAY10404, and cyclopamine for 5 days. As shown in Figure 7C, all 3 drugs induced significant growth inhibition and decreased viability of CD138− cells compared with untreated control cells.
Targeting Wnt and Hh signaling induces myeloma cell apoptosis in vitro and in the 5TGM1 myeloma mouse model. (A) Clonogenic assay shows the effect of ATRA, Wnt, and Hh inhibitors in RARα2 overexpressing OCI-MY5 and ARP1 cells (magnification ×40). (B) Real-time PCR shows that the expression of TCF4, LEF1, CD44, CCND1, c-Myc, SMO, and Gli1 in CD138− cells and CD138+ cells derived from ARK and KMS11 cell lines. (C) Cell growth and viability were evaluated in CD138− cells derived from KMS11 and ARK lines treated with ATRA, Wnt, and Hh inhibitors. Results are expressed as means ± SD of 3 independent experiments. (D) Kaplan-Meier curves show the 5TGM1 C57BL/KaLwRij mouse survival treated with CAY10404, cyclopamine, and itraconazole. (E) Tumor burden was examined idiotype lgG2b levels by ELISA in the 5TGM1 C57BL/KaLwRij mice treated with CAY10404, cyclopamine, and itraconazole. (F) The model of our working hypothesis shows potential mechanisms by which RARα2 maintains myeloma stem cell features.
Targeting Wnt and Hh signaling induces myeloma cell apoptosis in vitro and in the 5TGM1 myeloma mouse model. (A) Clonogenic assay shows the effect of ATRA, Wnt, and Hh inhibitors in RARα2 overexpressing OCI-MY5 and ARP1 cells (magnification ×40). (B) Real-time PCR shows that the expression of TCF4, LEF1, CD44, CCND1, c-Myc, SMO, and Gli1 in CD138− cells and CD138+ cells derived from ARK and KMS11 cell lines. (C) Cell growth and viability were evaluated in CD138− cells derived from KMS11 and ARK lines treated with ATRA, Wnt, and Hh inhibitors. Results are expressed as means ± SD of 3 independent experiments. (D) Kaplan-Meier curves show the 5TGM1 C57BL/KaLwRij mouse survival treated with CAY10404, cyclopamine, and itraconazole. (E) Tumor burden was examined idiotype lgG2b levels by ELISA in the 5TGM1 C57BL/KaLwRij mice treated with CAY10404, cyclopamine, and itraconazole. (F) The model of our working hypothesis shows potential mechanisms by which RARα2 maintains myeloma stem cell features.
To evaluate if the effects of Wnt and Hh inhibition also take place in the presence of a normal micro-environment, we examined their effects on MMSCs using the 5TGM1 myeloma mouse model in vivo. Wnt and Hh pathway genes are also increased in the CD138− fraction of 5TGM1 cells compared with the CD138+ cells (data not shown), indicating that the 5TGM1 model is a good substitute to test our overall hypothesis. A total of 0.5 × 106 of CD138+ or CD138− 5TGM1 cells was injected into C57BL/KaLwRij mice through the tail vein. One week after injection of the 5TGM1 murine myeloma cells, CAY10404 (20 mg/kg, I.P.) or cyclopamine (20 mg/kg, I.P.) was given to 5 mice in each group 3 times/week. The control groups, also containing 5 mice, received vehicle alone (DMSO/oil, volume/volume) at the same time points. The mice injected with MMSCs (CD138−) showed a significantly shorter survival than those injected with bulk MM cells (CD138+) (P < .05) (Figure 7D). Treatment with cyclopamine or CAY10404 significantly extended mouse survival (P < .05) (Figure 7D) and decreased tumor progression as measured by serum IgG2B levels (Figure 7E). In addition, we found that another Hh inhibitor, itraconazole (20 mg/kg, I.P.), was equally effective as cyclopamine in suppressing the growth of 5TGM1 cells in vivo.
Discussion
Most of our myeloma therapies exert their effects on the more differentiated and drug-sensitive myeloma cells. These therapies take advantages of key features of malignant cells, ie, they proliferate faster than normal cells, or they disrupt pathways and stromal interactions required for the survival of these more mature myeloma cells. In our opinion, there are currently no effective approaches available to deal with MMSCs (noncycling, highly drug-resistant MM cells), except for high-dose chemotherapy with drugs that are lethal to hematopoietic stem cells. The combination of ASCT with the newer drugs such as bortezomib, thalidomide, and lenalidomide has greatly improved the clinical outcome of MM patients, with median 5-year progression-free survivals of >50% and a median overall survival of more than 10 years.37 However, these agents still fail to completely eradicate the disease in many patients, most likely because of the persistence of a MMSC compartment, which survives myelo-ablative therapy. Therefore, a specific posttransplant therapy is needed to directly hit these virtually noncycling MMSCs.
In this study, we have demonstrated that CD138− myeloma cells had high expression of RARα2, and showed that artificial overexpression of RARα2 induced the typical features of stem cells, such as increased SP cells, clonogenesic potential and drug-resistance, and expression of prototypical stem cell genes, and activation of signaling pathways typically observed in CSC. The opposite effect was seen when RARα2 was knocked-down. Also, RARα2 expression was significantly increased in CD138 selected myeloma cells at relapse compared with those at diagnosis.9 We performed quantitative real-time PCR analysis to examine RARα2 expression in MM cell lines and a peripheral blood sample from a MM patient and found higher levels of RARα2 in the CD138− fraction of MM cell lines and SP/κ+ fraction in primary sample compared with non-SP/κ+ cells. We had previously shown that newly diagnosed MM patients with high baseline expression of RARα2 in CD138 selected myeloma cells had an inferior outcome.9 We now found that RARα2 expression in CD138 selected myeloma cells was positively correlated with Oct4, Sox2, Nanog, Lin28A, TCF1, CCND1, and ABCC3 expression in 23 primary myeloma samples (P < .05), explaining the poor prognosis of these patients. These observations strongly suggest that RARα2 may play a crucial role in maintaining MM stem cell features. Moreover, we observed that overexpression of RARα2 induced drug resistance by increasing levels of ALDH. We found that RARα2 interacts with Nanog, which is essential for embryonic stem cell and iPS cell pluripotency and maintaining an undifferentiated stem cell state. Western-blot assays showed that RARα2 upregulated expression of the drug-resistance genes ABCC3, Bcl-2, and Mcl-1. We further demonstrated that RARα2 overexpression increased Wnt activity, as evidenced by a higher level of nuclear β-catenin using western blots. Studies by Matsui20 reported that Hh signaling maintains MMSCs, while Noubissi et al14,38 showed that Wnt signaling stimulated the Hh pathway by stabilizing Gli1 mRNA. Therefore, we also probed the Hh genes in MMSCs and found that Gli1 and SMO showed higher levels of expression in RARα2 overexpressing cells. The ABC transporter members are direct targets of β-catenin.39 Thus, increased levels of nuclear β-catenin were associated with increased expression of the ABCC3 gene. Moreover, Nanog colocalizes with endogenous nuclear β-catenin in colorectal cancer cells and defines colon CSCs.40 It is known that β-catenin inhibits Tcf3-mediated repression of Nanog and activates targets together with Tcf1 to collectively facilitate embryonic stem cell self-renewal.41 Additionally, β-catenin interacts with and stabilizes Oct4 in a TCF-independent manner as an alternative strategy to enhance the pluripotent stem cell state.42 We propose that RARα2, in collaboration with stabilized β-catenin, can activate the core pluripotency factor Nanog. Our analyses of published chromatin occupancy experiments43 demonstrate that these proteins, together with accessory pluripotency factors such as Myc and Klf4, bind the promoters and regulatory elements of target genes such as CCND1 and CD44, thereby enabling a mechanism for propagating the MMSC state.
We used ATRA, a physiologically active derivative of vitamin A, to inhibit the overexpressed RARα2 receptor, and CAY10404, one of the most selective inhibitors of Cox-2, to block the Wnt pathway in MMSCs. Cyclopamine is a well-known SMO antagonist and prevents the accumulation of SMO in the Hh pathway.20,44 We used CAY10404 and cyclopamine to target both the Wnt and Hh pathways and evaluated the efficacy of MMSC inhibition in vitro and in vivo. We also discovered that itraconazole, another Hh inhibitor, showed a clear effect on mouse survival similar to that of cyclopamine.
In conclusion, this study demonstrates that RARα2 plays a crucial role in MMSCs and activates Wnt and Hh pathways, possibly by regulating the transcription of Nanog and other stem cell genes, thereby maintaining the stem cell state of these cells (Figure 7F).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Justin Fishbaugh, Heath Vignes, and George Rasmussen for assistance with the use of flow cytometer and cell sorting and to Dr Thai Cao (University of Utah) and Garry Hauser (DNA Facility) for RT-PCR support.
This work was supported by National Cancer Institute grants R01CA115399 (to G.T.), R01CA152105 (to F.Z.), and R21CA143887 (to F.Z.); the MMRF Senior (to F.Z., 2008 and 2010); the Leukemia Lymphoma Society TRP (to F.Z., 2010 and 2011); institutional start-up funds from the Department of Internal Medicine, Carver School of Medicine, University of Iowa (to F.Z. and G.T.); and the National Natural Science Foundation of China, China (no. 81228016 to F.Z. and J.S.).
Authorship
Contribution: F.Z., G. Tricot, and Y.Y. designed the research; F.Z., G. Tricot, and Y.Y. organized, analyzed, and interpreted the data; Y.Y., J.S., H.X., J.X., H.W., W.Z., Y.Z., S.D., and Z.G. performed the experiments; F.Z., G. Tricot, and Y.Y. drafted the manuscript; G. Tricot contributed to clinical samples; and G. Tolomelli and D.L. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fenghuang Zhan, Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City, IA 52242; e-mail: fenghuang-zhan@uiowa.edu; or Guido Tricot, Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City, IA 52242; e-mail: guido-tricot@uiowa.edu.
References
Author notes
Y-Y. and S-J. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal