Key Points
Anticoagulants inhibit release of angiogenic proteins from platelets.
Abstract
Platelets are a reservoir for angiogenic proteins that are secreted in a differentially regulated process. Because of the propensity for clotting, patients with malignancy are often anticoagulated with heparin products, which paradoxically offer a survival benefit by an unknown mechanism. We hypothesized that antithrombotic agents alter the release of angiogenesis regulatory proteins from platelets. Our data revealed that platelets exposed to heparins released significantly decreased vascular endothelial growth factor (VEGF) in response to adenosine 5′-diphosphate or tumor cells (MCF-7 cells) and exhibited a decreased angiogenic potential. The releasate from these platelets contained decreased proangiogenic proteins. The novel anticoagulant fondaparinux (Xa inhibitor) demonstrated a similar impact on the platelet angiogenic potential. Because these anticoagulants decrease thrombin generation, we hypothesized that they disrupt signaling through the platelet protease-activated receptor 1 (PAR1) receptor. Addition of PAR1 antagonists to platelets decreased VEGF release and angiogenic potential. Exposure to a PAR1 agonist in the presence of anticoagulants rescued the angiogenic potential. In vivo studies demonstrated that platelets from anticoagulated patients had decreased VEGF release and angiogenic potential. Our data suggest that the mechanism by which antithrombotic agents increase survival and decrease metastasis in cancer patients is through attenuation of platelet angiogenic potential.
Introduction
Cancer cells are surrounded by and interact with a complex milieu consisting of but not limited to endothelial cells, mast cells, macrophages, stromal cells, and lymphocytes. In fact, tumor cells live in intimate symbiosis with the rest of the body and appear to hijack normal physiological processes to aid their progression and growth. The recognition that tumor growth and metastasis is not exclusively an independent process for tumor cells suggests that disrupting the tumor microenvironment might provide a novel treatment modality for malignancy.
Although platelets are best known for their role in hemostasis and thrombosis, a substantial amount of data supports the idea that platelets play important roles in tumorigenesis, contributing to inflammation, angiogenesis, and metastatic dissemination of tumor cells.1 Platelet count can be a prognostic factor in cancer, patients presenting with thrombocytosis having a poor survival in a variety of cancers including breast cancer.2-5 Conversely, the presence of thrombocytopenia is associated with a survival benefit and decreased metastasis.6-9 For tumors to grow beyond 1 to 2 mm3, they must establish their own blood supply through angiogenesis, and there is substantial evidence that angiogenesis is regulated by platelets.10-12 In malignancy, platelets are the major serum source of many potent proangiogenic proteins, including more than 80% of circulating vascular endothelial growth factor (VEGF).13,14 Platelets may aid cancer cells in completing their journey to metastatic sites in a variety of ways, including coating tumor cells to help them evade the immune system, shielding tumor cells from high shear forces, aggregating tumor cells and platelets to embolize to new extravasation sites, and facilitating the adhesion of tumor cells to the vascular endothelium.15 Tumor cells are involved in platelet activation and aggregation, and a baseline level of platelet activation has been established in cancer.16,17 Tumor cells can aggregate platelets, and this ability correlates with the tumor’s metastatic potential.18-22
The association between hemostasis and malignancy was first recognized by Armand Trousseau in 1865.23 This relationship is strengthened by the observation that treatment with anticoagulants can improve survival.24-26 Several preclinical studies have shown that unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) have many anticancer properties that are not dependent on their anticoagulant functions.27-29 Because of the role that platelets play in both hemostasis/thrombosis and cancer, it is reasonable to hypothesize that one mechanism by which anticoagulants affect cancer outcome is by modulating platelet function. The direct effect of anticoagulants on the ability of platelets to regulate angiogenesis and tumorigenesis has not been determined.30-38 In most preclinical studies, the anticancer effects of LMWH and UFH did not affect the primary tumor directly, but rather reflected interference in the metastatic pathway.30-33
One of the mechanisms by which heparin regulates hemostasis is through thrombin inactivation. Thrombin, a potent platelet agonist, is essential for platelet and tumor cell interactions, initiating aggregation, adhesion, and metastasis formation.39 Secretion of thrombin from human tumor cells also directly activates platelets and recruits them to participate in tumor cell–mediated platelet responses.17 Several studies have shown that thrombin-induced activation of proteinase-activated receptors (PARs) on platelets can stimulate platelets to release angiogenic factors.40,41 In addition, thrombin is also generated in response to platelet activation by the agonist adenosine 5′-diphosphate (ADP), which results in subsequent activation of the PAR1 receptor.42 Therefore, anticoagulants could impact tumor cell and platelet interaction by inhibiting thrombin and interfering with PAR-mediated platelet activation. The importance of PAR blockade in angiogenesis was previously demonstrated by Ma and colleagues, who showed that PAR1 inhibition diminished angiogenic-dependent wound healing.43 Our group also previously revealed that direct platelet activation by PAR1 modulates differential platelet release of the proangiogenic protein VEGF with concurrent retention of the antiangiogenic protein endostatin.44,45 Thus, anticoagulants may diminish release of angiogenic proteins from platelets through modulation of thrombin-mediated PAR1 activation.
In this manuscript, we explored the impact of UFH, LMWH, and the Xa inhibitor fondaparinux on platelets’ angiogenic potential. To analyze this interaction both physiologically and pathologically, we examined the role of anticoagulants in platelets that have been activated with the agonist ADP and also exposed to MCF-7 tumor cells (a breast cancer cell line), respectively. We also demonstrated that one mechanism by which anticoagulants may impact platelet release of angiogenic proteins is through inhibition of thrombin-dependent PAR1 activation. Our in vitro observations are further supported by clinical data demonstrating inhibition of platelet-mediated angiogenesis in anticoagulated patients.
Methods
Preparation of resting platelets
Human blood collection was performed in accordance with the Declaration of Helsinki and ethics regulations with institutional review board approval. Platelets were isolated from 10 healthy volunteers as described.12 Volunteers did not ingest aspirin or nonsteroidal anti-inflammatory drugs for at least 10 days prior to blood collection. Platelet number was counted by fluorescence-activated cell sorting and adjusted to 2 × 108/mL. The resting state of platelets was confirmed by P-selectin antibody (BD Biosciences, San Jose, CA) labeling on flow cytometry. Determination of PAR1 cleavage by thrombin was demonstrated using SPAN12 monoclonal antibody (Beckman Coulter, Miami, FL) labeling by flow cytometry. In vitro anticoagulant exposure was performed by pretreating platelet-rich plasma (PRP) with UFH (1 U/mL), LMWH (dalteparin 5 U/mL), or fondaparinux (0.77 μg/mL). All anticoagulants were diluted in platelet buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 140 mM NaCl, 3 mM KCl, 0.5 mM MgCl2, 5 mM NaHCO3, 10 mM glucose). Platelets were washed extensively in wash buffer (140 mM NaCl, 5 mM KCl, 12 mM trisodium citrate, 10 mM glucose, 12.5 mM sucrose, pH = 6.0) to remove the remaining anticoagulant.
Activation of platelets
Release of α granule content was examined in vitro in response to 25 μM ADP (Biodata, Horsham, PA), TFLLR-NH2 (PAR1 agonist, 8 μM; Tocris Bioscience), ML161 (PAR1 antagonist, 10 μM; gift of Dr Robert Flaumenhaft), or exposure to 3 × 106/mL MCF-7 cells (ATCC, Manassas, VA). Platelets were exposed to agonist for 10 minutes at 37°C prior to collecting the releasate or processing for immunofluorescence microscopy.
Immunofluorescence microscopy
Mouse anti-VEGF antibody was obtained from Laboratory Vision (Fremont, CA) and rabbit anti-endostatin antibody was the generous gift of Vascular Biology, Children’s Hospital. Platelet immunofluorescence labeling was performed as previously described, and samples were analyzed on a Nikon TE 2000 Eclipse microscope equipped with a Nikon 100X/1.4 NA objective and a 100-W mercury lamp.12 Images were acquired with a Hamamatsu (Bridgewater, NJ) Orca IIER CCD camera and analyzed using Molecular Devices Metamorph software (Downington, PA).
Angiogenic protein quantification
VEGF and endostatin concentrations were determined in triplicate using the Quantikine human enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN) using 200 μL of platelet releasate. Concentrations of VEGF and endostatin were corrected for platelet count, and statistical significance was determined using the Student t test.
Angiogenesis antibody array
The RayBio Human Angiogenesis Antibody C-1000 (RayBiotech Inc., Norcross, GA) was screened according to the protocol.
Capillary tube formation assay
Capillary tube formation was used to assess the angiogenic potential of releasates generated from resting platelets or platelets stimulated with agonists using the Millipore Capillary Tube Formation Assay kit (Billerica, MA) in duplicate. Primary human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Walkersville, MD) and cultured in EBM media (Lonza) according to their protocol. After a 6-hour incubation of HUVEC cells with platelet releasate, capillary tube formation was quantified. Five fields were imaged (at ×4 and ×20 magnification) with differential-interference-contrast microscopy using a Zeiss Axiovert microscope, and the degree of tubulogenesis was quantified by counting branch points (nodes with 3 or more branches). Independent assays were averaged, and statistical analysis was performed using the Student t test.
HUVEC migration
The bottom chamber of a transwell plate (Corning Inc., Corning, NY) was precoated with 0.15% gelatin. HUVECs in serum-free media were seeded in the media, and 1 × 104/mL cells were inoculated into the upper chamber of each transwell with the releasate from 2 × 108/mL platelets generated under experimental conditions placed in the bottom chamber. After 24 hours of incubation, the cells were fixed and stained with Diff-quik (Siemens, Newark, DE), and the cells on the bottom of the filters were counted in 3 microscope fields. The results are shown as the number of cells that migrated to the bottom of the filter. Each experiment was performed in duplicate. Results from independent assays were averaged, and statistical analysis was performed using the Student t test.
Results
Release of VEGF from platelets is decreased by anticoagulants
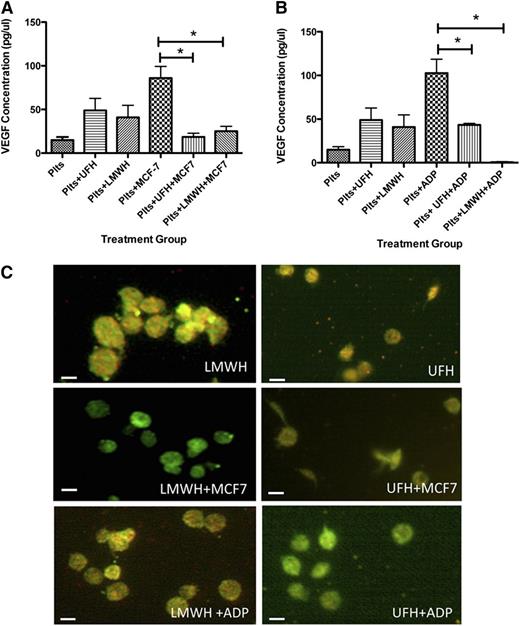
We previously demonstrated that platelets exposed to the platelet agonist ADP or stimulated by MCF-7 cells are induced to release the proangiogenic protein VEGF and retain the antiangiogenic protein endostatin.10 To determine whether anticoagulants could block the preferential release of angiogenesis regulatory proteins from human platelets, we pretreated PRP with heparin drugs (UFH or LMWH) prior to isolating platelets. Platelets were then washed, exposed to MCF-7 cells, and assayed for release of proangiogenic VEGF. Platelets exposed to anticoagulants prior to exposure to MCF-7 cells demonstrated significantly decreased VEGF release vs baseline exposure to MCF-7 cells (Figure 1A). At baseline, resting platelets (not exposed to MCF-7 cells) contained 14.89 ± 3.607 pg/μL of VEGF in their releasate. Platelets exposed to MCF-7 cells released significantly more VEGF; 85.9 ± 13.7 pg/μL VEGF was present in the releasate. Platelets pretreated with UFH without subsequent exposure to MCF-7 cells had 48.95 ± 13.79 pg/μL of VEGF in their releasate, whereas the platelets pretreated with UFH and then exposed to MCF-7 cells released only 18.6 ± 4.19 pg/μL of VEGF. Similar results were observed when platelets were pretreated with LMWH; platelets pretreated with LMWH released 40.8 ± 13.9 pg/μL of VEGF, and this did not increase in the presence of MCF-7 cells (25.1 ± 5.56 pg/μL of VEGF).
UFH and LMWH suppress VEGF release from human platelets after exposure to MCF-7 cells or ADP. (A) VEGF concentration in releasate generated from platelets resting or exposed to MCF-7 cells after exposure to UFH or LMWH. (B) VEGF concentration in the releasate generated from platelets activated with 25 μM ADP after exposure to UFH or LMWH. (C) Immunofluorescence of platelets resting, exposed to MCF-7 cells, or activated with 25 μM ADP after exposure to UFH or LMWH and labeled with endostatin (red) or VEGF (green). *P < .05. Bars represent 2 μM.
UFH and LMWH suppress VEGF release from human platelets after exposure to MCF-7 cells or ADP. (A) VEGF concentration in releasate generated from platelets resting or exposed to MCF-7 cells after exposure to UFH or LMWH. (B) VEGF concentration in the releasate generated from platelets activated with 25 μM ADP after exposure to UFH or LMWH. (C) Immunofluorescence of platelets resting, exposed to MCF-7 cells, or activated with 25 μM ADP after exposure to UFH or LMWH and labeled with endostatin (red) or VEGF (green). *P < .05. Bars represent 2 μM.
Similarly, platelets exposed to anticoagulants prior to stimulation with the physiological agonist ADP demonstrated significantly decreased VEGF release. Platelets exposed to ADP released 90.17 ± 15.1 pg/μL of VEGF (Figure 1B), whereas exposure to heparins significantly dampened the VEGF release; platelets pretreated with UFH or LMWH prior to activation with ADP released 43.33 ± 1.79 pg/μL and 0.6 pg/μL ± 0.633 of VEGF, respectively. These results suggest that the exposure of platelets to anticoagulants such as UFH or LMWH prior to activation with the physiological and pathological agonists ADP and MCF-7 tumor cells significantly reduces the release of VEGF.
To verify that the VEGF was retained and endostatin was released from the platelets upon activation with ADP or exposure to MCF-7 cells, we performed immunofluorescence labeling of VEGF and endostatin, which showed that VEGF (labeled green) was retained, whereas endostatin (labeled red) was released in the presence of both LMWH and UFH (Figure 1C).
Anticoagulants dramatically reduce the proangiogenic potential of platelets
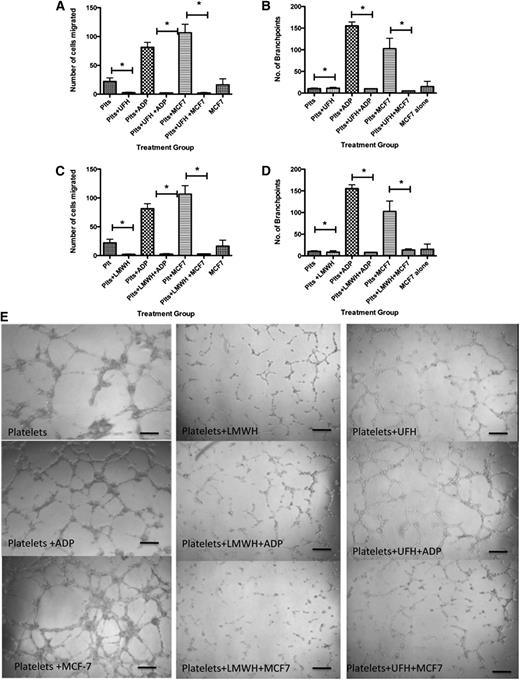
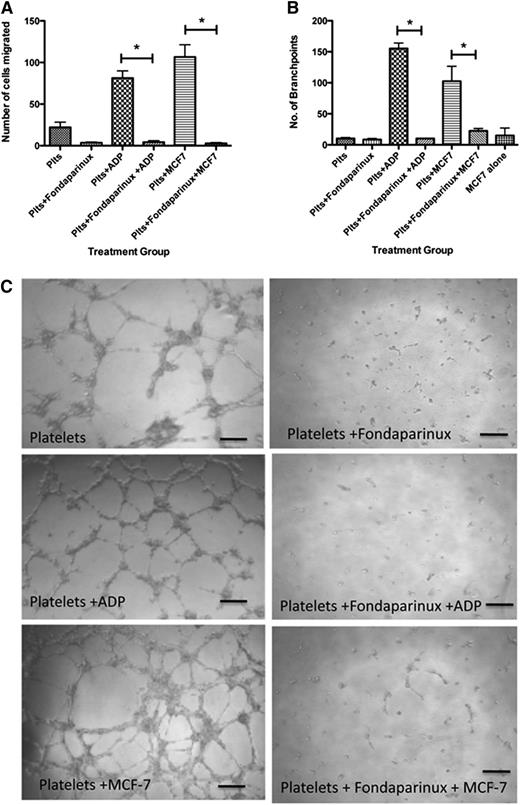
To examine the physiological relevance of our findings, we analyzed the capacity of specific platelet releasates to promote angiogenesis. Based on the decreased amount of VEGF in releasates generated by pretreatment with anticoagulants, we hypothesized that platelets exposed to anticoagulants prior to stimulation with MCF-7 cells would yield a decreased net angiogenic potential. To test this, we used a variety of known angiogenesis assays including the endothelial cell migration assay and the capillary tube formation assay. Releasates from platelets alone (no exposure to anticoagulants) or after exposure to ADP or MCF-7 cells yielded 22.11 ± 6.22, 81.25 ± 8.64, and 106.6 ± 14.7 migrating cells, respectively (Figure 2A). This response was significantly decreased by pretreatment with anticoagulants. Platelets exposed to UFH at rest had 2.66 ± 0.870 migrating cells, whereas those exposed to ADP after UFH had 2.278 ± 0.503 migrating cells, and in the presence of MCF-7 cells, only 2.22 ± 0.790 cells migrated (Figure 2A). Similarly, exposure to LMWH resulted in only 2.11 ± 0.522 cells having migrated at rest, addition of ADP after pretreatment with LMWH yielded only 2.57 ± 0.934 migrating cells, and exposure to MCF-7 in the presence of LMWH yielded 2.85 ± 0.524 migrating cells (Figure 2C). Thus, pretreatment with anticoagulants significantly decreased the migratory potential of endothelial cells in this assay.
UHF and LMWH inhibit platelet-mediated angiogenic response. (A) Endothelial cell migration after exposure to the releasate from control, resting platelets (Plts) or platelets activated with 25 μM ADP after exposure to UFH. (B) Quantification of branch points generated by the releasate from resting platelets or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to UFH. (C) Endothelial cell migration after exposure to the releasate from control, resting platelets (Plts) or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to LMWH. (D) Quantification of branch points generated by the releasate from resting platelets or platelets activated with 25 μM ADP after exposure to LMWH. (E) Representative images of capillary tube formation generated from the releasate of resting platelets or platelets exposed to ADP or MCF-7 cells with or without LMWH or UFH. *P < .05. Bars represent 100 μM.
UHF and LMWH inhibit platelet-mediated angiogenic response. (A) Endothelial cell migration after exposure to the releasate from control, resting platelets (Plts) or platelets activated with 25 μM ADP after exposure to UFH. (B) Quantification of branch points generated by the releasate from resting platelets or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to UFH. (C) Endothelial cell migration after exposure to the releasate from control, resting platelets (Plts) or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to LMWH. (D) Quantification of branch points generated by the releasate from resting platelets or platelets activated with 25 μM ADP after exposure to LMWH. (E) Representative images of capillary tube formation generated from the releasate of resting platelets or platelets exposed to ADP or MCF-7 cells with or without LMWH or UFH. *P < .05. Bars represent 100 μM.
To further interrogate the angiogenic potential of these releasates, capillary tube formation assays were performed. Platelets alone produced 10.27 ± 1.4 branch points, whereas platelets stimulated with ADP or MCF-7 cells produced 155.3 ± 8.9 and 102.5 ± 24.1 branch points, respectively (Figure 2B). There was significant suppression in the number of branch points in samples exposed to UFH or LMWH prior to activation with ADP or MCF-7 cells. In platelets treated with UFH, 11.3 ± 2.028 branch points were seen. Platelets exposed to UFH prior to activation with ADP produced 10.0 ± 0.0 branch points, and cells exposed to MCF-7 cells after exposure to UFH produced 5.00 ± 0.0 branch points (Figure 2B). Platelets treated with LMWH formed 8.40 ± 3.4 branch points, whereas those exposed to LMWH prior to exposure to ADP formed 8.00 ± 0.57 branch points and those exposed to LMWH prior to MCF-7 formed 13.60 ± 2.67 branch points (Figure 2D). Representative images depicting the decreased branch point formation in the presence of LMWH and UFH are shown in Figure 2E. These results indicate the potent ability of anticoagulants to decrease the angiogenic potential of platelets, as measured by both endothelial cell migration and capillary tube formation.
Releasates from platelets pretreated with LMWH demonstrated decreased proangiogenic protein content
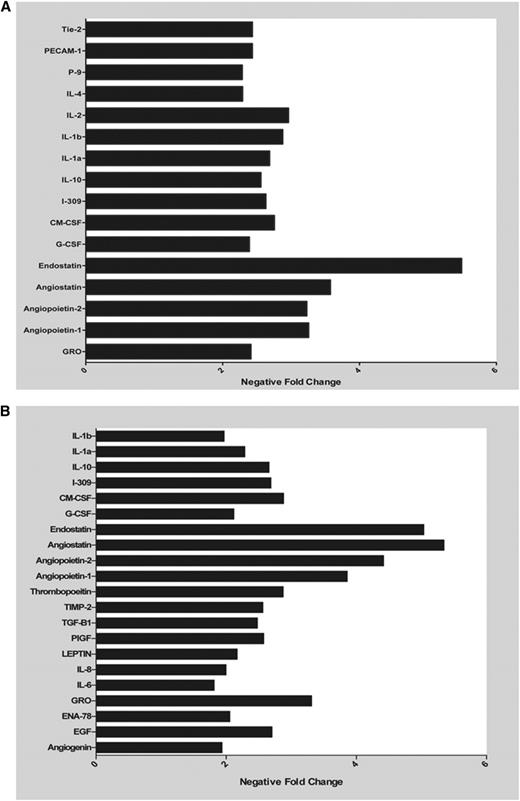
Because the net potential of releasates from platelets pretreated with LMWH was antiangiogenic, we wanted to assess the specific angiogenic proteins that elicit this response in the releasate of platelets pretreated with LMWH and then exposed to ADP or MCF-7 cells (Figure 3 and supplemental Figure 1; see the Blood Web site). Release of angiogenic proteins was detected using angiogenesis antibody arrays. After preincubation with LMWH and stimulation with ADP, there was a decrease in the concentration of proangiogenic proteins, making the overall milieu of the releasate less proangiogenic (Figure 3A). Similar results were seen in the releasate from platelets pretreated with LMWH prior to exposure to MCF-7 cells (Figure 3B).
LMWH decreases platelet release of angiogenic proteins. Representative graph of angiogenic factors that were found to have a 1.5-fold decrease as measured in the releasate from platelets exposed to MCF-7 cells (A) or ADP (B) with and without prior exposure to LMWH.
LMWH decreases platelet release of angiogenic proteins. Representative graph of angiogenic factors that were found to have a 1.5-fold decrease as measured in the releasate from platelets exposed to MCF-7 cells (A) or ADP (B) with and without prior exposure to LMWH.
Releasates from platelets exposed to fondaparinux also had a decreased angiogenic potential
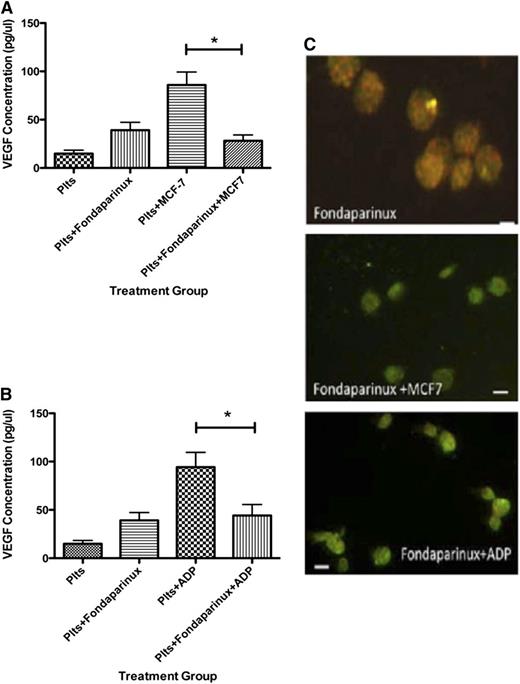
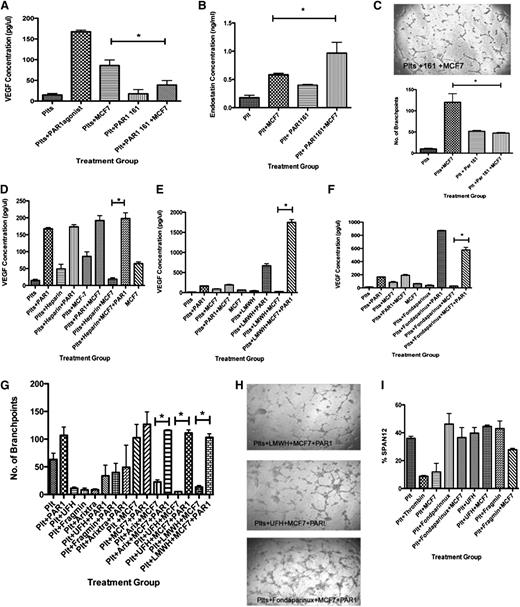
Next, we asked if the Xa inhibitor fondaparinux could similarly suppress the platelet angiogenic potential. Exposure to fondaparinux prior to MCF-7 cells significantly decreased VEGF in comparison with platelets exposed to MCF-7 cells alone, resulting in 28.08 ± 5.97 pg/μL of VEGF (Figure 4A). Similarly, platelets pretreated with fondaparinux prior to exposure to ADP released 44.20 ± 11.33 pg/μL. This was a statistically significant decrease in VEGF release compared with ADP-stimulated platelets without exposure to fondaparinux (Figure 4B).
Fondaparinux suppresses VEGF release from human platelets after exposure to MCF-7 cells or ADP. (A) VEGF concentration in the releasate generated from platelets resting or exposed to MCF-7 cells after exposure to fondaparinux. (B) VEGF concentration in the releasate generated from platelets activated with 25 μM ADP after exposure to fondaparinux. (C) Immunofluorescence of platelets resting, exposed to MCF-7 cells, or activated with 25 μM ADP after exposure to fondaparinux and labeled with endostatin (red) or VEGF (green). *P < .05. Bars represent 300 μM.
Fondaparinux suppresses VEGF release from human platelets after exposure to MCF-7 cells or ADP. (A) VEGF concentration in the releasate generated from platelets resting or exposed to MCF-7 cells after exposure to fondaparinux. (B) VEGF concentration in the releasate generated from platelets activated with 25 μM ADP after exposure to fondaparinux. (C) Immunofluorescence of platelets resting, exposed to MCF-7 cells, or activated with 25 μM ADP after exposure to fondaparinux and labeled with endostatin (red) or VEGF (green). *P < .05. Bars represent 300 μM.
Treatment with fondaparinux prior to exposure to ADP or MCF-7 cells shifted the angiogenic potential to become antiangiogenic. Using the releasate from platelets that were exposed to fondaparinux, only 3.5 ± 0.922 endothelial cells migrated. Adding ADP to the platelets pretreated with fondaparinux resulted in 4.33 ± 1.706 migrating cells (Figure 5A). Exposing platelets to MCF-7 cells after fondaparinux yielded only 2.667 ± 0.988 migrating endothelial cells. Capillary tube assays supported the results of the endothelial migration experiments. Platelets pretreated with fondaparinux had suppressed branch point formation; releasate from platelets exposed to fondaparinux produced 8.60 ± 1.60 branch points. Pretreatment with fondaparinux prior to exposure to ADP or MCF-7 cells resulted in 10.33 ± 0.333 or 22.6 ± 3.8 branch points (Figure 5B-C). These results clearly show that the angiogenic potential of platelets pretreated with the anticoagulant fondaparinux is suppressed.
Fondparinux inhibits platelet-mediated angiogenic response. (A) Endothelial cell migration after exposure to the releasate from control, resting platelets (Plts) or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to fondaparinux. (B) Quantification of branch points generated by the releasate from resting platelets or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to fondaparinux. (C) Representative images of capillary tube formation generated from the releasate of resting platelets or platelets exposed to ADP or MCF-7 cells with or without fondaparinux. *P < .05. Bars represent 100 μM.
Fondparinux inhibits platelet-mediated angiogenic response. (A) Endothelial cell migration after exposure to the releasate from control, resting platelets (Plts) or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to fondaparinux. (B) Quantification of branch points generated by the releasate from resting platelets or platelets activated with 25 μM ADP or stimulation with MCF-7 cells after exposure to fondaparinux. (C) Representative images of capillary tube formation generated from the releasate of resting platelets or platelets exposed to ADP or MCF-7 cells with or without fondaparinux. *P < .05. Bars represent 100 μM.
Platelet angiogenic potential is inhibited by PAR1 antagonist and rescued by addition of PAR1 agonist
Previously, it has been shown that platelets activated with the thrombin receptor PAR1 preferentially release VEGF and retain endostatin.12,43 In our system, 2 potential sources of thrombin include the MCF-7 cells, which secrete thrombin, and the platelets themselves.46,47 We therefore explored if suppression of VEGF release from platelets exposed to anticoagulants was mediated through mechanistic inhibition of PAR1 activation. To investigate if PAR1 disruption by anticoagulants could account for the inhibition of tumor cell/platelet–mediated angiogenesis, we repeated our experiments in the presence of direct PAR1 antagonists. As predicted, in the presence of the PAR1 antagonist, release of VEGF was suppressed. When platelets were preincubated with the PAR1 antagonist prior to exposure to MCF-7 cells, the amount of VEGF released decreased to 38.9 ± 10.76 (Figure 6A). In addition, the amount of endostatin released reciprocally increased; in the presence of the PAR1 antagonist, there was 0.540 ± 0.025 ng/μL of endostatin in the releasate from platelets incubated with MCF-7 cells vs 0.9670 ± 0.1905 ng/μL with exposure to the PAR1 antagonist prior to MCF-7 cells (Figure 6B). In the presence of the PAR1 antagonist, the overall angiogenic potential was decreased; capillary tube formation was suppressed similarly to results with anticoagulants (Figure 6C).
PAR1 antagonists decrease the platelet angiogenic potential. (A) VEGF concentration in releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with PAR1 antagonist 161. (B) Quantification of branch points generated by the releasate from resting platelets or platelets exposed to MCF-7 cells with and without preincubation with PAR1 antagonists. (C) Representative images of capillary tube formation generated from the releasate of resting platelets or platelets exposed to MCF-7 cells with and without preincubation with PAR1 antagonists. *P < .05. Bar represent 100 mm. (D) VEGF concentration in the releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with UFH and PAR1 agonist, TFLLR-NH2. (E) VEGF concentration in the releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with LMWH and PAR1 agonist, TFLLR-NH2. (F) VEGF concentration in the releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with fondaparinux and PAR1 agonist, TFLLR-NH2. (G) Quantification of branch points generated by the releasate from resting platelets or platelets exposed to MCF-7 cells with and without preincubation with PAR1 agonist TFLLR-NH2. (H) Representative images of capillary tube formation. (I) To determine if the anticoagulants disrupted cleavage of PAR1, the percentage of uncleaved PAR1 on the surface of the platelet was measured using the antibody SPAN12 by flow cytometry.
PAR1 antagonists decrease the platelet angiogenic potential. (A) VEGF concentration in releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with PAR1 antagonist 161. (B) Quantification of branch points generated by the releasate from resting platelets or platelets exposed to MCF-7 cells with and without preincubation with PAR1 antagonists. (C) Representative images of capillary tube formation generated from the releasate of resting platelets or platelets exposed to MCF-7 cells with and without preincubation with PAR1 antagonists. *P < .05. Bar represent 100 mm. (D) VEGF concentration in the releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with UFH and PAR1 agonist, TFLLR-NH2. (E) VEGF concentration in the releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with LMWH and PAR1 agonist, TFLLR-NH2. (F) VEGF concentration in the releasate generated from the platelets resting or exposed to MCF-7 cells with and without preincubation with fondaparinux and PAR1 agonist, TFLLR-NH2. (G) Quantification of branch points generated by the releasate from resting platelets or platelets exposed to MCF-7 cells with and without preincubation with PAR1 agonist TFLLR-NH2. (H) Representative images of capillary tube formation. (I) To determine if the anticoagulants disrupted cleavage of PAR1, the percentage of uncleaved PAR1 on the surface of the platelet was measured using the antibody SPAN12 by flow cytometry.
To determine whether PAR1 signaling was disrupted by the presence of anticoagulants, we demonstrated that addition of PAR1 agonist to anticoagulated samples rescues tumor cell/platelet–mediated angiogenesis. PRP was exposed to UFH, LMWH, or fondaparinux prior to MCF-7 cells, as described, prior to addition of the PAR1 agonist TFLLR-NH2 and concurrent exposure to MCF-7 cells. For all 3 anticoagulants, addition of PAR1 agonist in the presence of MCF-7 cells led to a significant increase in VEGF release (Figure 6D-F). The amount of VEGF measured from platelets exposed to MCF-7 cells with PAR1 agonist in the presence of UFH was 198.0 ± 28.78 pg/μL; for LMWH, the VEGF concentration was 1749 ± 120.5 pg/μL; and for fondaparinux, the VEGF concentration was 576.3 ± 70.1 pg/μL.
Because the VEGF concentration was increased by addition of PAR1 agonist to anticoagulated platelet samples, we hypothesized that the angiogenic potential would correspondingly be rescued. For all 3 anticoagulants, addition of PAR1 agonist in the presence of MCF-7 cells led to a significant increase in capillary tube formation in comparison with the releasate from anticoagulated platelets exposed to MCF-7 cells without addition of PAR1 agonist (Figure 6F-G).
To specifically determine whether or not signaling through the PAR1 receptor was disrupted by the presence of anticoagulants, the cleavage of PAR1 receptor was determined by flow cytometry using the SPAN12 antibody, which recognizes uncleaved PAR1, as described previously (Figure 6I).48 As expected, PAR1 is not cleaved in resting platelets, whereas addition of thrombin causes a decrease in the percentage of uncleaved PAR1. Platelets exposed to MCF-7 cells also demonstrate a decrease in the percentage of uncleaved PAR1. Interestingly, addition of anticoagulants suppressed the ability of MCF-7 cells to induce PAR1 cleavage. This is likely attributable to inhibition of MCF-7–derived thrombin. These data demonstrate that anticoagulants suppress PAR1 signaling by inhibiting its activation on the platelet surface.
Anticoagulants modulate the platelet angiogenic balance in the clinical setting
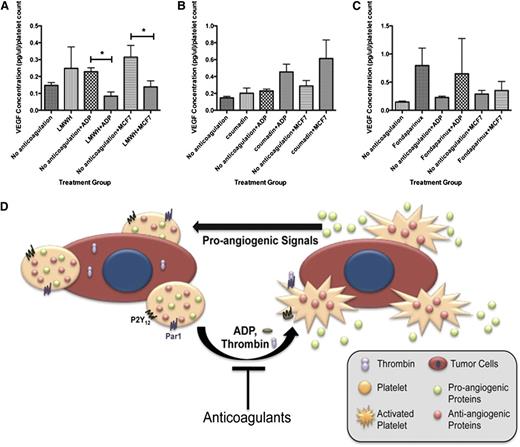
Currently, there are millions of individuals who require anticoagulation for a variety of conditions including atrial fibrillation, venous thromboembolic events, stroke, and hypercoagulable states. To determine whether our in vitro observations could be extended to the clinical environment, we asked whether platelets from patients medicated with a variety of oral and parenteral anticoagulants exhibited angiogenic suppression. These patients were enrolled from the Brigham and Women’s and Dana Farber Benign Hematology Clinics. All patients were on anticoagulants for management of venous thromboembolic disease and had no known evidence of malignancy at the time of enrollment. They were not taking antiplatelet agents. Patients anticoagulated with LMWH at therapeutic concentrations exhibited a suppression of VEGF release after exposure to ADP or MCF-7 cells, supporting our hypothesis that exposure to anticoagulants such as LMWH suppressed VEGF release from platelets, resulting in reduced angiogenic potential (Figure 7). Suppression of VEGF release was not seen when the patients were exposed to the oral anticoagulant warfarin (Figure 7B). Suppression of VEGF release was, however, less robust in patients anticoagulated with fondaparinux (Figure 7C).
Exposure to anticoagulants in vivo decreases platelet release of VEGF. (A) VEGF concentration in the releasate generated from the platelets of patients in response to activation with ADP or exposure to MCF-7 cells with and without LMWH anticoagulation. (B) VEGF concentration in the releasate generated from the platelets of patients in response to activation with ADP or exposure to MCF-7 cells with and without coumadin anticoagulation. (C) VEGF concentration in the releasate generated from the platelets of patients in response to activation with ADP or exposure to MCF-7 cells with and without fondaparinux anticoagulation. (D) Schematic model of tumor cell and platelet communication and impact on angiogenesis. In our model, platelets come in contact with tumor cells in the vasculature, which activates the platelets to release angiogenic proteins that can then affect tumor cell growth, the impact of which is diminished in the presence of anticoagulants (n = 5 for each treatment group).
