Key Points
Paneth cell numbers in the duodenum at onset of GVHD correlate with outcomes.
Paneth cells are easy to identify and quantify with light microscopy and may supplement histopathological grading of GI GVHD.
Abstract
Acute graft-versus-host disease (GVHD) of the gastrointestinal (GI) tract is an often lethal complication of allogeneic hematopoietic cell transplant. Clinical severity correlates with outcomes, but histopathologic grading is primarily used to confirm the clinical diagnosis. One barrier to using histopathologic grading to predict clinical outcomes is inter-grader variability among transplant centers. Recent experimental models have shown that the loss of Paneth cells, which are located in the small intestine and help regulate the GI microbiome by secreting antimicrobial peptides, correlates with clinical GVHD severity. Because Paneth cells are easy to identify and quantify by light microscopy, we evaluated the mean number of Paneth cells per high-powered field (hpf) in 116 duodenal biopsies obtained at diagnosis of GI GVHD at 2 different centers with their clinical outcomes. Paneth cell counts were reproducible between centers (r2 = 0.81; P < .0001). Lower numbers of Paneth cells at diagnosis correlated with clinically more severe GI GVHD (P < .0001) and less likelihood of response to GVHD treatment (P < .0001). A threshold of 4 Paneth cells per hpf stratified patients according to nonrelapse mortality (28% vs 56%; P = .004). We conclude that the enumeration of duodenal Paneth cells is a readily available index of disease severity that provides important information regarding GVHD prognosis.
Introduction
Acute graft-versus-host disease (GVHD) is a major cause of morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT).1 Although GVHD of any target organ can be resistant to treatment, gastrointestinal (GI) tract involvement is generally considered to the most serious acute GVHD manifestation, given its association with high rates of mortality.2,3 One obstacle to improving GI GVHD outcomes is that patients with similar presentations can have very different responses to treatment, a finding that highlights the limitations of the GI GVHD staging systems in current use that distinguish survival outcomes according to maximal GVHD grade.4,5 In fact, the clinical severity of GVHD at diagnosis does not reliably predict either the likelihood of response to treatment or mortality.3,6 The histological severity of GI GVHD is measured on the Lerner scale, which assigns a score of 1 to 4 based on a semiquantitative assessment of damage.7 Grades in this scoring system are defined as: 1) isolated apoptotic cells; 2) single crypt dropout; 3) adjacent crypt dropout; and 4) mucosal denudation. Considerable differences exist, however, for the minimal criteria required, such as the number of apoptotic bodies per slide or the number of serial sections examined. Many centers no longer even report the GI histopathological grade, because there is poor correlation between pathologic grade and clinical severity at the time of the biopsy and 2 different but simultaneous severity grades for the same patient can serve as a major source of confusion.8 Thus, there is a significant need for a histologic GI GVHD staging system that better correlates with both clinical stage and response to treatment and long-term outcomes.
Recently, we reported that plasma concentrations of regenerating islet derived protein-3α (REG3α) correlate with GI GVHD severity and help discriminate between lower GI GVHD and other causes of post-HCT diarrhea.2 REG3α is a secreted antimicrobial peptide that regulates intestinal gram-positive bacteria9-11 and is secreted by Paneth cells, which are primarily located in the small intestine. This previously unrecognized connection between Paneth cell function and intestinal GI GVHD has since been documented in an experimental GI GVHD model.12 Paneth cells, which are readily identified by their location and histochemical staining with lysozyme, were unaffected in mice without GVHD receiving T-cell–depleted bone marrow transplants but were nearly absent in mice with GVHD, thus confirming the inverse relationship between Paneth cell numbers and GI GVHD. Given the newly discovered relationship between Paneth cells and GI GVHD as well as the ease and reproducibility of Paneth cell quantification, we investigated the correlation of Paneth cell numbers with clinical GI GVHD and its outcomes. We hypothesized that Paneth cell numbers in the duodenum would inversely correlate with clinical GI GVHD severity and would be lowest in patients who did not respond to GVHD treatment and ultimately died of GVHD. We therefore retrospectively correlated Paneth cell numbers in duodenal biopsies with clinical severity, response to treatment, and survival at 2 BMT centers.
Materials and methods
Patients
Patients were studied with the approval of the Institutional Review Boards at both the University of Michigan and Regensburg University and all patients gave written informed consent in accordance with the Declaration of Helsinki. At the University of Michigan, patients who consented to a prospective observational trial of allogeneic HCT complications underwent allogeneic HCT from 2000 to 2009, developed GI GVHD or diarrhea suspicious for GVHD, and had a duodenal biopsy at the time of onset of symptoms available for analysis were included in the study population. At the University of Regensburg, study subjects for analysis were identified from a cohort of patients who underwent allogeneic HCT from October 2008 to January 2012 and consented to a prospective study assessing the prognostic significance of screening GI biopsies obtained from asymptomatic patients between day 20 and 40 after SCT, at the time of onset of symptoms, or as part of a comprehensive GVHD diagnostic work-up. Only patients with duodenal biopsies available for Paneth cell enumeration were included in this study. In patients with duodenal biopsies obtained on more than one date, only the first biopsy was included in the analysis. When available, colonic biopsies (n = 87) obtained at the same time as the duodenal biopsies were graded for GVHD severity.
GVHD outcome definitions
GI GVHD was staged according to the modified Keystone criteria.4 GI GVHD responses were assessed 4 weeks after treatment began. The response definitions are as follows: complete response was defined as GI GVHD stage zero, partial response as an improvement in GI GVHD stage but higher than zero, and no response as stable or worsening GI GVHD stage. Nonrelapse mortality (NRM) was defined as any death that occurred without intervening relapse of the underlying malignancy.
Histopathology
Paneth cells were counted in at least 3 high-powered fields (hpfs) in the area of each biopsy showing the largest number of Paneth cells per specimen. At the University of Michigan, an Olympus BX43 microscope was used. At the University of Regensburg, a Zeiss Axioskop 40 microscope was used. An hpf was defined as a 40× objective with the field diameter nearly identical between the 2 centers (Michigan: 0.345 mm2, Regensburg: 0.31 mm2). The counts from each hpf were then averaged to give the number of Paneth cells per hpf.
Statistical analysis
A Kruskal-Wallis test was used to assess the relationship of Paneth cells with clinical GVHD severity, maximum GVHD grade, and 4-week response to treatment. A χ-squared test of association was used to assess the relationship of pathologic grade with clinical GVHD severity. Proportional odds logistic regression was used to assess the joint association of Paneth cells and pathologic grade with clinical GVHD severity. Stepwise competing risks regression was used to develop a model for NRM based on clinical severity, Paneth cell count, and pathologic grade. Kaplan-Meier methods were used to assess the relationship of Paneth cells with overall survival and cumulative incidence of NRM was assessed using competing risks methodology, where relapse was treated as a competing risk. Statistical significance was defined as a P value < .05.
Results
Patients
We identified 116 patients (90 from the University of Michigan and 26 from the University of Regensburg) who had duodenal biopsies obtained at the time of GI GVHD-related symptoms as part of a diagnostic evaluation. There were 26 control patients. Fifteen patients from the University of Regensburg who had duodenal biopsies but did not have GI GVHD or symptoms were also included in the analyses as control subjects. In 8 cases, small intestine biopsies were obtained between day 20 and 30 posttransplant (median 26 days) as part of an institutional review board-approved GI GVHD screening protocol. An additional 7 patients, who had minimal or no GI symptoms at the time, had small intestine biopsies obtained as part of a comprehensive chronic GVHD work-up. Eleven patients at the University of Michigan who had duodenal biopsies performed as part of a work-up for diarrhea and were diagnosed with non-GVHD enteritis were included in the analysis as control subjects. Patient characteristics are shown in Table 1.
Patient characteristics
| Control patients (n = 26) | |
| Age (median), y | 3-68 (52) |
| Donor type | Unrelated 16, related 10 |
| HLA-match | Matched 21, mismatched 5 |
| Conditioning regimens* | Full intensity 8, reduced intensity 18 |
| Day of duodenal biopsy (median) | |
| Asymptomatic screening patients | 20-30 (26) |
| Chronic GVHD work-up | 133-882 (246) |
| Non-GVHD enteritis | 31-163 (37) |
| GI GVHD patients (n = 116) | |
| Age (median), y | 9-67 (52) |
| Donor type | Unrelated 70, related 40 |
| HLA-match | Matched 85, mismatched 31 |
| Conditioning regimens* | Full intensity 58, reduced intensity 58 |
| GI GVHD onset (median), d | 10-282 (37) |
| Day of duodenal biopsy (median) | 11-290 (40) |
| Control patients (n = 26) | |
| Age (median), y | 3-68 (52) |
| Donor type | Unrelated 16, related 10 |
| HLA-match | Matched 21, mismatched 5 |
| Conditioning regimens* | Full intensity 8, reduced intensity 18 |
| Day of duodenal biopsy (median) | |
| Asymptomatic screening patients | 20-30 (26) |
| Chronic GVHD work-up | 133-882 (246) |
| Non-GVHD enteritis | 31-163 (37) |
| GI GVHD patients (n = 116) | |
| Age (median), y | 9-67 (52) |
| Donor type | Unrelated 70, related 40 |
| HLA-match | Matched 85, mismatched 31 |
| Conditioning regimens* | Full intensity 58, reduced intensity 58 |
| GI GVHD onset (median), d | 10-282 (37) |
| Day of duodenal biopsy (median) | 11-290 (40) |
HLA, human leukocyte antigen.
Details of the conditioning regimens are provided in supplemental Table 1.
Diarrhea was the predominant GI symptom in the patients with GI GVHD. Only 7 patients (6%) presented with isolated upper GI GVHD symptoms (nausea, vomiting, and/or anorexia) and in each case, the diagnosis of GVHD was confirmed on biopsy. Patients with isolated GI symptoms were likely to undergo diagnostic biopsy at each center according to clinical practice. Thus, GI symptoms were the only clinical manifestation of GVHD in 82 of the 116 (71%) patients. Forty patients (34%) also had skin GVHD (stage 1 or 2 in 29, stage 3 in 11) present at the time that GI GVHD was diagnosed. Eleven cases of liver GVHD occurred at the time of GI GVHD: stage 1 in 5, stage 2 in 3, and stage 3 or 4 in 3 (who also had simultaneous stage 4 GI GVHD).
Paneth cell counts are very reproducible
We first confirmed that Paneth cell counts are reproducible by the same pathologist, who recounted Paneth cells of 41 coded samples. The correlation between the 2 evaluations was extremely high (r2 = 0.92; P < .0001). We then had the pathologist from the other center count Paneth cells on these slides, and inter-operator reproducibility remained very high (r2 = 0.81; P < .0001) (supplemental Figure 1).
The number of Paneth cells correlates with clinical GVHD severity
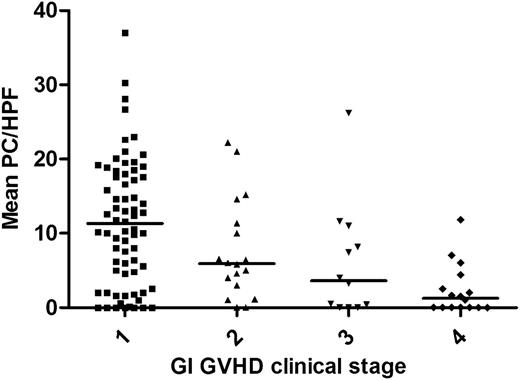
We found a strong statistical correlation between the number of Paneth cells per hpf and the clinical severity of GI GVHD at the time that the duodenal biopsy was obtained (P < .0001) (Figure 1). This finding is notable, because the clinical severity was primarily due to lower GI symptoms at the time of biopsy. Small intestine pathologic grade, according to the Lerner scale, correlated with clinical GVHD severity (P < .0001).
Paneth cell count correlates with GI GVHD clinical severity at onset. Mean Paneth cell counts per hpf (horizontal lines show mean of the means) were significantly different (P < .0001) in biopsies obtained at the onset of GI GVHD stage 1 (n = 69), 2 (n = 18), 3 (n = 12), and 4 (n = 16).
Paneth cell count correlates with GI GVHD clinical severity at onset. Mean Paneth cell counts per hpf (horizontal lines show mean of the means) were significantly different (P < .0001) in biopsies obtained at the onset of GI GVHD stage 1 (n = 69), 2 (n = 18), 3 (n = 12), and 4 (n = 16).
Given these results, we next determined which of these 2 variables (Paneth cell number or small intestine pathologic grade) correlated best with clinical GVHD severity. Proportional odds logistic regression analysis demonstrated that, after accounting for pathologic grade, Paneth cell numbers retain a strong correlation with GVHD severity (P = .008). However, once Paneth cell numbers were accounted for, pathologic grade no longer significantly correlated with GVHD severity (P = .27). Thus, Paneth cell quantification from the duodenum better correlates with clinical GI GVHD severity.
In 87 patients, colonic biopsies obtained at the same time of the duodenal biopsies were available for pathologic grading. Paneth cells are not present in the colon, and thus Paneth cell counts are not possible from this anatomic site; but the pathologic grade of the colonic biopsies correlated significantly with clinical severity (P < .001). We performed another proportional odds logistic regression analysis and found that, after accounting for the other variable, both lower GI pathologic grade and duodenal Paneth cell count correlated with clinical GI GVHD severity. Pathologic grade from the colon correlated with clinical GI GVHD severity (P = .005) better than duodenal Paneth cell counts (P = .057).
Paneth cell numbers correlate with GVHD outcomes
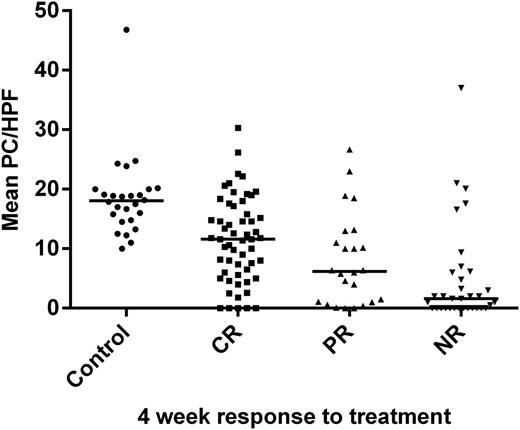
The response to treatment at 4 weeks is a good surrogate for long-term GVHD outcomes.6,13 As shown in Figure 2, the number of Paneth cell per hpf was highest in patients who had a complete response to treatment at 4 weeks (median 11.6, mean 11.5), lower in patients who had a partial response (median 6.4, mean 8.2), and lowest in patients who did not respond to treatment (median 1.6, mean 4.9), with a highly significant difference among the 3 treatment responses (P < .001), a finding that remained highly significant after statistical adjustment for clinical GVHD severity (P < .001). For comparison, the number of Paneth cells in the control patients without GI GVHD (median 18, mean 18.5) was significantly higher than that in patients with GI GVHD who completely responded to treatment (P < .0001). Although 44 patients also had skin and/or liver GVHD involvement, the other organs did not influence treatment responses, because patients who responded did so in all target organs.
Paneth cell count at onset correlates with GI GVHD response to treatment. Mean Paneth cell counts per hpf (horizontal lines show median of the means) were significantly different (P < .0001) in biopsies obtained from control patients without GI GVHD (n = 26) and at the onset of GI GVHD in patients whose 4-week response to treatment was complete response (CR, n = 56), partial response (PR, n = 24), or no response/progression (NR, n = 34). The interquartile range of mean Paneth cell counts per hpf was 5.7 to 16.9 for CR, 1 to 12.5 for PR, and 0 to 6.1 for NR.
Paneth cell count at onset correlates with GI GVHD response to treatment. Mean Paneth cell counts per hpf (horizontal lines show median of the means) were significantly different (P < .0001) in biopsies obtained from control patients without GI GVHD (n = 26) and at the onset of GI GVHD in patients whose 4-week response to treatment was complete response (CR, n = 56), partial response (PR, n = 24), or no response/progression (NR, n = 34). The interquartile range of mean Paneth cell counts per hpf was 5.7 to 16.9 for CR, 1 to 12.5 for PR, and 0 to 6.1 for NR.
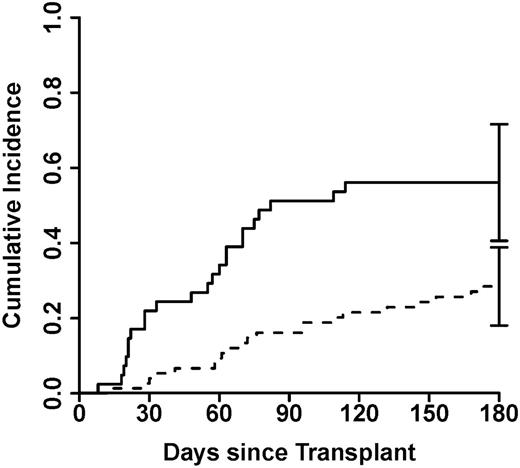
Given these results, we explored whether a threshold number of Paneth cells could predict NRM. A receiver operating characteristic curve for Paneth cells discriminated according to NRM with an AUC of 0.68 (supplemental Figure 2). We found that a mean <4 Paneth cells per hpf at onset of GVHD categorized patients for high risk of NRM and worse overall survival. The 42 patients with <4 Paneth cells per hpf were more than twice as likely to die of nonrelapse causes within 6 months than the 75 patients with ≥4 Paneth cells per hpf (55% vs 23%; P < .0001) (Figure 3). This large difference in NRM translated into a significant difference in overall survival at 6 months from the onset of GI GVHD (16% vs 42%; P = .01). Finally, we verified that Paneth cell counts are informative for NRM even when other risk factors (clinical GI GVHD severity, duodenal and colonic pathologic grade) are taken into account. Because these risk factors are correlated with each other, we used stepwise competing risks regression to model for NRM. The best model for predicting NRM included Paneth cell count <4 (hazard ratio 2.5; P = .02) and colonic pathologic grade 3 to 4 (hazard ratio 4.2; P < .001).
Cumulative incidence of NRM after GVHD onset, stratified by Paneth cell count. Patients with a mean Paneth cell count per hpf ≥4 (n = 75, solid line) at the time of GI GVHD onset experienced significantly less NRM than the 42 patients (dashed line) with a mean Paneth cell count per hpf <4 (28% vs 56%; P = .004).
Cumulative incidence of NRM after GVHD onset, stratified by Paneth cell count. Patients with a mean Paneth cell count per hpf ≥4 (n = 75, solid line) at the time of GI GVHD onset experienced significantly less NRM than the 42 patients (dashed line) with a mean Paneth cell count per hpf <4 (28% vs 56%; P = .004).
Discussion
GI GVHD can be difficult to diagnose at the onset of symptoms and has a high rate of treatment failure. The current GI GVHD pathologic grading system has major limitations. First, although in this study the severity of colonic changes correlated with clinical GVHD severity, others have reported different results and thus many pathologists do not report pathologic grade in order to avoid confusion by clinicians. Second, the grading system has not been standardized. For example, the number of biopsy sections or hpfs to review is not consistent at each center or even among different pathologists. Finally, there is no definition on how to resolve the variable severity within biopsy sections from the same patient. In this study, we show that quantification of Paneth cell numbers in the duodenum, which is easily accomplished with light microscopy, can aid in establishing the diagnosis of GI GVHD and has prognostic importance. Patients with low numbers of Paneth cells had more severe GI GVHD symptoms, were less likely to respond to treatment, and were much more likely to die of nonrelapse causes.
Recent research supports the correlation between GI GVHD and Paneth cells that secrete antimicrobial peptides such as α-defensins and REG3α.12 These peptides regulate the noncommensal bacteria while generally sparing commensal bacteria. A loss of diversity in intestinal microbiota was characterized by the expansion of Lactobacillus species, and contracture of the commensal bacterial flora has been observed in both mice and patients with GVHD.14 Decreased numbers of Paneth cells has been independently reported in a different experimental GVHD model.12 Other immune-mediated inflammatory conditions, such as inflammatory bowel disease, also result in reduced numbers of Paneth cells and changes in the intestinal microbiota.15,16 Finally, we have reported that the plasma concentrations of REG3α are higher in patients with GI GVHD-related diarrhea compared with patients with diarrhea from other causes. Interestingly, Paneth cell counts do not correlate with plasma concentrations of REG3α (data not shown). We explain this surprising result by postulating that elevated REG3α in the plasma derives from REG3α in the mucus covering the luminal surface of enterocytes. Due to its large size, REG3α concentrates in the mucus, where it prevents enterococci from adhering to enterocytes.9 We hypothesize that when enterocytes apoptose during GVHD, the mucus containing REG3α drains into the villus capillaries and systemic circulation. Experiments are in progress using mouse models of GVHD to further quantify the relationships between REG3α, production, Paneth cells, intestinal stem cells, and GVHD histology.
Paneth cells are normally not present in the distal colon, but it is perhaps not surprising that changes within the small intestine should influence a disease with predominantly lower GI symptoms. The small intestine contributes to the volume of diarrhea and is typically a target organ in experimental GVHD.17,18 Small intestinal pathology causes diarrhea in a number of disease conditions such as viral gastroenteritis,19 celiac disease,20 and some cases of bacterial overgrowth.21 Although Paneth cell quantification is less subjective and variable than the current histologic grading system, it too has its limitations. First, Paneth cell counts require acquisition of biopsies from the small intestine, which is not a universal practice. Second, Paneth cell counts will not predict skin and liver GVHD treatment response, which may also contribute to NRM. Lastly, there was a wide range of Paneth cell counts for each outcome. The threshold of 4 Paneth cells per hpf that divided this population into high and low risk for NRM should be validated in a prospective study before it can be recommended for clinical use. If validated, the additional expense and/or risk associated with upper GI or ileal biopsy may be offset by improved early risk stratification when interventions are most likely to be effective.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the physicians, laboratory staff, and data management teams at the University of Michigan: Craig Byersdorfer, Sung Choi, James Connelly, Carrie Kitko, John Magenau, Shin Mineishi, Sophie Paczesny, Attaphol Pawarode, Edward Peres, Pavan Reddy, Gregory Yanik, Katherine Archangeli, Sean Kelley, Ryan McKendree, Rachel Young, and Charlotte Zinkus, and at the University of Regensburg: Karin Schmid, Daniel Wolff, Julia Ammer, Daniela Sporrer, Conny Winter, and Sigrun Gleich.
This work was supported by National Institutes of Health grants CA46592, P01-CA039542 (National Cancer Institute), and T32-HL007622 (National Heart, Lung, and Blood Institute), and by the Hartwell Foundation and the Judith Devries fund.
Authorship
Contribution: J.E.L., J.K.G., J.L.M.F., and E. Holler planned the study, interpreted the data, and wrote the manuscript; A.C.H. performed research, performed data collection and quality assurance, and wrote the manuscript; E. Huber, S.T.G.H., and J.K.G. performed pathology evaluations and wrote the manuscript; and T.M.B. was the study statistician and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John E. Levine, Blood and Marrow Transplant Program, University of Michigan Comprehensive Cancer Center, Room 5303, 1500 E Medical Center Dr, Ann Arbor, MI 48109-5941; e-mail: jelevine@umich.edu.
References
Author notes
J.L.M.F. and E.H. contributed equally to this study.