Key Points
Immunological tolerance can be achieved by direct modulation of the intrinsic apoptosis pathway in peripheral lymphocytes.
Abstract
Induction of mixed hematopoietic chimerism results in donor-specific immunological tolerance by apoptosis-mediated deletion of donor-reactive lymphocytes. A broad clinical application of this approach is currently hampered by limited predictability and toxicity of the available conditioning protocols. We developed a new therapeutic approach to induce mixed chimerism and tolerance by a direct pharmacological modulation of the intrinsic apoptosis pathway in peripheral T cells. The proapoptotic small-molecule Bcl-2 inhibitor ABT-737 promoted mixed chimerism induction and reversed the antitolerogenic effect of calcineurin inhibitors by boosting the critical role of the proapoptotic Bcl-2 factor Bim. A short conditioning protocol with ABT-737 in combination with costimulation blockade and low-dose cyclosporine A resulted in a complete deletion of peripheral donor-reactive lymphocytes and was sufficient to induce mixed chimerism and robust systemic tolerance across full major histocompatibility complex barriers, without myelosuppression and by using moderate doses of bone marrow cells. Thus, immunological tolerance can be achieved by direct modulation of the intrinsic apoptosis pathway in peripheral lymphocytes—a new approach to translate immunological tolerance into clinically applicable protocols.
Introduction
Induction of allograft tolerance, a state in which the immune system accepts donor organs but normally responds to foreign antigens, represents the ideal solution for preventing rejection after solid-organ transplantation without immunosuppression-related toxicity. Among the different experimental models to induce tolerance, very few were successful in large animals and only one strategy—namely, the induction of mixed chimerism by combined transplantation of a solid organ and hematopoietic stem cells from the same donor—was successful in clinical pilot studies.1
The main mechanism of tolerance in mixed chimeras is central deletion of newly arising donor-reactive lymphocytes induced by the presence of donor-derived antigen-presenting cells in central lymphatic organs.2,3 Preexisting mature donor-reactive T cells are not exposed to this tolerization process and need to be removed from the T-cell repertoire by a conditioning therapy to prevent rejection. In the first clinical mixed chimerism studies, peripheral T-cell tolerization was obtained by unselective lymphocyte depletion through irradiation or profound T-cell depletion by antibodies.4-7 This approach was effective, but inevitably induced pancytopenia and with some protocols also engraftment syndrome. Moreover, in nonhuman primates and patients, hematopoietic chimerism was not always stable over time, and in some protocols chimerism was only transiently detectable (apparently without precluding tolerance maintenance).4,5 Other groups reported the establishment of full chimerism, a condition that, however, bears a high risk for graft-vs-host disease.6
The hope for a selective and less toxic option to clonally delete peripheral donor-reactive T cells was provided by the development of immunomodulatory strategies based on costimulation blockade. Blocking CD28/CD80/CD86 and CD154/CD40 signaling induces anergy and deletional tolerance by activation of the apoptosis cascade in alloreactive T cells.3,8,9 However, this strategy displayed a reduced efficacy in combination with standard immunosuppression by calcineurin inhibitors10,11 and was not sufficient to induce tolerance in case of a high donor-reactive T-cell precursor frequency.12
Resistance to anti-CD154-induced or CTLA4Ig-induced tolerance in mice overexpressing the antiapoptotic factor Bcl-xL indicates that the regulation of the intrinsic apoptosis pathway by the Bcl-2 family is pivotal in this setting.13,14 The recent advent of selective Bcl-2 family inhibitors offers new pharmacological options to modulate these physiological processes. Of particular clinical interest are the small molecules ABT-737 and ABT-263 (navitoclax),15,16 rationally designed molecules with antineoplastic17 and immunomodulatory properties.18-20 ABT-737 acts as a “sensitizer BH3-only protein”: it inhibits the antiapoptotic Bcl-2 factors Bcl-2, Bcl-xL, and Bcl-w and enhances the effect of proapoptotic endogenous “activator BH3-only proteins,” such as Bid or Bim.15 ABT-737 has a selective proapoptotic activity on peripheral lymphocytes and platelets, but does not induce myelosuppression, as shown by the unaffected numbers of granulocytes and erythrocytes in peripheral blood.18,20 This activity profile resulted in a favorable therapeutic index for ABT-263 in first clinical cancer trials.21 Moreover, ABT-737 inhibited allogeneic immune responses through apoptosis induction in donor-reactive T cells.20 This effect was markedly increased in combination with cyclosporine A (CsA), because calcineurin inhibition prevented resistance of activated T cells to ABT-737 in the first days after antigen recognition by blocking the expression of the antiapoptotic factor Bcl-2A1, which is not antagonized by ABT-737.22 Furthermore, CsA reduced the expression of Bcl-2 in lymphocytes and therefore increased the proapoptotic effect of ABT-737 without affecting its selectivity profile.23
In this study we evaluated Bcl-2 inhibition as a novel option to induce donor-specific tolerance in the context of mixed chimerism protocols. We found that Bcl-2 inhibition promoted tolerance by supporting the deletion of donor reactive T cells in combination with costimulation blockade. The tolerogenic effect of ABT-737 on peripheral lymphocytes was mediated by a pharmacological boosting of the proapoptotic factor Bim, which was identified as a critical player for establishment of mixed chimerism. By targeting the intrinsic apoptosis pathway, we developed a novel protocol to achieve complete deletion of donor-reactive T cells with a minimal toxicity and to induce robust systemic tolerance without myelosuppression,
Methods
Mice
C57BL/6 (B6, H-2b), CBA (H-2k), BALB/c (H-2d), BM3.3 (CBA, H-2k), and Bim knock-out mice (Bim−/−, H-2b) were housed in specific pathogen-free conditions at the University of Zürich. The BM3.3 mouse24 —which expresses on all CD8 T cells a transgenic T-cell receptor (TCR) selective for a naturally processed octapeptide bound to the allogeneic major histocompatibility complex (MHC) class I molecule H-2Kb—was kindly provided by A.-M. Schmitt-Verhulst.25 Bim−/− mice were kindly provided by Andreas Strasser.26 All animal experiments were performed according to protocols approved by the legal authority (Veterinary Office, Canton of Zürich, Switzerland).
Conditioning and bone marrow (BM) procedures
Different conditioning protocols were tested as indicated. In general, B6-recipient mice received 1.5 or 3 Gy total body irradiation (TBI) from a 137Caesium irradiator on day −1 with respect to bone marrow transplantation (BMT). Hamster anti-mouse CD154 (MR1, 2 mg; purchased from Bio-X-cell, West Lebanon, NH) was administered intraperitoneally (i.p.) 6 to 12 hours before transplantation with 25 × 106 fully MHC-mismatched CBA BM cells by tail vein injection. In some experiments, CTLA4Ig (abatacept, 0.5 mg; provided by Bristol-Myers Squibb, New York, NY) was administered at day 2 after BMT. ABT-737, provided by Abbott Bioresearch (Worcester, MA), was dissolved in polyethylene glycol, Tween 80, dextrose solution, and dimethylsulfoxide and injected intraperitoneally at 50 mg/kg. CsA (Sigma-Aldrich, Buchs, Switzerland) was dissolved in ethanol and cremaphor EL (Sigma-Aldrich), then diluted in phosphate-buffered saline and injected subcutaneously at 10 mg/kg. Both ABT-737 and CsA were administered daily from day −3 to day 12; on day −2 and day −1, mice received 2 injections for a total of 100 mg/kg of ABT-737 and 20 mg/kg of CsA. On day 0, ABT-737 and CsA were not administered. To monitor the deletion of donor-reactive CD8 T cells, we adoptively transferred 20 × 106 syngeneic BM3.3 splenocytes to CBA recipients before starting the conditioning protocol with B6 BM cells. The transgenic H-2Kb reactive BM3.3 CD8 T cells were monitored over time in peripheral blood in a fluorescence-activated cell sorter (FACS) using the clonotypic antibody Ti98,27 kindly provided by A.-M. Schmitt-Verhulst, and stained with a secondary phycoerythrin (PE) rat anti-mouse IgG, purchased from Becton Dickinson (Basel, Switzerland).
Skin grafting
Mice were shaved and anesthetized with ketamine/xylazine. Full thickness tail skin (about 1 cm2) from CBA (BM donor) or BALB/c (3rd party) mice were grafted 3 to 6 weeks after BMT and considered rejected when <10% of the graft remained viable. In some experiments a second skin grafting was performed using the same procedure.
Flow cytometric analysis of chimerism and detection of allospecific antibodies
Flow cytometric (FACS) analyses were performed with a BD-FACSCanto (Becton Dickinson, Basel, Switzerland). Chimerism was analyzed in white blood cells at different time points after BMT, in spleen and thymus as indicated. Donor-derived cells were identified by fluorescein isothiocyanate (FITC)–conjugated anti-H-2Dk or anti-H-2Kk (Becton Dickinson). The cells were counterstained with anti-CD4-PE, anti-CD8-APC, anti-B220-PE, anti-CD11b-APC, anti-CD11c-APC, anti-CD49b-PE (for natural killer [NK] cells) antibodies purchased from eBioscience (Frankfurt, Germany). Background signal measured in a naïve B6 mouse was subtracted to determine the percentage of donor-derived cells. Allospecific antibodies were measured in indirect FACS: CBA, and Balb/c splenocytes were incubated with recipients’ serum (1:5 dilution in FACS buffer) and subsequently stained with a secondary anti-mouse FITC-conjugated IgG antibody (eBioscience). Mean fluorescence intensity was determined in FACS gating on CD8-positive cells.
Mixed lymphocyte reaction (MLR)
MLR were performed in 96 wells plates with responder splenocytes stimulated by splenocytes from BM-donor, 3rd party, or syngeneic mice at a final concentration of 4 × 106 cells/mL in Roswell Park Memorial Institute medium containing 10% fetal bovine serum, penicillin 100 U/mL, streptomycin 100 μg/mL, and 2-mercaptoethanol 50 μM. T-cell proliferation was measured by incorporation of 3H-thymidine (PerkinElmer, Waltham, MA) added to the culture on day 4 after stimulation. For the selective analysis of alloreactive CD8 T cells in vitro, BM3.3 spenocytes were stimulated with CD8 T-cell-depleted splenocytes from B6 (allogeneic) or CBA (syngeneic) mice and analyzed in FACS gating on CD8 T cells. After cell permeabilization, the level of different Bcl-2 factors in alloreactive CD8 T cells was detected in FACS, as previously described.23 Bcl-2 was stained using an anti-mouse Bcl-2-PE from Becton Dickinson, Bcl-xL with an Alexa Fluor 488 conjugated antibody from Cell Signaling Technology (Danvers, MA). Mcl-1 was detected with a monoclonal rabbit anti-mouse Mcl-1 antibody from Abcam (Cambridge, United Kingdom), Bim with a polyclonal rabbit antibody detecting total Bim (EL, L, and S isoforms) purchased from Cell Signaling, and then stained with a FITC anti-rabbit IgG (eBioscience). Splenocytes were sorted by automatic magnetic cell separation using an autoMACS proseparator according to the protocols of Miltenyi Biotec (Bergisch Gladbach, Germany). For polyclonal stimulation of splenocytes, anti-CD3 and anti-CD28 antibodies were used (eBioscience). Cell viability was measured by propidium iodide exclusion in FACS.
Statistics
Student t test was used to compare values between groups. A P value < .05 was considered significant. Graph Pad Prism Software version 5.0 (San Diego, CA) was used for calculations.
Results
Tolerogenic effect of Bcl-2 inhibition by ABT-737
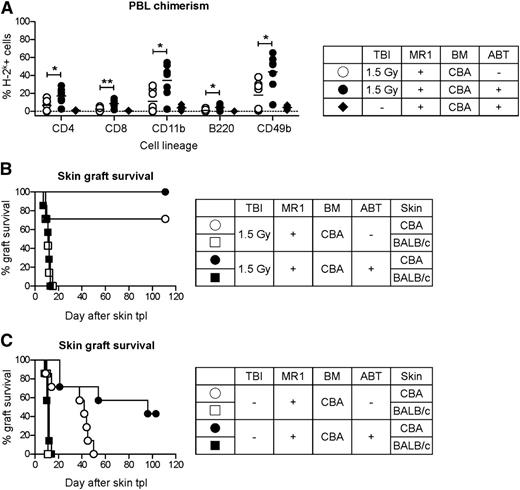
The effect of Bcl-2 inhibitors on BM engraftment and tolerance induction was first assessed by adding a short course of ABT-737 (50 mg/kg/day, from day −3 to day 12 after BMT) to a reduced conditioning protocol consisting of low-dose TBI (1.5 Gy) on the day before BMT, a single injection of anti-CD154 (MR1, 2 mg) and 25 × 106 fully MHC-mismatched CBA BM cells to B6 recipients. ABT-737 increased the percentage of chimeric mice and induced a higher level of chimerism in all hematopoietic cell lineages (Figure 1A). All chimeric animals accepted donor-type skin grafts for more than 100 days and promptly rejected third-party grafts (BALB/c) (Figure 1B). In a second experiment, mice were treated with the same conditioning protocol including ABT-737, MR1, and BM cells, but without TBI. Although this protocol was not sufficient to allow BM engraftment (Figure 1A), skin transplantation (performed 6 weeks after BMT) revealed a marked and lasting donor-specific hyporesponsiveness in comparison with mice treated with MR1 and BM only (median survival time, 42 vs 96 days) (Figure 1C). Nevertheless, donor grafts were eventually rejected (graft survival 21, 21, 54, 96, >100, >100, and >100 days). Thus, inhibition of Bcl-2, Bcl-xL, and Bcl-w was not detrimental for survival and engraftment of donor-derived hematopoietic stem cells. Furthermore, ABT-737 in combination with CD154 blockade led to some degree of donor-specific hyporesponsiveness, but it was not sufficient to induce stable tolerance in an irradiation-free conditioning protocol.
ABT-737 facilitates mixed chimerism induction. B6 recipients (H-2b) were treated with a conditioning protocol including TBI (1.5 Gy), MR1 (2 mg), and 25 × 106 fully MHC-mismatched BM cells from CBA donors (H-2k). Six weeks after BMT, skin transplantation from CBA and BALB/c (3rd party, H-2d) donors was performed. (A) Adding a short course of ABT-737 to the conditioning regimen (ABT; 50 mg/kg/day, from day −3 to day 12 after BMT) resulted in a higher number of chimeric animals and significantly increased the percentage of donor-derived cells in different cell lineages in peripheral blood leukocytes (PBL; 10 weeks after BMT). CD11b for neutrophils; CD49b for natural killer cells; *P < .05; **P < .01. N = 7. (B) After skin transplantation all chimeric mice accepted donor grafts and promptly rejected 3rd-party grafts, demonstrating that donor-specific tolerance had been induced. N = 7-8 per group. (C) Using the same experimental protocol but without TBI, we obtained a pronounced donor-specific hyporesponsiveness, but tolerance was not achieved, as shown by the slow rejection of donor skin grafts over time. N = 7-8 per group. Representative results of 2 independent experiments are shown. tpl, transplantation.
ABT-737 facilitates mixed chimerism induction. B6 recipients (H-2b) were treated with a conditioning protocol including TBI (1.5 Gy), MR1 (2 mg), and 25 × 106 fully MHC-mismatched BM cells from CBA donors (H-2k). Six weeks after BMT, skin transplantation from CBA and BALB/c (3rd party, H-2d) donors was performed. (A) Adding a short course of ABT-737 to the conditioning regimen (ABT; 50 mg/kg/day, from day −3 to day 12 after BMT) resulted in a higher number of chimeric animals and significantly increased the percentage of donor-derived cells in different cell lineages in peripheral blood leukocytes (PBL; 10 weeks after BMT). CD11b for neutrophils; CD49b for natural killer cells; *P < .05; **P < .01. N = 7. (B) After skin transplantation all chimeric mice accepted donor grafts and promptly rejected 3rd-party grafts, demonstrating that donor-specific tolerance had been induced. N = 7-8 per group. (C) Using the same experimental protocol but without TBI, we obtained a pronounced donor-specific hyporesponsiveness, but tolerance was not achieved, as shown by the slow rejection of donor skin grafts over time. N = 7-8 per group. Representative results of 2 independent experiments are shown. tpl, transplantation.
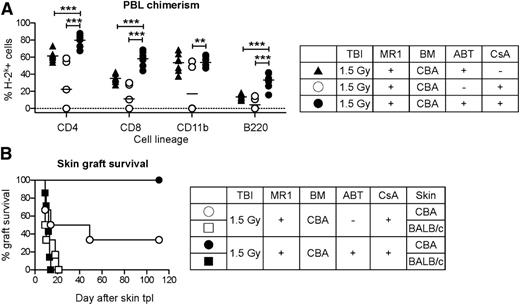
We previously demonstrated that the proapoptotic effect of ABT-737 on naïve and activated lymphocytes can be potentiated in combination with CsA.22,23 Therefore, we added a short course of low-dose CsA (10 mg/kg/day subcutaneously) to the mixed chimerism protocol introduced above (1.5 Gy TBI, MR1, 25 × 106 BM cells from CBA donors). In previous studies, treatment with CsA alone had an antitolerogenic effect and resulted in a reduced number of tolerant mice and lower chimerism levels (Figure 2A-B).8,10,11 However, the combination of CsA with ABT-737 completely prevented this phenomenon and induced a high level of chimerism (Figure 2A; supplemental Figure 1 for kinetics of multilineage chimerism) and donor-specific tolerance in all recipients, as shown by the long-term survival of donor-type CBA skin grafts (Figure 2B). Importantly, the combination of ABT-737 with CsA not only prevented the antitolerogenic effect of calcineurin inhibitors, it resulted in an even higher chimerism level in comparison with ABT-737 alone, thereby reversing the antitolerogenic effect of CsA in a paradoxical synergism (Figure 2A). The mechanism underlying this clinically important finding was further investigated.
ABT-737 reverses the antitolerogenic effect of CsA. B6 recipients (H-2b) were treated with a conditioning protocol including TBI (1.5 Gy), MR1 (2 mg), and 25 × 106 fully MHC-mismatched BM cells from CBA donors (H-2k). Six weeks after BMT, skin transplantation from CBA and BALB/c (3rd party, H-2d) donors was performed. (A) CsA (10 mg/kg/day, from day −3 to day 12 after BMT) had a deleterious impact on mixed chimerism induction, but this effect was reversed in combination with ABT-737 (ABT, 50 mg/kg/day, from day −3 to day 12 after BMT), as shown in the level of chimerism in different hematopoietic cell lineages in peripheral blood leukocytes (20 weeks after BMT). Notably, the chimerism level in the combination group was even higher than in the group treated with ABT-737 alone. **P < .01; ***P < .001; N = 7-8. (B) Most recipients receiving CsA alone rejected CBA skin grafts, whereas all mice treated with a combination of CsA and ABT-737 accepted CBA and promptly rejected BALB/c skin grafts. N = 7-8/group.
ABT-737 reverses the antitolerogenic effect of CsA. B6 recipients (H-2b) were treated with a conditioning protocol including TBI (1.5 Gy), MR1 (2 mg), and 25 × 106 fully MHC-mismatched BM cells from CBA donors (H-2k). Six weeks after BMT, skin transplantation from CBA and BALB/c (3rd party, H-2d) donors was performed. (A) CsA (10 mg/kg/day, from day −3 to day 12 after BMT) had a deleterious impact on mixed chimerism induction, but this effect was reversed in combination with ABT-737 (ABT, 50 mg/kg/day, from day −3 to day 12 after BMT), as shown in the level of chimerism in different hematopoietic cell lineages in peripheral blood leukocytes (20 weeks after BMT). Notably, the chimerism level in the combination group was even higher than in the group treated with ABT-737 alone. **P < .01; ***P < .001; N = 7-8. (B) Most recipients receiving CsA alone rejected CBA skin grafts, whereas all mice treated with a combination of CsA and ABT-737 accepted CBA and promptly rejected BALB/c skin grafts. N = 7-8/group.
The critical role of the proapoptotic factor Bim in deletional tolerance
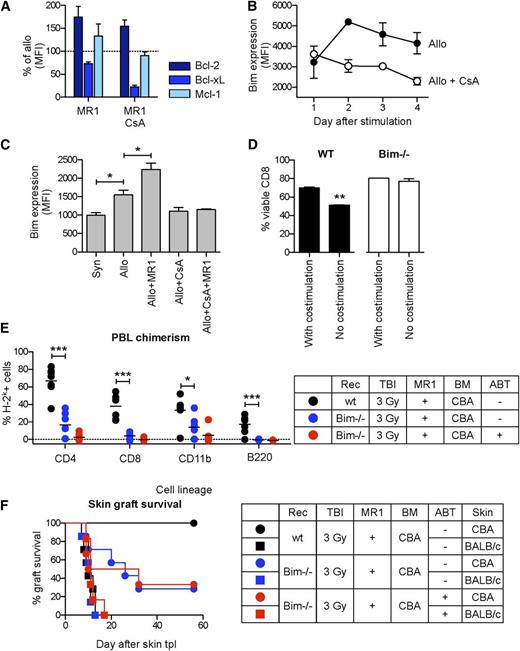
Because ABT-737 inhibits antiapoptotic Bcl-2 factors with high selectivity,15 its tolerogenic effect is likely to result from a direct interaction with the regulation of the intrinsic apoptosis pathway in alloreactive lymphocytes. We hypothesized that ABT-737 might reverse the antitolerogenic effect of CsA by compensating a dysregulation of the intrinsic apoptosis pathway in activated CD8 T cells determined by signal 1 inhibition.28 First, we aimed to identify an antiapoptotic factor of the Bcl-2 familiy, whose expression in donor-reactive T cells was reduced under MR1 alone, but not in combination with CsA. To monitor the regulation of Bcl-2 factors in a homogeneous population of alloreactive CD8 T cells, we took advantage of the BM3.3 transgenic mouse, which expresses on all CD8 T cells a transgenic TCR specific for the MHC class I molecule H-2Kb and can be detected by the clonotypic antibody Ti98. BM3.3 splenocytes were stimulated with CBA (syngeneic) or B6 (allogeneic) splenocytes under the effect of CsA and MR1 in classical MLR experiments, and the expression of different Bcl-2 factors was measured in FACS. Among the most important antiapoptotic Bcl-2 factors, only the expression of Bcl-xL was reduced under the effect of MR1, but this process was not reversed in combination with CsA (Figure 3A). This finding was consistent with the previously reported critical role of Bcl-xL in this model,13 but did not explain the effect of CsA and ABT-737. However, we found that CsA had a major impact on the regulation of Bim, a fundamental proapoptotic Bcl-2 factor in T cells. Alloantigen stimulation induced a transitory upregulation of Bim in CD8 T cells (Figure 3B). The initial upregulation phase was dependent on signal 1 (and was blocked by CsA), whereas the subsequent downregulation was influenced by costimulatory signals. As a result, the level of Bim remained low in cells activated in the presence of CsA, and—starting at day 3 after stimulation—was significantly higher in CD8 T cells treated with MR1 (Figure 3C). This correlated with the viability of polyclonally stimulated alloreactive CD8 T cells: costimulation blockade did not influence alloreactive CD8 T cell viability during the first 2 days of culture (not shown), but a progressive loss of viability in alloreactive CD8 T cells devoid of costimulation was registered at days 3 and 4 after stimulation. This process was completely dependent on Bim, as shown in experiments using Bim−/− CD8 T cells under the same experimental conditions (Figure 3D). Notably, the dysregulation of Bim under the effect of CsA was not influenced by MR1 (Figure 3C).
Bim is required for induction of mixed chimerism. BM3.3 splenocytes were stimulated in vitro with CD8-depleted B6 (allo) or CBA (syn) splenocytes. The expression of different Bcl-2 factors in transgenic alloreactive BM3.3 CD8 T cells was monitored by FACS. (A) In comparison with allostimulated cells without additional pharmacological treatment, after 4 days of MLR, cells exposed to MR1 expressed higher levels of Bcl-2 and Mcl-1 and lower levels of Bcl-xL. The expression of these antiapoptotic factors was not influenced by an additional treatment with CsA. Percentages of mean fluorescence intensity (MFI) values in comparison with allostimulated cells without pharmacological treatment are shown. (B) Allostimulation induced a transient upregulation of Bim, with a peak after 2 days of culture. CsA inhibited the initial upregulation of Bim, and MR1 prevented its downregulation in the late activation phase. (C) As a result, after 4 days the level of Bim was low in cells stimulated in the presence of CsA and high with MR1. Statistical comparison with syn: *P < .01. (D) The relevance of these processes on CD8 T-cell viability after polyclonal stimulation was assessed culturing WT and Bim−/− splenocytes in the presence of anti-CD3 with anti-CD28 antibodies or without anti-CD28 and MR1. After 4 days, absence of costimulation reduced the viability of WT CD8 T cells, but the same phenomenon was not observed using Bim−/− cells, suggesting that the downregulation of Bim (C) was important for the viability of activated T cells. **P < .01. (E) In vivo, a standard conditioning protocol (3 Gy TBI, MR1, 25 × 106 CBA BM cells) induced mixed chimerism in all WT B6 mice, but was not successful in the majority of Bim−/− mice, as shown by the levels of chimerism 15 weeks after BMT and (F) by the rejection of CBA skin grafts. Similar results were obtained if ABT-737 was added to the same conditioning protocol (E-F). Statistical comparison WT vs Bim−/−: *P < .05; ***P < .001; N = 6-7 per group.
Bim is required for induction of mixed chimerism. BM3.3 splenocytes were stimulated in vitro with CD8-depleted B6 (allo) or CBA (syn) splenocytes. The expression of different Bcl-2 factors in transgenic alloreactive BM3.3 CD8 T cells was monitored by FACS. (A) In comparison with allostimulated cells without additional pharmacological treatment, after 4 days of MLR, cells exposed to MR1 expressed higher levels of Bcl-2 and Mcl-1 and lower levels of Bcl-xL. The expression of these antiapoptotic factors was not influenced by an additional treatment with CsA. Percentages of mean fluorescence intensity (MFI) values in comparison with allostimulated cells without pharmacological treatment are shown. (B) Allostimulation induced a transient upregulation of Bim, with a peak after 2 days of culture. CsA inhibited the initial upregulation of Bim, and MR1 prevented its downregulation in the late activation phase. (C) As a result, after 4 days the level of Bim was low in cells stimulated in the presence of CsA and high with MR1. Statistical comparison with syn: *P < .01. (D) The relevance of these processes on CD8 T-cell viability after polyclonal stimulation was assessed culturing WT and Bim−/− splenocytes in the presence of anti-CD3 with anti-CD28 antibodies or without anti-CD28 and MR1. After 4 days, absence of costimulation reduced the viability of WT CD8 T cells, but the same phenomenon was not observed using Bim−/− cells, suggesting that the downregulation of Bim (C) was important for the viability of activated T cells. **P < .01. (E) In vivo, a standard conditioning protocol (3 Gy TBI, MR1, 25 × 106 CBA BM cells) induced mixed chimerism in all WT B6 mice, but was not successful in the majority of Bim−/− mice, as shown by the levels of chimerism 15 weeks after BMT and (F) by the rejection of CBA skin grafts. Similar results were obtained if ABT-737 was added to the same conditioning protocol (E-F). Statistical comparison WT vs Bim−/−: *P < .05; ***P < .001; N = 6-7 per group.
The critical role of Bim for deletional tolerance during mixed chimerism induction was confirmed in vivo by applying an established conditioning protocol (TBI 3 Gy, MR1) to Bim−/− recipients. In comparison with wild-type (WT) animals, Bim−/− mice displayed a marked resistance to mixed chimerism induction (Figure 3E). This was reflected in skin graft survival: in Bim−/− mice the majority of donor-type CBA skin grafts were rejected within 50 days in comparison with indefinite survival in WT mice (Figure 3F). Notably, ABT-737 failed to promote tolerance when added to the same conditioning protocol in Bim−/− recipients (Figure 3F-G). These data are consistent with the involvement of Bcl-xL and Bim in anti-CD154-dependent deletional tolerance, support the thesis that the antitolerogenic effect of CsA is related to a dysregulation of Bim, and indicate that ABT-737 promotes deletional tolerance and reverses the antitolerogenic effect of CsA by enhancing the function of Bim. This novel mechanism for inducing tolerance was explored to aim for a myelosuppression-free tolerance-induction protocol.
Irradiation- and myelosuppression-free tolerance induction by targeting the apoptosis pathway
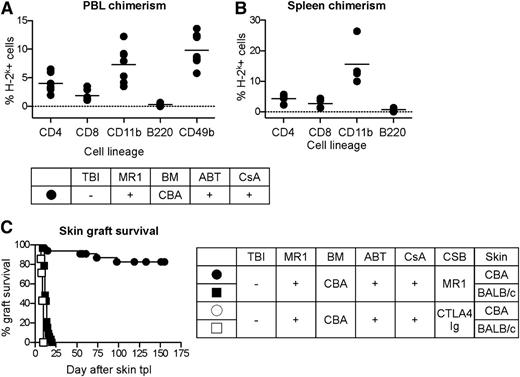
WT B6 mice were treated with ABT-737 (50 mg/kg/day) and low-dose CsA (10 mg/kg/day) for 2 weeks starting at day −3 with respect to BMT, and an additional dose of both drugs was administered on day −2 in order to achieve more pronounced peripheral lymphocyte depletion. Subsequently, MR1 was injected 6-12 hours before 25 × 106 BM cells from fully MHC-mismatched CBA donors. This protocol led to multilineage mixed chimerism in 29 out of 33 mice in 5 independent experiments. Similar to previous attempts of mixed chimerism induction without myelosuppressive conditioning,29 the level of donor chimerism was rather low in comparison with TBI-based protocols (Figure 4A-B). However, chimerism remained stable over time (supplemental Figure 2): 33 weeks after BMT, we measured a 4.4% of donor-derived CD4, 2.7% CD8, and 15.6% CD11b in the spleen. Interestingly, the B-cell compartment did not show any macrochimerism, either in the spleen or in peripheral blood at any time point after BMT. Donor-type CBA skin grafts were accepted indefinitely with a maximal observation time of 269 days, whereas all third-party grafts (BALB/c) were promptly rejected, therefore demonstrating donor-specific tolerance (Figure 4C). Notably, the additional dose of ABT-737 on day −2 was not sufficient to induce mixed chimerism without CsA (supplemental Figure 3)
Myelosuppression-free tolerance induction with ABT-737 and CsA. B6 recipients (H-2b) were treated with an irradiation-free conditioning protocol including ABT-737 (50 mg/kg/day), CsA (10 mg/kg/day) from day −3 to day 12, costimulation blockade (CSB) with MR1 (2 mg on day −1), and 25 × 106 fully MHC-mismatched BM cells from CBA donors (H-2k). Four to 6 weeks after BMT, skin transplantation from CBA and BALB/c (3rd party, H-2d) donors was performed. (A-B) A significant myeloid (CD11b+) and T-cell chimerism, but no chimerism in the B-cell compartment, was measured in peripheral blood (FACS at week 10 after BMT shown in panel A) and in the spleen (FACS at 33 weeks after BMT shown in panel B). N = 7 per group. Representative results of 5 independent experiments are shown. (C) Donor skin grafts were indefinitely accepted by 29 out of 33 mice in 5 independent experiments, whereas 3rd-party grafts (BALB/c) were promptly rejected. Combined data of 5 independent experiments are shown. N = 33. The same conditioning protocol failed to induce tolerance, when MR1 was replaced by CTLA4Ig (0.5 mg on day 2).
Myelosuppression-free tolerance induction with ABT-737 and CsA. B6 recipients (H-2b) were treated with an irradiation-free conditioning protocol including ABT-737 (50 mg/kg/day), CsA (10 mg/kg/day) from day −3 to day 12, costimulation blockade (CSB) with MR1 (2 mg on day −1), and 25 × 106 fully MHC-mismatched BM cells from CBA donors (H-2k). Four to 6 weeks after BMT, skin transplantation from CBA and BALB/c (3rd party, H-2d) donors was performed. (A-B) A significant myeloid (CD11b+) and T-cell chimerism, but no chimerism in the B-cell compartment, was measured in peripheral blood (FACS at week 10 after BMT shown in panel A) and in the spleen (FACS at 33 weeks after BMT shown in panel B). N = 7 per group. Representative results of 5 independent experiments are shown. (C) Donor skin grafts were indefinitely accepted by 29 out of 33 mice in 5 independent experiments, whereas 3rd-party grafts (BALB/c) were promptly rejected. Combined data of 5 independent experiments are shown. N = 33. The same conditioning protocol failed to induce tolerance, when MR1 was replaced by CTLA4Ig (0.5 mg on day 2).
Although the effect of CTLA4Ig on the regulation of Bcl-2 factors in vitro was promising (supplemental Figure 4), the success of this protocol was dependent on inhibition of the CD40/CD154 signaling, because the same conditioning regime failed to induce mixed chimerism and tolerance, when MR1 was replaced by CTLA4Ig (0.5 mg on day 2 after BMT; Figure 4C). The mechanisms of tolerance induction and maintenance in mice treated with this protocol were further investigated.
Robust peripheral and central deletional tolerance
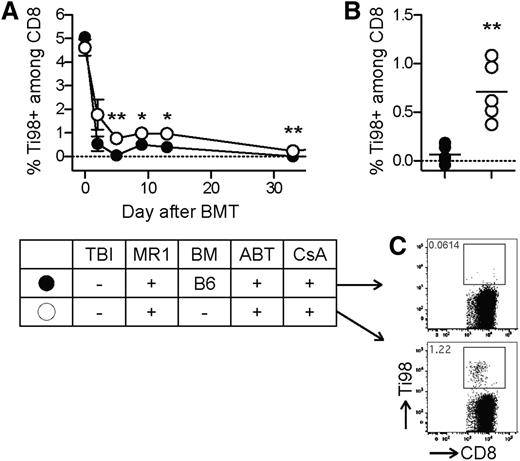
First, tolerization of preexisting peripheral alloreactive T cells was assessed using the transgenic BM3.3 system described above. Before starting the tolerance induction protocol, 20 × 106 BM3.3 splenocytes were transferred into syngeneic CBA recipients, which allowed monitoring alloreactive BM3.3 CD8 T cells in blood over time using the clonotypic antibody Ti98. In mice treated with the full conditioning protocol including ABT-737 (50 mg/kg/day), low-dose CsA (10 mg/kg/day), MR1, and 25 × 106 B6 BM cells, donor-reactive CD8 T cells completely disappeared from the peripheral T-cell repertoire in the first 2 weeks and did not recover after resolution of lymphopenia (Figure 5A). In contrast, in mice treated with the same pharmacological conditioning regimen, but without BMT, Ti98+ cells were markedly reduced, but still detectable in peripheral blood. The complete deletion of Ti98+ cells in mice exposed to the full protocol was confirmed by FACS analysis of the spleen after rechallenging the recipient mice with B6 splenocytes (106 cells IV) (Figure 5B-C). Thus, exposure to donor-derived BM cells under the effect of MR1 and ABT-737 induced a complete peripheral deletion of alloreactive T cells during the mixed chimerism induction phase, and this was not impaired by CsA.
Peripheral deletion of alloreactive CD8 T cells. To monitor a well-defined population of alloreactive CD8 T cells during tolerance induction with ABT-737 and CsA, we adoptively transferred CBA recipients (H-2k) with BM3.3 splenocytes (transgenic TCR specific for H-2Kb on all CD8 T cells) and subsequently treated with our irradiation-free conditioning protocol including ABT-737 (50 mg/kg/day), CsA (10 mg/kg/day) from day −3 to day 12, MR1 (2 mg), and 25 × 106 fully BM cells from B6 donors (H-2b). In a control group, BM cells were not administered (conditioning only). (A) Transgenic donor-reactive BM3.3 CD8 T cells (Ti98+) were reduced in both groups, but only in mice receiving the full protocol was a complete deletion achieved. Statistical comparison of the 2 groups: *P < .05; **P < .01; N = 5. (B-C) After rechallenging with donor antigens (106 B6 splenocytes IV at day 37 after BMT), Ti98+ cells were readily detectable in the conditioning-only group, but had completely disappeared from the peripheral T-cell repertoire after exposure to the full protocol. **P < .01; N = 5 per group.
Peripheral deletion of alloreactive CD8 T cells. To monitor a well-defined population of alloreactive CD8 T cells during tolerance induction with ABT-737 and CsA, we adoptively transferred CBA recipients (H-2k) with BM3.3 splenocytes (transgenic TCR specific for H-2Kb on all CD8 T cells) and subsequently treated with our irradiation-free conditioning protocol including ABT-737 (50 mg/kg/day), CsA (10 mg/kg/day) from day −3 to day 12, MR1 (2 mg), and 25 × 106 fully BM cells from B6 donors (H-2b). In a control group, BM cells were not administered (conditioning only). (A) Transgenic donor-reactive BM3.3 CD8 T cells (Ti98+) were reduced in both groups, but only in mice receiving the full protocol was a complete deletion achieved. Statistical comparison of the 2 groups: *P < .05; **P < .01; N = 5. (B-C) After rechallenging with donor antigens (106 B6 splenocytes IV at day 37 after BMT), Ti98+ cells were readily detectable in the conditioning-only group, but had completely disappeared from the peripheral T-cell repertoire after exposure to the full protocol. **P < .01; N = 5 per group.
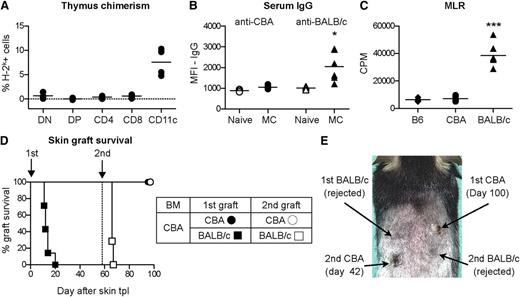
Second, the mechanism of long-term maintenance of tolerance was investigated in mice receiving our irradiation-free protocol. Mixed chimeras maintain donor-specific tolerance through central deletion of donor-reactive T cells, and the presence of donor-derived antigen-presenting cells in the thymus is critical in this setting.30 Using the above-mentioned irradiation-free protocol, we consistently detected about 5% of donor-derived antigen presenting cells (CD11c+) in the thymus as demonstrated by thymic FACS analysis 33 weeks after BMT (Figure 6A), a finding consistent with central clonal deletion. The presence of a sustained systemic tolerance was further confirmed by different immunological tests: donor-specific B-cell tolerance was demonstrated by the absence of donor-specific IgG alloantibodies, whereas 3rd party–reactive IgG was readily detectable several weeks after skin grafting (Figure 6B). A lack of donor-specific T-cell responses was measured in classical MLR experiments (Figure 6C), confirming at a functional level a complete deletion of donor-reactive T cells from the peripheral repertoire. Finally, 58 days after the first skin transplantation, tolerant mice were rechallenged with a second skin graft from donor and 3rd-party control. All mice accepted the secondary CBA graft and rejected the BALB/c graft (Figure 6D-E). Taken together, a 2-week conditioning protocol with ABT-737 and CsA in combination with MR1 induced mixed chimerism and stable systemic T- and B-cell tolerance without myelosuppressive treatment and with moderate doses of BM cells.
Robust, systemic tolerance after mixed chimerism induction with ABT-737 and CsA. B6 recipients were treated with an irradiation-free conditioning protocol including ABT-737 (50 mg/kg/day), CsA (10 mg/kg/day) from day −3 to day 12, MR1 (2 mg), and 25 × 106 BM cells from CBA donors. Six weeks after BMT, skin transplantation from CBA and BALB/c donors was performed. (A) FACS analysis 33 weeks after BMT revealed a significant percentage of donor-derived antigen-presenting cells (CD11c+) in the thymus, but not in T cells and in T-cell precursors (DN, double negative; DP, double positive). (B) Serum samples were collected 60 days after skin transplantation and analyzed by indirect FACS using CBA and BALB/c cells: a complete absence of CBA-reactive IgG and a normal seroconversion toward BALB/c were measured. MFI, mean fluorescence intensity. Statistical comparison between mixed chimeras (MC) and a group of naïve mice is shown. *P < .05; N = 5. (C) Thirty-three weeks after BMT, recipient mice were killed and their splenocytes stimulated in vitro with irradiated B6 (syn), CBA (BM donor), and BALB/c (3rd party) splenocytes in a classical MLR. T-cell proliferation analysis, measured by 3H-thymidin incorporation, revealed a complete lack of T-cell reactivity against CBA and a normal response toward BALB/c. CPM, counts per minute; ***P < .001; N = 5 per group. (D) A group of mice received a second skin graft 58 days after initial transplantation. All BALB/c grafts were rejected within 10 days, whereas CBA grafts were accepted for more than 40 days without signs of rejection. N = 7 per group. (E) Photograph of a representative example at day 100 after first transplantation.
Robust, systemic tolerance after mixed chimerism induction with ABT-737 and CsA. B6 recipients were treated with an irradiation-free conditioning protocol including ABT-737 (50 mg/kg/day), CsA (10 mg/kg/day) from day −3 to day 12, MR1 (2 mg), and 25 × 106 BM cells from CBA donors. Six weeks after BMT, skin transplantation from CBA and BALB/c donors was performed. (A) FACS analysis 33 weeks after BMT revealed a significant percentage of donor-derived antigen-presenting cells (CD11c+) in the thymus, but not in T cells and in T-cell precursors (DN, double negative; DP, double positive). (B) Serum samples were collected 60 days after skin transplantation and analyzed by indirect FACS using CBA and BALB/c cells: a complete absence of CBA-reactive IgG and a normal seroconversion toward BALB/c were measured. MFI, mean fluorescence intensity. Statistical comparison between mixed chimeras (MC) and a group of naïve mice is shown. *P < .05; N = 5. (C) Thirty-three weeks after BMT, recipient mice were killed and their splenocytes stimulated in vitro with irradiated B6 (syn), CBA (BM donor), and BALB/c (3rd party) splenocytes in a classical MLR. T-cell proliferation analysis, measured by 3H-thymidin incorporation, revealed a complete lack of T-cell reactivity against CBA and a normal response toward BALB/c. CPM, counts per minute; ***P < .001; N = 5 per group. (D) A group of mice received a second skin graft 58 days after initial transplantation. All BALB/c grafts were rejected within 10 days, whereas CBA grafts were accepted for more than 40 days without signs of rejection. N = 7 per group. (E) Photograph of a representative example at day 100 after first transplantation.
Discussion
In this study we explored the intrinsic apoptosis pathway as a novel pharmacological target to induce mixed chimerism and allograft tolerance. We observed a critical role of the proapoptotic Bcl-2 family member Bim for deletional tolerance using costimulation blockade, and we therapeutically exploited this finding using the BH3-mimetic ABT-737 to establish a new irradiation- and myelosuppression-free protocol to induce mixed chimerism with a moderate dose of BM cells.
The tolerization of the peripheral T-cell compartment in mixed chimerism induction protocols based on costimulation blockade depends on the complex regulation of apoptosis in T cells after antigen recognition.13 Focusing on the intrinsic pathway, the initial upregulation of Bim is counteracted by a simultaneous regulation of antiapoptotic factors and is required for T-cell activation.28,31 Three to 4 days after antigen-recognition stimuli by costimulatory molecules and interleukins are required to maintain cell survival by a downregulation of Bim and an upregulation of Bcl-xL.32,33 In the absence of an adequate antiapoptotic signal through costimulation, Bim prevails and activated T cells die by apoptosis.
Bcl-2 inhibitors offer the opportunity to selectively interact with these mechanisms. In our model, ABT-737 promoted peripheral T-cell tolerization by at least 2 complementary effects. First, it reduced the precursor frequency by a peripheral depletion of lymphocytes. Second, it led to a more efficient clonal deletion of alloantigen-reactive T cells by directly boosting the critical role of Bim in combination with anti-CD154. Additional effects of Bcl-2 inhibitors on regulatory T cells cannot be excluded. The beneficial effect of the combination of ABT-737 with CsA is multifactorial. CsA potentiated the proapoptotic effect of ABT-737 on lymphocytes and blocked the upregulation of A1, thereby preventing resistance to ABT-737 in T cells after antigen recognition.22,23 On the other hand, ABT-737 reversed the antitolerogenic effect of CsA by compensating the missing upregulation of Bim after allostimulation under calcineurin blockade (Figure 3). As a result, the combination of CsA and ABT-737 resulted in a paradoxical synergistic effect. This finding is of critical clinical relevance: because graft lost because of acute rejection cannot be ethically accepted in the modern transplantation era, a clinically applicable tolerance induction protocol has to foresee a combination of initial standard immunosuppressive and tolerance-induction regimes in a preventive or therapeutic setting, ideally without the a priori exclusion of calcineurin inhibitors.34
A short induction phase with ABT-737, CsA, and MR1 induced a complete deletion of peripheral donor-reactive T cells and allowed the engraftment of a sufficient number of donor-derived stem cells to maintain a durable mixed hematopoietic chimerism. Notably, because hematopoietic stem cells express high levels of Mcl-1,35 ABT-737 does not induce apoptosis in this cell population and is not expected to “create space” in stem cell niches, a factor that was originally thought to be required for BM engraftment.36,37 A significant level of chimerism without myelosuppressive conditioning was previously achieved using megadoses of BM, but has never been reported using clinically applicable BM doses.29 Because our conditioning protocol was sufficient to induce a stable level of chimerism >5% with moderate doses of BM, we speculate that ABT-737 and CsA might promote BM engraftment by influencing the complex interaction of immunological, stromal, and stem cells in the stem cell niche38 or by promoting the physiological niche recycling.39 These aspects might assume a general relevance for BM transplantation and the underlying mechanisms merit further investigation.
The sustained detection of donor-derived granulocytes over more than 8 months clearly indicated that engraftment of donor stem cells or very early progenitor cells had occurred. However, the level of hematopoietic chimerism was not uniform among different cell lineages with a complete absence of donor-derived B cells and higher levels of granulocytes. This could be explained by the engraftment of myeloid-biased hematopoietic stem cells.40,41 The donor-derived hematopoiesis was sufficient to maintain a pool of donor-derived antigen-presenting cells in the thymus to establish central deletional tolerance and—in a clinical perspective—may be favorable, because it reduces the risk of graft-vs-host disease.
The tolerance induction protocol described here provides a solution to several problems currently precluding a broad clinical application of the mixed chimerism approach. The general toxicity of the conditioning regimen is low in comparison with protocols including myelosuppressive drugs or TBI.4 In phase I clinical trials, ABT-263 displayed a favorable toxicity profile, and its application for a short conditioning therapy seems to be adequate also for nonmalignant conditions.21 Depleting antibodies—notably more efficient in mice than in humans42 —are not required, but a blockade of the CD40/CD154 pathway was critical in our model. The recent report of antibodies blocking CD40 may represent an ideal solution for blocking this pathway without the thromboembolic side effect reported in primates after exposure to anti-CD154.43-46 Importantly, in contrast to previous reports of mixed chimerism induction without myelosuppression,29,47 our conditioning protocol was successful using a clinically relevant dose of BM cells and without additional cell-based therapy.
In summary, we established a novel and reliable approach to induce mixed chimerism and allograft tolerance by pharmacological modulation of the intrinsic apoptosis pathway. This approach allowed induction of mixed chimerism using a nontoxic, nonmyelosuppressive conditioning protocol with potential clinical applicability.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Andreas Strasser and Martin Hausmann for providing the Bim−/− mouse, Anne-Marie Schmitt-Verhulst for the BM3.3 mouse and the Ti98 antibody, and Markus Manz for critical review of the manuscript.
The project was supported by the Swiss National Science Foundation (grant 323530-133893 to P.E.C., grant 310000-121979 to T.F.) and by the Olga Mayenfisch Stiftung.
Authorship
Contribution: P.E.C. and T.F. designed and performed experiments and wrote the paper; J.C., S.S.G., and A.K.K. performed experiments; A.B. and T.W. provided important scientific input; P.D.B., A.G., and T.W. provided important reagents; and R.P.W. supervised the project.
Conflict-of-interest disclosure: P.D.B. is an employee of Abbott, which developed and provided ABT-737; however, no financial sponsoring was received for this study. The remaining authors declare no competing financial interests.
Correspondence: Thomas Fehr, Division of Nephrology, University Hospital, Rämistrasse 100, CH-8091 Zürich, Switzerland; e-mail: thomas.fehr@uzh.ch.