Exposure to anticoagulants in vivo decreases platelet release of VEGF. (A) VEGF concentration in the releasate generated from the platelets of patients in response to activation with ADP or exposure to MCF-7 cells with and without LMWH anticoagulation. (B) VEGF concentration in the releasate generated from the platelets of patients in response to activation with ADP or exposure to MCF-7 cells with and without coumadin anticoagulation. (C) VEGF concentration in the releasate generated from the platelets of patients in response to activation with ADP or exposure to MCF-7 cells with and without fondaparinux anticoagulation. (D) Schematic model of tumor cell and platelet communication and impact on angiogenesis. In our model, platelets come in contact with tumor cells in the vasculature, which activates the platelets to release angiogenic proteins that can then affect tumor cell growth, the impact of which is diminished in the presence of anticoagulants (n = 5 for each treatment group).
Discussion
We have shown that anticoagulants, including UFH, LMWH, and fondaparinux, can disrupt the angiogenic balance of platelets’ releasate (Figure 7D). Exposure to all of these agents leads to a shift toward a more antiangiogenic releasate upon platelet activation. Because the majority of patients are currently anticoagulated in the outpatient setting using LMWH, and the initial survival advantage studies used LMWH as the anticoagulant of choice, we chose to focus on LMWH for this investigation. Using releasate from platelets that were pretreated with LMWH prior to exposure to ADP or MCF-7 cells, we were able to reveal an overall dimunition in proangiogenic protein release in these platelets. This suggests that the presence of anticoagulant resulted in a more antiangiogenic platelet releasate.
In order to measure the angiogenic potential of releasate generated in the presence of anticoagulation, we employed 2 established methods of measuring angiogenic potential: endothelial cell migration and capillary tube formation. Using these methods, it was clear that pretreatment of platelets with anticoagulant substantially decreased the angiogenic potential. Previously, it has been shown that anticoagulants including heparin can have an impact on endothelial cell growth and migration independently.49 In our experiments, however, the anticoagulant exposure occurred for a designated period of time (referred to as preincubation), after which the platelets were extensively washed to remove exogenous anticoagulant prior to platelet stimulation and releasate collection. This suggests that the anticoagulants directly affected the platelets themselves and were not responsible for a more general effect. The impact of the anticoagulant on the platelet itself was further demonstrated by the shift in angiogenic protein release.
Next, we explored the impact of anticoagulation on platelet releasate in vivo. To examine the angiogenic potential of platelets in the setting of anticoagulation, platelets were analyzed from patients who were currently therapeutically anticoagulated. When standardized to platelet count, it was clear that the presence of anticoagulant decreased the amount of VEGF measured in the platelet releasate. This occurred in response to activation with the physiological agonist ADP or stimulation with pathological agonist MCF-7 cells. Interestingly, the same effect was not seen when the patients were anticoagulated with warfarin. This suggests a thrombin-dependent mechanism.
Differential release of angiogenic proteins has also been seen with the thrombin receptors PAR1 and PAR4.12,43 One of the main angiogenic proteins that is differentially released upon activation with PAR1 is proangiogenic VEGF, whereas activation with PAR4 leads to release of antiangiogenic endostatin. Previously, heparin has been shown to inhibit thrombin-mediated platelet activation through disruption of PAR1 cleavage and internalization.48,50 Our data demonstrate that in platelets exposed to PAR1 antagonists, tumor cell/platelet–mediated angiogenic potential is disrupted and can be rescued by the addition of PAR1 agonists. This suggests that one mechanism by which anticoagulants may limit the angiogenic potential of platelet and tumor cell interaction is through inhibiting PAR1-mediated platelet release of proangiogenic factors such as VEGF. Similar mechanisms may account for the anticoagulant effect when the platelets are activated with ADP. Recently, Jiang and colleagues demonstrated that platelets activated with ADP generate thrombin, which propagates platelet activation through thrombin-mediated PAR1 activation.42 Platelets exposed to anticoagulants prior to activation with ADP also exhibit impaired thrombin/PAR1 signaling. Therefore, PAR1 antagonists may represent a novel therapeutic target for regulation of tumor growth and metastasis. The effects of these medications on metastasis and survival in breast cancer patients have yet to be explored.
This manuscript further elucidates the interplay between breast cancer cells and platelets and defines the role of antithrombotic agents in platelet-regulated tumor angiogenesis. Understanding platelet and tumor cell communication, as well as its modulation by anticoagulation, may lead to targeted drug design regulating platelet-mediated angiogenesis as a means of limiting metastatic disease progression in breast cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jean Connors and Ms Lisa Stewart for help with patient recruitment.
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (K08HL097070) (E.M.B.) (HL68130 and PO1 CA045548-21) (J.E.I.), Susan G. Komen for the Cure (E.M.B.), Karin Grunebaum Research Foundation (E.M.B.), and the Malcolm Hecht Jr. Hematology Research Fund (E.M.B.).
Authorship
Contribution: E.M.B. designed and performed the research, analyzed the data, and wrote and revised the manuscript; B.A.M., K.R.M., and R.A.K. performed experiments; R.F. provided expertise related to PAR1 experiments; and J.E.I. provided mentorship for the group and expertise in preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabeth M. Battinelli, Division of Hematology, Department of Internal Medicine, Brigham and Women’s Hospital, Karp, Fifth Floor, 1 Blackfan Circle, Boston, MA 02115; e-mail:ebattinelli@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal