Key Points
Anti-KEL alloantibodies generated after exposure to paternally derived RBC antigens during pregnancy result in fetal anemia.
This is the first animal model of pregnancy associated HDFN, with transfusion and pregnancy resulting in boostable anti-KEL alloantibodies.
Abstract
Exposure to nonself red blood cell (RBC) antigens, either from transfusion or pregnancy, may result in alloimmunization and incompatible RBC clearance. First described as a pregnancy complication 80 years ago, hemolytic disease of the fetus and newborn (HDFN) is caused by alloimmunization to paternally derived RBC antigens. Despite the morbidity/mortality of HDFN, women at risk for RBC alloimmunization have few therapeutic options. Given that alloantibodies to antigens in the KEL family are among the most clinically significant, we developed a murine model with RBC-specific expression of the human KEL antigen to evaluate the impact of maternal/fetal KEL incompatibility. After exposure to fetal KEL RBCs during successive pregnancies with KEL-positive males, 21 of 21 wild-type female mice developed anti-KEL alloantibodies; intrauterine fetal anemia and/or demise occurred in a subset of KEL-positive pups born to wild type, but not agammaglobulinemic mothers. Similar to previous observations in humans, pregnancy-associated alloantibodies were detrimental in a transfusion setting, and transfusion-associated alloantibodies were detrimental in a pregnancy setting. This is the first pregnancy-associated HDFN model described to date, which will serve as a platform to develop targeted therapies to prevent and/or mitigate the dangers of RBC alloantibodies to fetuses and newborns.
Introduction
Blood groups A, B, and O are well known to clinicians across all specialties. However, there are hundreds of other less well-known blood group antigens on red blood cells (RBCs) and other hematopoietic cells.1,2 These antigens are capable of stimulating alloantibody formation in individuals whose immune system recognizes them as foreign, with subsequent antigen/antibody interactions potentially causing harm in settings including transfusion, pregnancy, and transplantation. Alloantibodies to such antigens on RBCs, including those in the Rh, KEL, Kidd, and Fy families, may lead to hemolytic transfusion reactions or hemolytic disease of the newborn (HDFN).
HDFN was first described in the 1930s, and was realized to be an antibody-mediated process a decade later.3 Since that time, more than 50 antigens have been associated with HDFN, which affects more than 6 of 1000 live births.4,5 In HDFN, IgG alloantibodies against blood group antigens cross the placenta and bind to RBCs in the fetal circulation, potentially resulting in hemolysis, reticulocytopenia, and fetal death in severe cases. Some women enter pregnancy with pre-existing RBC alloantibodies from transfusion, whereas other women become alloimmunized to foreign paternally derived RBC antigens present on the RBCs of the fetus during gestation/delivery. RBC phenotypic differences between partners are not routinely taken into consideration prior to conception, and thus RBC alloimmunization and HDFN are potential risks in nearly all pregnancies. Surviving children may require simple or exchange RBC transfusion, intravenous immunoglobulin, and/or phototherapy; severely affected children may be affected by developmental delay and cerebral palsy.6,7
With the exception of polyclonal anti-D (RhoGam), there are no known therapies to prevent RBC alloimmunization or to mitigate the dangers of existing RBC alloantibodies. With the introduction of anti-D, Rh(D) pregnancy associated alloimmunization has decreased by 95%.8 In fact, anti-D is one of the most successful immunotherapies in use today. However, its mechanism of action remains ill-defined. Furthermore, no monoclonal anti-D preparation has been deemed safe and effective enough to be licensed by the Food and Drug Administration for use in a pregnancy setting.9,10 Limitations in the understanding of the mechanism of action of anti-D, as well as in the development of therapeutic options to prevent pregnancy associated RBC alloimmunization, is due in part to a lack of in vivo experimental models. The generation of transgenic animals with RBC specific expression of the human Rh(D) antigen has remained elusive, due in part to the genetic complexities of Rh(D).
The “Kell factor” was initially described half a century ago, after hydropic fetal complications11 and fatal transfusion reactions.12 It is now appreciated that the Kell factor is actually a family of antigens, with Kell being a glycoprotein with endopeptidase activity.2 Multiple epitopes on the Kell protein have been defined as clinically significant antigens, including KEL1/KEL2, Jsa/b, and Kpa/b.13 Approximately 91% of whites and 98% of African Americans lack the KEL1 antigen on their RBCs, thus putting them at risk of alloimmunization with exposure to antigen-positive RBCs, whether it is through transfusion or pregnancy. Today, KEL alloantibodies are a leading cause of antibody-mediated transfusion and pregnancy-associated morbidity/mortality.5,14-18
To our knowledge, no animal model to date has been generated in which pregnancy-associated RBC alloantibodies lead to adverse fetal outcomes. Limited knowledge of the RBC antigen systems of animals, in combination with lack of clinical significance of alloantibodies against these antigens, has contributed to the lack of model development. We have recently described a mouse model in which transgenic animals have RBC specific expression of the clinically significant human KEL glycoprotein antigen.19 Herein, we demonstrate that paternally derived KEL antigens on fetal RBCs lead to maternal anti-KEL RBC alloimmunization through pregnancy/delivery, with subsequent pregnancies and transfusions being adversely affected by these boostable anti-KEL glycoprotein alloantibodies. This model thus lays the groundwork to investigate pregnancy-associated alloantibody formation and subsequent RBC clearance mechanisms, as well as targeted therapeutic strategies to prevent or mitigate the dangers of pregnancy-associated alloantibodies.
Methods
Mice
Mice transgenic for the KEL human RBC antigen (specifically the KEL2 or Cellano antigen with mid-levels of expression near 1300 copies/RBC, previously described as “KEL2B”) were generated as previously described,19 using a B-globin promoter. C57BL/6 mice were purchased from the National Cancer Institute (Fredricksburg, MD); MuMT, ubiquitin C green fluorescence protein (uGFP), and triple congenic IgHa (IgHa × Thy1.1 × GPI) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). For breeding experiments, C57BL/6 or MuMT females at 2 to 3 months of age were mated with heterozygous KEL or homozygous uGFP males; all protocols were approved by the Emory University Institutional Animal Care and Use Committee.
Blood collection, labeling, and transfusion
Donor blood was collected in the anticoagulant preservative solution ACD (BD, Fisher Scientific). After being washed twice with phosphate-buffered saline, KEL RBCs were labeled with the lipophilic dye chloromethylbenzamido 1,1’-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) control C57BL/6 RBCs were labeled with 3,3′-dihexadecyloxacarbocyanine perchlorate (DiO), as previously described20-23 (Molecular Probes, Eugene, OR). C57BL/6 recipients were transfused via lateral tail vein with a mixture of 50 to 75 μL of KEL and C57BL/6 RBCs, diluted with saline to a total volume of 300 μL. Post-transfusion RBC recovery was determined by flow cytometry at multiple time points post-transfusion. A subset of recipients were pretreated with 100 µg of poly (I:C) (Amersham, Piscataway, NY) 4 hours prior to transfusion.
Hematologic evaluation, flow cytometry, and antibody generation/detection
Wright-Giemsa stained smears were evaluated using an Olympus BX41 microscope; cells were quantitated using APC Calibrite beads (BD Biosciences) and stained with thiazol orange. Serum bilirubin was evaluated by QuantiChrom Assay (BioAssay Systems, Hayward, CA). RBC bound anti-KEL was detected on transfused DiI-labeled RBCs using an IgG secondary reagent conjugated to allophycocyanin (APC). To detect serum anti-KEL, the serum was titrated and cross matched with KEL or control C57BL/6 RBCs, and APC-conjugated goat anti-mouse IgG (BD Biosciences) or horseradish peroxidase-conjugated goat anti-mouse IgG subtypes (Bethyl Laboratories, Montgomery, TX) plus anti-HRP Cy5.5 (Jackson Immunoresearch Laboratories, West Grove, PA) were used. An adjusted mean fluorescence intensity was determined by subtracting the background binding signal with control RBCs from that of target RBCs.
For experiments involving fetal livers, APC-conjugated CD4, CD3, B220, CD11b, CD11c, and CD41 were used to exclude non-RBC precursors; fluorescein isothiocyanate -conjugated TER119 and phycoerythrin-conjugated CD71 were used to identify fetal liver erythroid precursor populations24,25 ; polyclonal anti-KEL generated in IgHa recipients, biotin-conjugated mouse anti-mouse IgG1a (BD Biosciences), PerCP-Cy5.5-conjugated Strepavidin (Ebioscience, San Diego, CA), and anti-mouse immunoglobulins (Igs) (BD Biosciences) were also used. Fluorescein isothiocyanate-conjugated TER119 and APC-conjugated CD44 (BD Biosciences) were used to stain bone marrow, with CD45 and CD11 exclusion gates. All results were acquired on a BD FacsComp flow cytometer, and analyzed using FloJo Software (Treestar, Ashland, OR).
Tissue antigen detection by reverse transcription (transcriptase)-polymerase chain reaction
KEL animals were fully myeloablated with split doses of total body irradiation totaling 900 cGY, and transplanted with 5 × 106 splenocytes from C57BL/6 animals. After ensuring full RBC engraftment, organs were harvested, placed into RNAlater Stabilization Reagent (Qiagen, Valencia, CA) and homogenized; RNA was isolated by phenol-chloroform extraction using TRIzol Reagent (Invitrogen, Carlsbad, CA). An RNeasy Mini Kit (Qiagen) was used to digest contaminating DNA and to isolate bone marrow RNA. For polymerase chain reaction (PCR), complementary DNA was synthesized using AccuScript Reverse Transcriptase (Stratagene, Santa Clara, CA) and amplified using PCR Master Mix (Promega, Madison, WI). Primers specific for Kell (5′-GGGGGATCCGCCACCATGGAAGGTGGGGACCAAAGTG and 3′-TTGGAACAGAAGCAGAAAGGAA) and control primers specific for mouse G3PDH (5′-ACCACAGTCCATGCCATCAC and 3′-TCCACCACCCTGTTGCTGTA) were used for amplification.
MRI imaging
All magnetic resonance imaging (MRI) data were acquired at 9.4 T on a BioSpec 94/20 spectrometer (Bruker; Billerica, MA) using a radio frequency volume coil with 72 mm inner diameter. After isofluorane anesthesia, T2-weighted images were obtained using a fast spin-echo sequence with the following parameters: repetition time/echo time = 3800/84 ms, field of view = 51.2 × 36 mm2, matrix = 256 × 180, resolution = 200 × 200 µm2 in plane, slice thickness = 0.8 mm with 0.2 mm inter slice gap, average = 12, rapid acquisition with relaxation enhancement factor = 8. MRIs were generated using the Paravison 5.1 software package (Bruker BioSpec; Billerica, MA).
Statistical analysis
Statistical analysis and graphing were performed using Graph Pad Prism software (San Diego, CA). Statistically significant differences between 2 groups were compared using an unpaired t test, and differences between 3 or more groups were calculated by a one-way analysis of variance with a Tukey post test. A Fisher’s exact test used to evaluate differences between proportions. In all instances, statistical significance was defined as P < .05.
Results
Smaller successive litters with fewer KEL-positive pups are born to wild-type females mated with KEL males
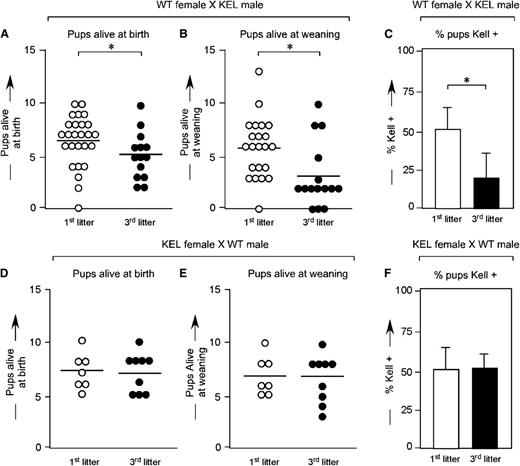
Wild-type C57BL/6 females were bred with KEL heterozygous males, and pregnancy outcomes were documented. In a total of 59 pregnancies, the mean number of live births was 6.3 (first litters), 5.3 (second litters), and 4.2 (third litters) (Figure 1A). The number of pups alive at weaning also declined with successive pregnancies (Figure 1B), as did the percentage of KEL-positive pups alive at weaning (Figure 1C, mean 53.1% in first litters, compared with 19.3% in third litters). The observed decrease in litter size was not an artifact of advanced maternal age, as 16 control pregnancies between KEL heterozygous females and wildtype C57BL/6 males showed no statistically significant declines in litter sizes at birth (Figure 1D) or weaning (Figure 1E) over time. Furthermore, there were no statistically significant differences in percentages of KEL-positive pups with successive pregnancies in these control animals (Figure 1F).
Smaller successive litters with fewer KEL-positive pups are born to wild-type females mated with KEL males. (A) Number of pups alive at birth, (B) number of pups alive at weaning, and (C) percentage of KEL-positive pups in first and third litters of wild-type females mated with KEL males. (D) Number of pups alive at birth, (E) number of pups alive at weaning, and (F) percentage of KEL-positive pups in first and third litters of control KEL females mated with wild-type males. (A-C) Data are a compilation of 40 total pregnancies; (D-F) data are a compilation of 16 total pregnancies. *P < .05.
Smaller successive litters with fewer KEL-positive pups are born to wild-type females mated with KEL males. (A) Number of pups alive at birth, (B) number of pups alive at weaning, and (C) percentage of KEL-positive pups in first and third litters of wild-type females mated with KEL males. (D) Number of pups alive at birth, (E) number of pups alive at weaning, and (F) percentage of KEL-positive pups in first and third litters of control KEL females mated with wild-type males. (A-C) Data are a compilation of 40 total pregnancies; (D-F) data are a compilation of 16 total pregnancies. *P < .05.
All pale/hydropic pups are KEL-positive by PCR
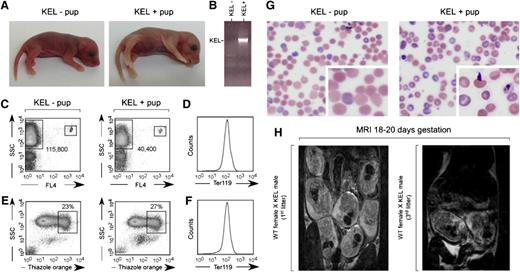
Approximately 10% of pups born in third and beyond litters to wild-type females bred with KEL males were stillborn, pale, and/or hydropic appearing (Figure 2A depicts littermates born in a third litter). All stillborn pups were screened for RBC expression of the human KEL antigen by PCR (Figure 2B). Documentation of the degree of anemia in stillborn pups or those who died within hours of birth was inherently difficult; however, Figure 2C documents anemia in the pale pup shown using Trucount beads (small boxes) and counting TER 119-positive events (large boxes). This pale pup also had a subtle, nonstatistically significant increase in thiazol orange reticulocyte staining at birth (Figure 2E-F), with an increase in thiazol orange staining noted in this and other pale KEL-positive pups compared with KEL-negative pups within the first week of life and returning to baseline by weaning. There were no statistically significant differences in serum bilirubin between KEL-positive and KEL-negative pups (mean 1.5 vs 1.3 mg/dL), although one severely anemic pup did have a level greater than the reference range. Blood smears of KEL-positive pups born to multiparous C57BL/6 females (Figure 2G) showed Howell-Jolly bodies, nuclear extrusion, polychromasia, and basophilic stippling.
A subset of pups born to multiparous wild-type females mated with KEL males are stillborn or pale. (A) Photograph of a representative pink KEL-negative and pale KEL-positive pup, hours after birth to a multiparous (third litter) wild-type female mated with a KEL male. (B) Representative KEL-specific PCR. (C) Flow cytometric analysis of the blood of the pups shown, with 1000 Trucount beads and RBCs gated. (D) TER119 positivity of gated RBCs. (E) Thiazole orange reticulocyte staining and (F) TER119 staining of the blood of the same pups. (G) Blood smear of representative KEL-negative and KEL-positive pups. (H) MRI of first (left) or third (right) pregnancies of representative wild-type females bred with KEL males.
A subset of pups born to multiparous wild-type females mated with KEL males are stillborn or pale. (A) Photograph of a representative pink KEL-negative and pale KEL-positive pup, hours after birth to a multiparous (third litter) wild-type female mated with a KEL male. (B) Representative KEL-specific PCR. (C) Flow cytometric analysis of the blood of the pups shown, with 1000 Trucount beads and RBCs gated. (D) TER119 positivity of gated RBCs. (E) Thiazole orange reticulocyte staining and (F) TER119 staining of the blood of the same pups. (G) Blood smear of representative KEL-negative and KEL-positive pups. (H) MRI of first (left) or third (right) pregnancies of representative wild-type females bred with KEL males.
Although 100% (10 of 10) of swollen/hydropic-appearing pups were KEL-positive by PCR, not all stillborn pups or those who died between birth and weaning were KEL-positive. Cesarean sections of 2 mothers at 18 to 20 days of gestation revealed ongoing reabsorption of deceased fetuses, with surrounding areas of fibrosis involving fetuses in close uterine proximity (data not shown), suggesting a “bystander” effect. To allow for longitudinal evaluation of pups during pregnancy, MRI was completed on pregnant mothers using a 9.4 T imaging machine. Although classic signs of “hydrops” could not be observed in the pups of the mothers imaged likely due in part to resolution considerations, fewer pups were observed in third compared with first litters (Figure 2H).
KEL expression is RBC-specific and present on early RBC precursors
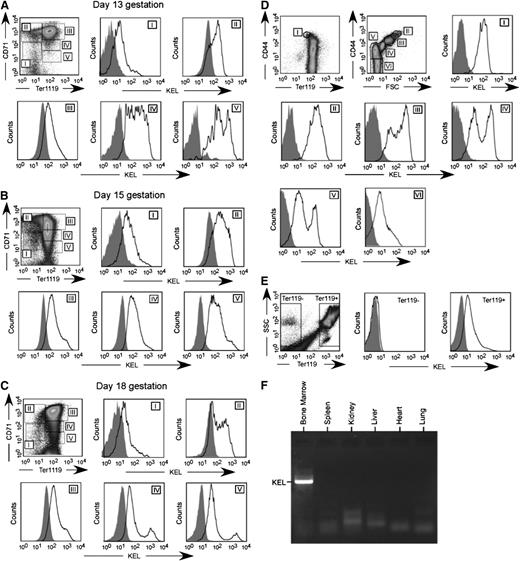
The KEL transgenic animals were made using a B-globin specific promoter,19 and thus expression of the KEL antigen would be predicted to be RBC specific. This B-globin promoter has been shown to be expressed on approximately day 1426 ; thus, KEL expression was investigated on RBC precursors from postconception (p.c.) day 13 through delivery. Fetal livers were stained with TER119, CD71, and anti-KEL, with exclusion gates. As early as p.c. day 13, KEL expression was detected on all stages of RBC precursors (Figure 3A-C). To further characterize KEL expression, bone marrow was stained with TER119, CD44, and anti-KEL with exclusion gates.27,28 All evaluated RBC precursors in the bone marrow also had detectable KEL expression (Figure 3D), although this KEL expression was not uniform throughout RBC precursor maturation. In addition to fetal liver and BM RBC precursors, all circulating TER119-positive but not TER119-negative cells had detectable KEL expression (Figure 3E).
KEL is expressed on all RBC precursors in the fetal liver and bone marrow of transgenic animals, in an RBC-specific fashion. (A-C) Fetal liver cells from KEL transgenic fetuses, with anti-KEL staining (solid) or secondary only staining (shaded) of RBC precursor populations (I = primitive erythroid progenitor cells, II = proerythroblasts and early basophilic erythroblasts, III = later basophilic erythroblasts, IV = chromatophilic and orthochromatophilic erythroblasts, and V = late orthrochromatophlic erythroblasts and reticulocytes). (D) Bone marrow from KEL transgenic animals, with anti-KEL staining (solid) or secondary only staining (shaded) of RBC populations (I = proerythroblasts, II = basophilic erythroblasts, III = polychromatic erythroblasts, IV = orthochromatic erythroblasts, V = reticulocytes, VI = mature RBCs). (E) Anti-KEL staining of peripheral blood cells. (F) Bone marrow and organs of a KEL-positive animal were evaluated for KEL expression by PCR. Results are from 2 independent experiments, with 1 to 2 mice per group.
KEL is expressed on all RBC precursors in the fetal liver and bone marrow of transgenic animals, in an RBC-specific fashion. (A-C) Fetal liver cells from KEL transgenic fetuses, with anti-KEL staining (solid) or secondary only staining (shaded) of RBC precursor populations (I = primitive erythroid progenitor cells, II = proerythroblasts and early basophilic erythroblasts, III = later basophilic erythroblasts, IV = chromatophilic and orthochromatophilic erythroblasts, and V = late orthrochromatophlic erythroblasts and reticulocytes). (D) Bone marrow from KEL transgenic animals, with anti-KEL staining (solid) or secondary only staining (shaded) of RBC populations (I = proerythroblasts, II = basophilic erythroblasts, III = polychromatic erythroblasts, IV = orthochromatic erythroblasts, V = reticulocytes, VI = mature RBCs). (E) Anti-KEL staining of peripheral blood cells. (F) Bone marrow and organs of a KEL-positive animal were evaluated for KEL expression by PCR. Results are from 2 independent experiments, with 1 to 2 mice per group.
To evaluate whether KEL was expressed in nonhematopoietic tissues,29 organs were harvested and evaluated for KEL by reverse transcription (transcriptase)-PCR. As predicted, KEL expression was observed in the bone marrow, but not in the spleen, kidney, liver, heart, and lung (Figure 3F). Studies were also undertaken in KEL transgenic animals that had been transplanted with C57BL/6 marrow under fully myeloablative conditions, to eliminate the possibility of KEL reticulocyte contamination. After engraftment and after confirming a lack of circulating KEL RBCs, organ reverse transcription (transcriptase)-PCR was completed. As expected, the KEL transgene, which is driven by the human B-globin promoter cassette,19,26 was RBC-specific with a lack of KEL expression on the organs or in the bone marrow of transplanted animals.
Anti-KEL is generated by wild-type females mated with KEL males
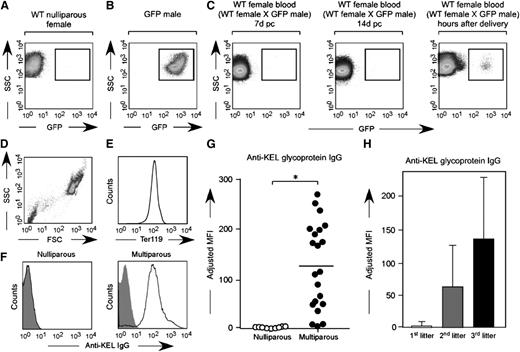
To test the hypothesis that the immune response hypothesized to occur in response to fetal/maternal RBC transfer is antibody-mediated, serum from wild-type C57BL/6 females mated with KEL males was evaluated for anti-KEL glycoprotein antibodies prior to and at weekly intervals during pregnancy and after delivery. Consistent with the finding that no pregnant female had detectable fetal RBCs in circulation until after delivery (Figure 4A-C), fewer than 5% (2 of 42) developed anti-KEL glycoprotein antibodies during their first pregnancies; this antibody was low titer and litter sizes were normal. However, essentially all mice had detectable anti-KEL glycoprotein antibodies after their third pregnancies (Figure 4D-G) (mean adjusted mean fluorescence intensity of 1.3 in nulliparous vs 124.7 in multiparous females). An increase in maternal antibody anti-KEL titer was observed with sequential pregnancies/deliveries (Figure 4H); no female with an anti-KEL IgG titer below 1:32 had any affected anemic pups. Taken together, these data suggest fetal/maternal RBC transfer of RBCs expressing a paternally derived antigen (KEL) foreign to the mother leads to maternal alloimmunization, with the largest detectable RBC transfer occurring near the time of delivery.
Fetal RBCs can be detected in the maternal circulation after delivery, with anti-KEL RBC antibodies detectable in the serum of wild-type females after multiple pregnancies with KEL-positive males. (A) Unstained RBCs from a nulliparous wild-type female. (B) Unstained RBCs from a homozygous uGFP male. (C) Unstained RBCs from a representative wild-type female bred with a homozygous uGFP male, p.c. day 7, p.c. day 14, and hours after delivery. (D-E) Serum from nulliparous or multiparous animals was cross matched with TER119-positive RBCs. (F) Representative flow cross match against wild-type (shaded) or KEL (solid) RBCs, from a nulliparous and a multiparous female, with anti-mouse IgG as a secondary reagent. (G) Anti-KEL IgG in the serum of nulliparous or multiparous (3 pregnancies) females. (H) Anti-KEL IgG in the serum of females after 1, 2, or 3 deliveries; 42 total pregnancies are shown. *P < .05.
Fetal RBCs can be detected in the maternal circulation after delivery, with anti-KEL RBC antibodies detectable in the serum of wild-type females after multiple pregnancies with KEL-positive males. (A) Unstained RBCs from a nulliparous wild-type female. (B) Unstained RBCs from a homozygous uGFP male. (C) Unstained RBCs from a representative wild-type female bred with a homozygous uGFP male, p.c. day 7, p.c. day 14, and hours after delivery. (D-E) Serum from nulliparous or multiparous animals was cross matched with TER119-positive RBCs. (F) Representative flow cross match against wild-type (shaded) or KEL (solid) RBCs, from a nulliparous and a multiparous female, with anti-mouse IgG as a secondary reagent. (G) Anti-KEL IgG in the serum of nulliparous or multiparous (3 pregnancies) females. (H) Anti-KEL IgG in the serum of females after 1, 2, or 3 deliveries; 42 total pregnancies are shown. *P < .05.
Anti-KEL glycoprotein IgG crosses the placenta and binds to the RBCs of KEL-positive pups
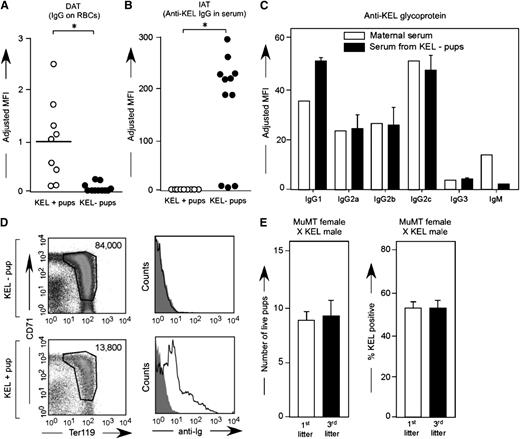
To determine whether anti-KEL crossed the placenta and bound to KEL RBCs in KEL-positive pups, peripheral blood samples were taken at weaning from pups born to alloimmunized wild-type females and were stained with anti-mouse IgG in a direct antiglobulin test or DAT (Figure 5A). The binding was KEL-specific, as the antibody did not bind to antigen-negative RBCs greater than levels of secondary antibody alone, and KEL-negative pups had largely negative direct antiglobulin tests. No anti-KEL was detected in the circulation via indirect antiglobulin test in KEL-positive pups (Figure 5B) presumably due to RBC saturation; consistent with this, KEL-negative pups had positive indirect antiglobulin tests. Additional studies were completed using flow-based cross match to evaluate the ability of maternal anti-KEL IgG subtypes to cross the placenta. Anti-KEL IgG1, IgG2a, IgG2b, IgG2c, and IgG3 were detected in alloimmunized multiparous mothers, with all IgG subtypes also being detected in the neonatal circulation at the time of birth (Figure 5C).
Maternal anti-KEL IgG crosses the placenta and binds to KEL RBCs of pups. (A) RBCs from KEL-positive and KEL-negative pups born to mothers immunized through pregnancy were evaluated for bound anti-KEL IgG by flow cytometric cross matching. (B) Serum from these same animals was evaluated for anti-KEL IgG in a direct antiglobulin test. Results are representative of 3 independent breeding experiments (n = 20 pups). *P < .05. (C) Anti-KEL subtypes in the serum of a representative alloimmunized mother compared with the serum of her KEL-negative pups. (D) Newborn liver cells from a representative KEL-negative and pale KEL-positive pup born to an alloimmunized mother, quantitated by Trucount beads (left column) or stained with anti-mouse Igs (right column). (E) Total number of pups and percentage of KEL-positive pups was evaluated in first and third litters of MuMT females bred with KEL males (n = 8 total pregnancies). P is not significant. No anti-KEL antibodies were detected in any MuMT female; error bars indicate standard deviation.
Maternal anti-KEL IgG crosses the placenta and binds to KEL RBCs of pups. (A) RBCs from KEL-positive and KEL-negative pups born to mothers immunized through pregnancy were evaluated for bound anti-KEL IgG by flow cytometric cross matching. (B) Serum from these same animals was evaluated for anti-KEL IgG in a direct antiglobulin test. Results are representative of 3 independent breeding experiments (n = 20 pups). *P < .05. (C) Anti-KEL subtypes in the serum of a representative alloimmunized mother compared with the serum of her KEL-negative pups. (D) Newborn liver cells from a representative KEL-negative and pale KEL-positive pup born to an alloimmunized mother, quantitated by Trucount beads (left column) or stained with anti-mouse Igs (right column). (E) Total number of pups and percentage of KEL-positive pups was evaluated in first and third litters of MuMT females bred with KEL males (n = 8 total pregnancies). P is not significant. No anti-KEL antibodies were detected in any MuMT female; error bars indicate standard deviation.
Decreased numbers of newborn liver RBC precursors are detected in KEL-positive pups born to alloimmunized mothers
To further investigate the in utero effect of maternal anti-KEL IgG, RBC precursors from newborn livers were evaluated on surviving newborn pups using Trucount beads. In 3 of 4 pregnancies, KEL-positive pups born to alloimmunized mothers had significantly decreased numbers of all stages of liver RBC precursors compared with their KEL-negative littermates (Figure 5D, left column). Furthermore, these liver RBC precursors were reactive by direct antiglobulin test (Figure 5D, right column). Although the exact mechanism of fetal anemia in this model remains under investigation, suppression of erythropoiesis during in utero development likely plays a contributory role.
MuMT females bred multiple times with KEL males have healthy pups
To further evaluate the role of maternally derived anti-KEL alloantibody in the observed fetal demise, MuMT females that lack mature B cells were bred with KEL males 3 times and litter statistics were evaluated. Consistent with their phenotype, no anti-KEL could be detected in the serum of these MuMT females after any pregnancy. Furthermore, the MuMT females had a similar number of pups in first (mean 8) compared with third litters (mean: 8.5) with approximately 50% KEL-positive pups (Figure 5E) in contrast to the decrease in litter size and percent KEL positivity observed over time in wild-type females bred with KEL males (Figure 1A). In combination, these data lend further support that the anti-KEL alloantibody generated by wild-type females bred with KEL males likely plays a central role in the observed fetal/neonatal demise.
Anti-KEL generated through pregnancy leads to clearance of transfused KEL RBCs and a proinflammatory serum cytokine storm
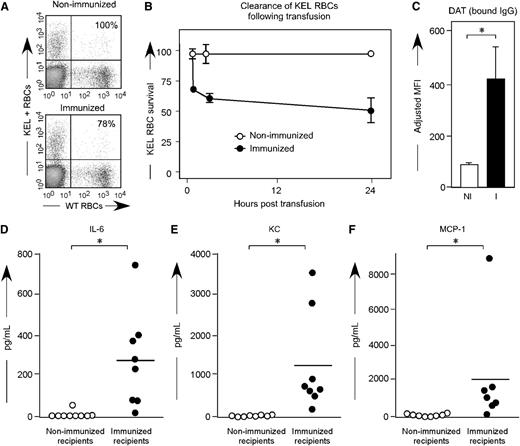
To investigate whether anti-KEL generated in pregnancy was capable of leading to clearance of KEL RBCs, a transfusion based approach was taken. For these experiments, KEL RBCs were labeled with the lipophilic dye Di, and control wild-type C57BL/6 RBCs were labeled with the lipophilic dye DiO. A mixture of KEL and control RBCs was transfused into C57BL/6 females who had previously been pregnant 3 or more times with KEL-positive pups and who had significant levels of anti-KEL glycoprotein IgG, but minimal levels of anti-KEL glycoprotein IgM; control recipients were nulliparous females. Clearance of the KEL-positive cells was determined by comparing the percentage of circulating KEL DiI-labeled RBCs to C57BL/6 DiO-labeled RBCs over time (Figure 6A). In 3 of 3 experiments (n = 21 animals total), rapid initial clearance of “incompatible” KEL RBCs was observed, followed by persistent circulation of a subset of KEL RBCs (Figure 6B, solid circles). In contrast, control nulliparous animals did not demonstrate preferential clearance of transfused DiI-labeled KEL RBCs compared with wild-type RBCs (Figure 6B, open circles). The clearance observed in alloimmunized females was presumably antibody-mediated, as transfused KEL RBCs had significant levels of anti-KEL IgG bound within 10 minutes post-transfusion (Figure 6C).
Transfused KEL RBCs are selectively cleared in alloimmunized animals, with recipient proinflammatory cytokine response. (A) KEL and wild-type RBCs were labeled with DiI and DiO, respectively, prior to transfusion into females alloimmunized through pregnancy or into control recipients. (B) Post-transfusion survival and recovery of KEL RBCs was determined by comparing a ratio of DiI KEL to DiO wild-type RBCs. (C) RBC bound IgG was evaluated by flow cytometry 10 minutes post-transfusion. (D-F) Serum cytokine responses in alloimmunized animals 90 to 120 minutes after a KEL RBC transfusion. Results are representative (A-C) or a compilation (D-F) of 3 experiments (n = 18 animals). *P < .05.
Transfused KEL RBCs are selectively cleared in alloimmunized animals, with recipient proinflammatory cytokine response. (A) KEL and wild-type RBCs were labeled with DiI and DiO, respectively, prior to transfusion into females alloimmunized through pregnancy or into control recipients. (B) Post-transfusion survival and recovery of KEL RBCs was determined by comparing a ratio of DiI KEL to DiO wild-type RBCs. (C) RBC bound IgG was evaluated by flow cytometry 10 minutes post-transfusion. (D-F) Serum cytokine responses in alloimmunized animals 90 to 120 minutes after a KEL RBC transfusion. Results are representative (A-C) or a compilation (D-F) of 3 experiments (n = 18 animals). *P < .05.
Within 2 hours of transfusion of “incompatible” KEL RBC transfusions, alloimmunized animals became transiently hunched and ill-appearing. In a compilation of 3 experiments (n = 9 alloimmunized compared with 9 controls), elevations of serum cytokines were observed with respect to serum levels of IL-6 (Figure 6D), keratinocyte-derived cytokine (Figure 6E), and monocyte chemoattractant protein-1 (Figure 6F). In addition, elevations were observed in serum levels of macrophage-inflammatory protein--1B (mean: 150 vs 19.7 pg/mL; P < .05) and in tumor necrosis factor-α (mean: 79.8 vs 53.5 pg/mL; P < .05); no significant differences were observed in serum levels of IFN-γ. However, it cannot be inferred that the mechanisms leading to clearance of transfused incompatible KEL RBCs are necessarily the same as that observed during the development of KEL-positive pups in the uterus of alloimmunized mothers.
Transfusion-associated KEL alloimmunization is boostable, and leads to adverse pregnancy outcomes
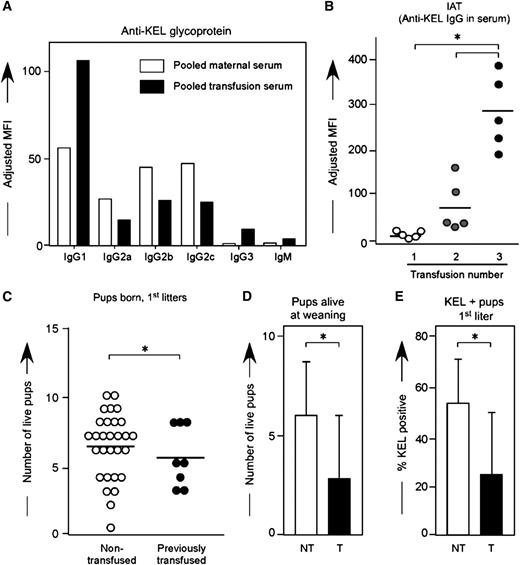
Multiple factors in humans prevent a direct juxtaposition between the dangers to fetuses of RBC alloantibodies generated through pregnancy compared with those generated through prior transfusion. However, either method of exposure and antibody acquisition can be detrimental to the human fetus.5 To investigate whether the method by which a female is exposed to an RBC antigen determines the clinical significance or subtype of the alloantibody in this reductionist murine system, wild-type C57BL/6 animals were transfused every 2 to 3 weeks with KEL RBCs in the presence of the double stranded RNA poly (I:C); anti-KEL responses were evaluated by flow cytometric cross match 2 weeks after each transfusion. The distribution of anti-KEL IgG subtypes was similar but not identical between animals immunized through transfusion compared with pregnancy (Figure 7A); recent studies in our laboratory have more fully characterized the time course of IgG and IgM antibody responses post-transfusion.30
KEL RBC transfusions induce boostable anti-KEL alloantibodies, which result in adverse pregnancy outcomes. (A) Control animals were transfused 3 times with KEL RBCs, with anti-KEL IgG subtypes and IgM measured in pooled serum and compared with pooled serum from 12 females alloimmunized through pregnancy. (B) Anti-KEL IgG measured in transfusion recipients 2 weeks after each transfusion. (C) Naïve or transfused females were bred to KEL males, with total pups born enumerated. (D) percentage of pups alive at weaning evaluated, and (E) percentage of KEL-positive pups determined. (B) Results are representative of 3 independent experiments. (C-E) Results are a compilation of 37 pregnancies. *P < .05.
KEL RBC transfusions induce boostable anti-KEL alloantibodies, which result in adverse pregnancy outcomes. (A) Control animals were transfused 3 times with KEL RBCs, with anti-KEL IgG subtypes and IgM measured in pooled serum and compared with pooled serum from 12 females alloimmunized through pregnancy. (B) Anti-KEL IgG measured in transfusion recipients 2 weeks after each transfusion. (C) Naïve or transfused females were bred to KEL males, with total pups born enumerated. (D) percentage of pups alive at weaning evaluated, and (E) percentage of KEL-positive pups determined. (B) Results are representative of 3 independent experiments. (C-E) Results are a compilation of 37 pregnancies. *P < .05.
In 3 of 3 experiments (n = 30 recipients total), 100% of recipients made detectable anti-KEL glycoprotein immunoglobulins after an initial transfusion, which increased further in response to subsequent transfusions (Figure 7B). This boostable response was not simply due to a continued increase in response after the initial transfusion, as antibody responses peaked between 14 and 21 days after the initial transfusion; furthermore, this boostable response was not dependent on poly (I:C) pretreatment, as animals transfused in the absence of poly (I:C) also had a boostable anti-KEL response.30
To determine whether transfusion induced anti-KEL alloantibodies were detrimental to KEL-positive fetuses, wild-type C57BL/6 females who had previously been transfused 3 times with KEL RBCs in the presence of poly (I:C) were bred with KEL-positive males, and pregnancy outcomes were compared with pregnancy outcomes in control females never previously transfused or pregnant. Females immunized through transfusion had smaller litters compared with nonimmunized females (Figure 7C) (5.5 vs 6.3 pups), fewer total pups alive at weaning per litter (Figure 7D) (2.6 vs 5.9 pups; P < .05), and fewer KEL-positive pups (Figure 7E) (22.6 vs 53.1%; P < .05). Hydropic-appearing pups, all documented to be KEL-positive by PCR, were noted in a minority of deliveries. Thus, similar to what has been described in humans, transfusion-induced anti-KEL alloimmunization is detrimental to KEL-positive fetuses, as is pregnancy-induced anti-KEL alloimmunization.
Discussion
Herein, we have described what to the best of our knowledge is the first animal model of pregnancy-induced RBC alloimmunization to a paternally derived, clinically significant RBC antigen (KEL). In this model, the human KEL antigen is present on transgenic murine RBCs, and fetal/maternal blood transfer during pregnancy and delivery leads to anti-KEL glycoprotein alloantibodies. These anti-KEL alloantibodies cross the placenta and bind to KEL expressing RBCs and RBC precursors in the fetal liver and the peripheral circulation of fetuses and neonates. The end result of anti-KEL RBC binding includes intrauterine fetal demise or hydropic stillborn pups; a subset of KEL-positive pups born to mothers with low titers of anti-KEL survive to weaning. Control MuMT females, incapable of making anti-KEL glycoprotein antibodies, have unaffected pups when bred with KEL transgenic males, thus lending further support to the antibody-mediated nature of the observed fetal demise.
KEL is known to be one of the most clinically significant RBC antigens in humans, both from a transfusion and HDFN perspective. In humans, anti-KEL1 antibodies lead to clearance of KEL-positive RBCs and fatal hemolytic transfusion reactions2 ; in our murine model, anti-KEL antibodies also lead to clearance of transfused KEL-positive RBCs, with a proinflammatory cytokine storm. In humans, KEL is expressed on early RBC precursors with anti-KEL1 antibodies leading to in utero suppression of erythroid progenitor cells and potentially severe HDFN.17,31 In our transgenic murine model, human KEL is expressed on murine RBC precursors as early as p.c. day 13, with anti-KEL antibodies also leading to in utero suppression of KEL erythroid progenitors. Of note, murine KEL is also expressed on RBCs during early during fetal development,27 although the antibodies generated in wild-type C57BL/6 females after exposure to fetal murine RBCs expressing the human KEL antigen are reactive with the human but not the murine KEL antigen. Consistent with suppression of erythropoiesis as a contributing factor to fetal anemia and HDFN, KEL-positive pups born to alloimmunized mothers lacked significant reticulocytosis or hyperbilirubinemia at the time of birth. However, these pups developed a compensatory reticulocytosis soon after birth, which is similar to that observed in human infants born to alloimmunized mothers.
Assay limitations prevented visualization of the fate of circulating KEL fetal cells in our model when the the pups were in utero, although transfusion of “incompatible” KEL RBCs allowed for an alternate method of visualization. Fifty percent of KEL RBCs transfused into females alloimmunized through pregnancy bound antibody, cleared rapidly, and resulted in a proinflammatory serum cytokine storm, with the other 50% remaining in circulation. This lack of complete KEL RBC clearance was not due to insufficient antibody, as similar observations were made in experiments in which large amounts of polyclonal anti-KEL glycoprotein immunoglobulins were passively transferred to naïve animals prior to KEL RBC transfusion (data not shown). Similar passive transfer experiments have revealed an antibody threshold below which no RBC clearance occurs, likely explaining the survival of a subset of KEL-positive pups born to alloimmunized mothers. Determination of the mechanism of action of incompatible RBC clearance (and clearance resistance) in both transfusion and pregnancy settings is ongoing.
Although our described model is the first animal model of clinically significant RBC alloantibodies generated through pregnancy, rabbit models dating back to the 1950s have demonstrated the dangers of maternal RBC alloantibodies.3,32,33 A model developed in the 1990s documented fetal hydrops, anemia, and reticulocytosis in response to maternal RBC antibody bound to fetal RBCs.34,35 This rabbit model significantly increased knowledge about placental antibody transfer and effect on RBCs, although maternal alloantibody could be stimulated only through repeat immunizations with complete and incomplete Freund’s adjuvant mixed with the antigen. Therefore, the process of antibody formation through pregnancy to a clinically significant human RBC antigen has never been studied in an animal model.
Polyclonal anti-D is perhaps one of the most successful targeted immumodulatory therapies in existence,8,36 yet its mechanism of action remains elusive.8,37,38 Many monoclonal anti-D antibodies have been generated and characterized in depth,10 although none to date have proven as efficacious as polyclonal anti-D at preventing Rh(D) alloimmunization. The lack of a mechanistic understanding of polyclonal anti-D is due, at least in part, to a lack of a reductionist model system in which to test the hypotheses. In addition to the polyclonal anti-KEL glycoprotein preparation that can be generated through transfusion or pregnancy, a number of monoclonal preparations targeting epitopes on the human KEL glycoprotein are already in existence. Thus, the newly described KEL system will offer the opportunity to test the abilities and mechanisms of such antibodies in preventing alloimmunization, with the realization that these findings may be applicable to some but not all human RBC antigens.
Limitations to the current studies must always be considered. This KEL model more closely resembles the human scenario of Rh(D) than the human KEL1/KEL2 scenario in the sense that the paternally derived foreign antigen (human KEL glycoprotein) is absent from the RBCs of the mother. However, the HDFN and antibody characteristics in this model are more similar to those described in the human KEL system. Other considerations include difficulties in fully visualizing and evaluating fetal KEL-positive RBCs in the maternal circulation, as well as differences in murine and human fetal/maternal interfaces.39,40 Although the fate of transfused RBCs into alloimmunized females was evaluated as a surrogate, differences in circulatory half-life and clearance patterns between fetal and adult KEL RBCs cannot be ruled out. Furthermore, differences in antibody-mediated clearance patterns based on RBC dose and KEL-antigen density on RBCs must also be considered, although circulating RBCs in adult transgenic mice all appear to have similar levels of KEL-antigen expression. Another consideration is that the males used in this study were KEL heterozygous, allowing for comparison studies between KEL-positive and KEL-negative littermates. It cannot be ruled out, however, that alloimmune responses may be different with KEL homozygous RBCs. Finally, ongoing studies involving intrauterine fetal imaging and autopsy may shed further light on the timing of intrauterine fetal death and fetal pathology, including the possibility that fetal resorption, in addition to fetal/maternal hemorrhage, may be contributing to maternal alloimmunization.
In summary, we have described the first animal model of RBC alloantibodies generated through pregnancy against the human KEL RBC antigen. The antibody response, which can be generated not only through pregnancy/delivery but also through transfusion, is boostable and clinically significant in both settings. This model thus provides a platform to study not only the induction, placental transfer, and consequences of RBC alloantibodies, but also potential therapeutics and their mechanism(s) of action. Long-term translational goals of this work include decreasing the dangers of RBC alloantibodies to developing fetuses and neonates through targeted maternal immunomodulatory therapies.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL092959 and HL115696), and a grant from the Emory-Children’s Pediatric Research Center (J.E.H.). The KEL animals were created through support from Immucor to JCZ.
Authorship
Contribution: S.R.S., K.L.H., N.H.S., K.E.H., J.C.P., A.M.B., K.R.G.-P., C.M.A., S.T.B., and J.E.H. performed experiments. J.C.Z. and N.H.S. generated the KEL animals, and G.R.H. contributed valuable reagents and intellectual input. S.R.S. and J.E.H. wrote the initial and final drafts of the manuscript and analyzed/interpreted the data; all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeanne E. Hendrickson, 101 Woodruff Circle, 7105B Woodruff Memorial Building, Atlanta, GA 30322; jeanne.hendrickson@emory.edu.