Key Points
Lysosomal storage affects NK-cell frequency, development, and function.
Lysosomal calcium is important for NK-cell degranulation.
Abstract
Niemann-Pick type C (NPC) is a neurodegenerative lysosomal storage disorder caused by defects in the lysosomal proteins NPC1 or NPC2. NPC cells are characterized by reduced lysosomal calcium levels and impaired sphingosine transport from lysosomes. Natural killer (NK) cells kill virally infected/transformed cells via degranulation of lysosome-related organelles. Their trafficking from lymphoid tissues into the circulation is dependent on sphingosine-1-phosphate (S1P) gradients, sensed by S1P receptor 5 (S1P5). We hypothesized that NK-cell function and trafficking could be affected in NPC disease due to the combined effects of the lysosomal calcium defect and sphingosine storage. In an NPC1 mouse model, we found the frequency of NK cells was altered and phenocopied S1P5-deficient mice, consistent with defects in S1P levels. NK cells from NPC1 mice also had a defect in cytotoxicity due to a failure in degranulation of cytotoxic granules, which was associated with reduced lysosomal calcium levels. Affected NPC1 patients and NPC1 heterozygote carriers had reduced NK-cell numbers in their blood and showed similar phenotypic and developmental changes to those observed in the NPC1 mouse. These findings highlight the effects of lysosomal storage on the peripheral immune system.
Introduction
Lysosomal storage diseases are inherited metabolic diseases caused by defects in lysosomal enzymes, transporters, channels, or regulatory proteins.1 Niemann-Pick type C (NPC) disease is a neurodegenerative lysosomal storage disease with heterogeneous presentation including seizures, ataxia, dysarthria, and dysphagia leading to premature death in childhood or young adulthood.2 Inflammation is present in the central nervous system (CNS) and influences disease progression.3-5 Mouse models of NPC disease phenocopy the human disorder and serve as authentic models of human disease. Treatment of an NPC mouse model with anti-inflammatory therapies improved function and lifespan,6 implicating inflammation as an active contributor to pathogenesis. Because there is communication between the peripheral immune system and the CNS, changes in the peripheral immune system might influence CNS inflammation, as has been reported in other neurodegenerative disorders.7
NPC disease is caused by mutations in 1 of 2 genes: NPC1 (95% of cases) or NPC2.8 Because clinical disease and cellular phenotypes are the same irrespective of the gene mutated, it has been suggested that these proteins function in the same pathway.9 The NPC1 gene encodes a transmembrane protein of the limiting lysosomal membrane, whereas the NPC2 gene encodes a soluble lysosomal cholesterol-binding protein.10 Dysfunction of the NPC1 protein leads to a lysosomal calcium defect in which the store fails to fill, causing reduced calcium release, which in turn blocks the fusion between late endosomes and lysosomes and leads to the storage of multiple lipids.11 NPC1 is involved in the efflux of sphingosine from the lysosome, which could impact sphingosine-1-phosphate (S1P) levels, as demonstrated by reduced cellular S1P levels after NPC1 inactivation.11
We hypothesized that natural killer (NK) cell biology may be altered in NPC1 disease due to the potential reduction in S1P gradients and defects in acidic store calcium filling11 resulting in defective lysosome-related organelle degranulation. NK cells are lymphocytes that play an important role in the early response to viral infection by directly killing infected or transformed cells via the release of lysosome-related organelles.12 NK cells develop in the bone marrow from the common lymphoid precursor, and the earliest committed NK-cell precursors are identified by their expression CD122.13,14 As with other lymphocyte lineages, NK cells migrate in response to S1P gradients, with lower concentrations found within lymphoid tissues and higher concentrations in circulating extracellular fluids.15 In contrast to T and B cells, which use the S1P receptor 1 (S1P1),15 NK cells sense S1P gradients via S1P receptor 5 (S1P5).16 In mice lacking S1P5, NK cells are “trapped” in the lymph nodes and bone marrow and are consequently depleted in the blood, spleen, and lungs.16 In addition to S1P5 expression, NK-cell tissue distribution is also influenced by chemokine receptors.17
We have found that the frequency, maturation, and phenotype of NK cells from the NPC1 mouse are altered compared with control animals and the frequency phenocopied which has been reported for the S1P5 knockout mouse. Similar alterations in frequency and phenotype were also identified in NPC1 patients and to a lesser extent in heterozygous carriers of the NPC1 mutation. Furthermore, NK cells from the NPC1 mouse demonstrated defective cytotoxicity, which was the result of reduced lysosome calcium content/release of NPC1 NK cells. These findings have important clinical implications for the treatment and management of NPC1 patients and also identify NK cells as a novel clinical biomarker for NPC disease.
Materials and methods
Animals
The NPC1 mouse BALB/cNctr-Npc1m1N/J (The Jackson Laboratory, Charles River, UK), was maintained by heterozygote brother/sister matings and genotyped as previously described.18 All animal studies were approved by the UK Home Office (Animal Scientific Procedures Act, 1986).
Cell preparation
Single-cell suspensions of lymph nodes (pooled inguinal), spleen, and bone marrow were prepared according to standard procedures. For lung and liver, they were obtained following saline perfusion and digested with collagenase prior to Percoll discontinuous gradient (67.5%/44%) to isolate lymphocytes. Blood was collected by cardiac puncture, red blood cells were lysed, and the equivalent of 100 μL whole blood was used for staining.
Human blood samples
Venous blood was collected into EDTA tubes and mononuclear cells were isolated using Histopaque 1077. All samples were obtained with informed consent/assent in accordance with the Declaration of Helsinki and ethical approval from participating centers.
NK cytotoxicity
Single-cell suspensions were prepared from the spleen of 5-week-old animals and activated for 6 days with 500 U/mL human interleukin-2 (IL-2; Peprotech) in complete medium. YAC-1 target cells were labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester and were added to 96-well round-bottom plates at effector to target ratios indicated for 4 hours at 37°C. The cells were washed twice with ice-cold fluorescence-activated cell sorter buffer and then resuspended in 200 μL Live/Dead far red (Invitrogen). Cells were fixed with BD Cell Fix prior to acquisition, where 2000 target cells were collected.
Mouse NK-cell degranulation and IFN-γ production
Splenocytes were prepared and activated as above. Degranulation and interferon-γ (IFN-γ) assays were performed as previously described,16 with full details provided in the supplemental Methods (available on the Blood Web site).
Single-cell calcium determination
Single-cell suspensions were prepared from 5-week-old animals. NK cells were enriched by negative selection using a cocktail of antibodies (CD4, CD8, major histocompatibility complex class II, CD19, CD45R, and TER119). NK cells were used immediately for single-cell Ca2+ imaging, loaded with 5 μM fluo-4/AM (Invitrogen) plus 0.03% Pluronic F127 in RPMI 1640 for 30 minutes at room temperature, and mounted on the stage of a Zeiss LSM510 Meta confocal laser-scanning microscope (Ex 488 nm, Em >505 nm) equipped with a ×40 objective. Experiments were conducted in RPMI 1640 at room temperature with an image collected every 1 to 5 seconds. The fluorescence of single cells was measured and expressed as fold changes over basal (F/F0). Calcium release was achieved with the application of glycyl-l-phenylalanine-2-napthylamide (GPN; Sigma-Aldrich) followed by ionomycin (Calbiochem). NK cells were identified at the end of the run by labeling with 1 μg/mL Alexa 647–conjugated anti-NKp46 antibody with only the NKp46+ cells included for intracellular [Ca2+] measurements.
Staining and flow cytometry analysis
Samples were blocked with 1 μg mouse BD FC Block (mouse samples) or 5% normal mouse serum (human samples) for 10 minutes prior to addition of multicolor antibody cocktails using titrated amounts to give saturating binding; dead cells were excluded using Live/Dead aqua (Invitrogen). All samples were acquired on a FACSCanto II (BD Biosciences). For all mouse and human phenotyping, gating was established using fluorescence minus 1 controls with the appropriate isotype control added. All samples were analyzed using FlowJo unless otherwise stated. For human T, B, and NK-cell (TBNK) analysis, whole-blood samples were tested with BD 6 color TBNK kit with TruCount tubes according to the manufacturer’s instructions. The instrument was set up using BD 7 color setup beads, and samples were acquired and analyzed using BD FACSCanto clinical software.
Lipid analysis
Pooled inguinal lymph nodes were homogenized in phosphate-buffered saline and a protein assay was performed. For mass spectrometry (MS) determination of lipid content, proteins were precipitated with methanol and the supernatant subjected to analysis using online column trapping liquid-chromatography tandem MS (API-4000 spectrometer; Applied Biosystems, Foster City, CA) with internal standards and sphingolipid standards for calibration obtained from Matreya (Pleasant Gap, PA) and Avanti Polar Lipids (Alabaster, AL) (details in the supplemental Methods).
Statistical analysis
Data were analyzed using Prism v4 (Graph Pad) statistical methods as indicated in the figure legends.
Results
Altered NK-cell frequency in NPC1 mouse model
NK cells were identified using NKp46,19 due to the BALB/c background, defined as NKp46+CD3−CD19− and determined as the percentage of CD45+ cells. The percentages of NK cells in the bone marrow, spleen, lymph node, liver, lung, and blood showed progressive changes with disease progression in Npc1−/− mice compared with controls (supplemental Figure 1). At end stage (10 weeks), the alterations to the NK-cell percentage were identical to that observed previously in S1P5-deficient mice,16 with increased NK cells in the bone marrow and lymph node and decreased NK cells in spleen, lung, and blood (Figure 1A). In addition, we determined the absolute number of NK cells in the spleen and lymph node and observed that the alterations in NK-cell percentages were mirrored in modified NK-cell numbers (Figure 1B).
NK-cell percentage and number in multiple organs from control and NPC1 animals at end stage. (A) NK cells as a percentage of CD45+ live cells and (B) NK-cell number per spleen or per 2 inguinal lymph nodes. Data were gated on forward scatter height (FSC-H) vs forward scatter area (FSC-A) singlets and dead cells were excluded. CD45+ cells were then selected and NK cells defined at NKp46+CD3− cells within that gate. Clear bars represent control Npc1+/+ animals and solid bars represent affected Npc1−/− animals. Data are presented as mean ± standard error of the mean (SEM), n = 3 to 10 animals with 2 independent determinations of NK-cell frequency per animal, or n = 5 for NK-cell number. *P < .05, **P < .01, and ***P < .001, calculated with the Mann-Whitney U test using GraphPad Prism v4.
NK-cell percentage and number in multiple organs from control and NPC1 animals at end stage. (A) NK cells as a percentage of CD45+ live cells and (B) NK-cell number per spleen or per 2 inguinal lymph nodes. Data were gated on forward scatter height (FSC-H) vs forward scatter area (FSC-A) singlets and dead cells were excluded. CD45+ cells were then selected and NK cells defined at NKp46+CD3− cells within that gate. Clear bars represent control Npc1+/+ animals and solid bars represent affected Npc1−/− animals. Data are presented as mean ± standard error of the mean (SEM), n = 3 to 10 animals with 2 independent determinations of NK-cell frequency per animal, or n = 5 for NK-cell number. *P < .05, **P < .01, and ***P < .001, calculated with the Mann-Whitney U test using GraphPad Prism v4.
To determine if the altered NK cell distribution was due to alterations in S1P gradients, we performed lipidomic analysis on inguinal lymph nodes from NPC1 mice. The almost 10-fold elevation in sphingosine was not mirrored by a comparable increase in S1P, consistent with sphingosine trapped in the lysosome unavailable for phosphorylation. The level of S1P was also increased 3-fold in NPC1 lymph nodes (Table 1), suggesting S1P may be trapped in the tissue and could result in a defect in establishing the gradient between the lymphatic fluid and the lymph node resulting in the failure of NK cells to migrate.
Sphingolipid and glycosphingolipid content of control and NPC1 lymph nodes
| Lipid content (ng/mg protein) . | SPH . | S1P . | Charged GSLs . | Neutral GSLs . |
|---|---|---|---|---|
| Control | 5.26 ± 0.17 | 0.21 ± 0.01 | 13.3 | 63.0 |
| NPC1 | 53.54 ± 4.53 | 0.57 ± 0.09 | 14.4 | 88.2 |
| Lipid content (ng/mg protein) . | SPH . | S1P . | Charged GSLs . | Neutral GSLs . |
|---|---|---|---|---|
| Control | 5.26 ± 0.17 | 0.21 ± 0.01 | 13.3 | 63.0 |
| NPC1 | 53.54 ± 4.53 | 0.57 ± 0.09 | 14.4 | 88.2 |
Liquid-chromatography tandem MS data were normalized to protein content of pooled inguinal lymph nodes; average values ± SD from 2 replicate experiments are shown (glycosphingolipid determinations were performed only once). Internal standards were used for the quantitation of lipids.
SPH, sphingosine.
Altered NK-cell development and phenotype in NPC1 mouse model
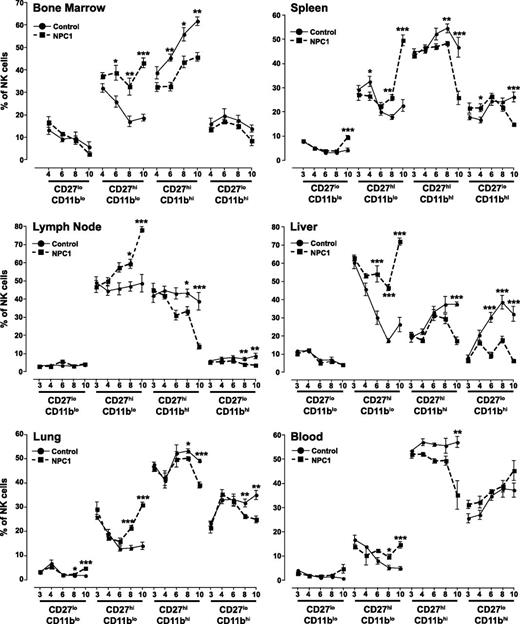
Using CD27 and CD11b, 4 developmental subsets of NK cells can be defined20 (supplemental Figure 2). In bone marrow, the percentage of immature CD27hiCD11blo NK cells was increased in Npc1−/− animals, and consequently the percentage of CD27hiCD11bhi NK cells were reduced (Figure 2). In lymph node, the percentage of CD27hiCD11blo NK increased in Npc1−/− animals with age, with a significant increase over Npc1+/+ animals at 8 and 10 weeks of age. Concomitantly, the percentage of more mature CD27hiCD11bhi and CD27loCD11bhi NK cells were decreased in Npc1−/− animals at 8 and 10 weeks of age (Figure 2). At 4 weeks of age, Npc1−/− spleens contained more mature CD27loCD11bhi and less immature CD27hiCD11blo NK cells (Figure 2). However, at 8 and 10 weeks, the NK cells in the Npc1−/− spleen shifted to a more immature phenotype (Figure 2). Liver NK cells from 6-week-old Npc1−/− animals were increased in CD27hiCD11blo cells with a decrease in the CD27hiCD11bhi and CD27loCD11bhi frequency (Figure 2). Lung NK cells from Npc1−/− mice had a higher frequency of more immature CD27loCD11blo and CD27hiCD11blo cells and a decreased frequency of mature CD27hiCD11bhi and CD27loCD11bhi NK cells at 8 and 10 weeks (Figure 2). Similarly, in blood, there was a shift to a higher frequency of more immature CD27hiCD11blo NK cells at 8 and 10 weeks and decreased CD27hiCD11bhi NK cells in Npc1−/− animals at 10 weeks compared with controls (Figure 2).
Development of mouse NK cells determined by CD27 and CD11b expression in multiple mouse organs. NK cells were identified as FSC-H vs FSC-A singlet and viable cells and then as CD45+NKp46+CD3− cells. Quadrant gates were set using fluorescence minus 1 with isotype controls (for example, see supplemental Figure 2).Circles represent control Npc1+/+ animals and squares represent affected Npc1−/− animals; ages are ±1 day. Data are presented as mean ± SEM, n = 3 to 7 animals.*P < .05, **P < .01, and ***P < .001, calculated by an unpaired t test comparing to the age-matched controls, using GraphPad Prism v4.
Development of mouse NK cells determined by CD27 and CD11b expression in multiple mouse organs. NK cells were identified as FSC-H vs FSC-A singlet and viable cells and then as CD45+NKp46+CD3− cells. Quadrant gates were set using fluorescence minus 1 with isotype controls (for example, see supplemental Figure 2).Circles represent control Npc1+/+ animals and squares represent affected Npc1−/− animals; ages are ±1 day. Data are presented as mean ± SEM, n = 3 to 7 animals.*P < .05, **P < .01, and ***P < .001, calculated by an unpaired t test comparing to the age-matched controls, using GraphPad Prism v4.
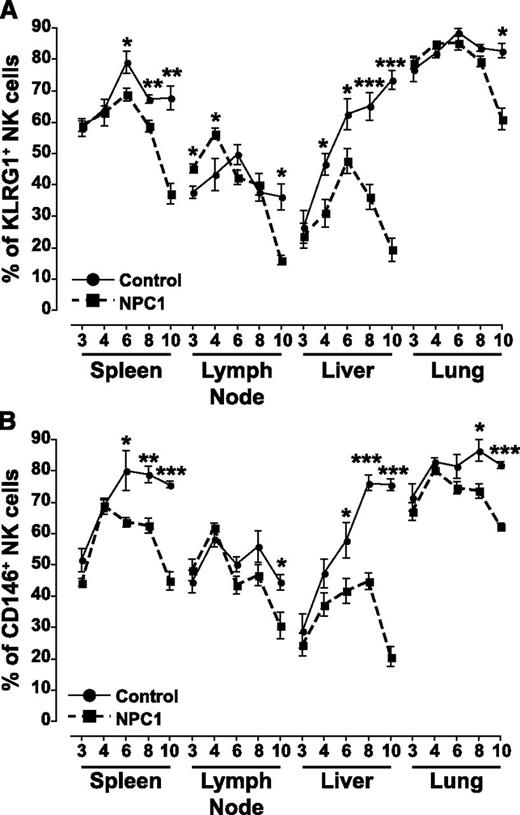
Two additional markers were used to assess the development of NK cells, killer cell lectin-like receptor G1 (KLRG1)21 and CD146,22 and a similar trend was observed with both markers. NK cells from the spleen of Npc1−/− animals expressed significantly less KLRG1 compared with control animals from 6 weeks of age (Figure 3A) and had decreased numbers of CD146+ NK cells from 6 weeks of age (Figure 3B). In the lymph node at early time points in development, there was an increase in KLRG1+ NK cells in Npc1−/− animals, but at 10 weeks the percentage of KLRG1+ NK cells (Figure 3A) and CD146+ NK cells (Figure 3B) was decreased. In the liver, from 4 weeks of age the percentage of KLRG1+ NK cells was decreased in Npc1−/− animals (Figure 3A), and from 6 weeks of age the percentage of CD146+ NK cells were decreased in Npc1−/− animals (Figure 3B). Finally, in the lung, KLRG1+ NK cells were decreased at 10 weeks of age (Figure 3A) and CD146+ NK cells from 8 weeks of age in Npc1−/− animals (Figure 3B).
Expression of developmental markers on NK cells from multiple organs. NK cells were identified as FSC-H versus FSC-A singlet and viable cells and then as NKp46+CD3− cells. Positive gates were set using fluorescence minus 1 with isotype controls. Circles represent control Npc1+/+ mice and squares represent affected Npc1−/− mice; ages are ±1 day. Data are presented as mean ± SEM, n = 3 to 7 animals. *P < .05, **P < .01, and ***P < .001, calculated by an unpaired t test comparing to the age-matched controls, using GraphPad Prism v4.
Expression of developmental markers on NK cells from multiple organs. NK cells were identified as FSC-H versus FSC-A singlet and viable cells and then as NKp46+CD3− cells. Positive gates were set using fluorescence minus 1 with isotype controls. Circles represent control Npc1+/+ mice and squares represent affected Npc1−/− mice; ages are ±1 day. Data are presented as mean ± SEM, n = 3 to 7 animals. *P < .05, **P < .01, and ***P < .001, calculated by an unpaired t test comparing to the age-matched controls, using GraphPad Prism v4.
The expression of DX5 (CD49b), another indicator of NK-cell maturation,23 the IFN-γ–inducible chemokine receptor CXCR3,24 and a NK-cell activation marker, CD69, were also tested. These markers all demonstrated age-related changes in expression (supplemental Figure 3). Taken together, these results demonstrate that alongside the alteration to the frequency of NK cells in multiple tissues of NPC1 animals compared with controls, there are also age-related developmental and phenotypic alterations.
NK-cell function in NPC1 mice
We investigated the function of IL-2–activated splenic NK cells from NPC1 mice. Due to the differences in maturation stages, we performed the experiments on NK cells isolated from mice at 5 weeks of age when there was no difference in CD27 and CD11b expression relative to controls (data not shown). Using the prototypic NK-cell target, YAC-1 cells, we found a significant defect in cytotoxicity of IL-2–activated NK cells generated from Npc1−/− splenocytes (Figure 4A). Because we have shown that there is a decrease in the NK-cell percentage in the spleens of Npc1−/− mice after 6 weeks, we tested the frequency of NKp46+CD3− cells and used this to normalize the values. Even after this adjustment, there was still a significant decrease in the cytotoxicity of the NK cells derived from Npc1−/− mice (supplemental Figure 4).
Functional assays and calcium levels in NK cells derived from mouse spleen. (A) Killing of YAC-1 cells by NK cells prepared from control Npc1+/+ or affected Npc1−/− mouse splenocytes. YAC-1 cells were identified as carboxyfluorescein diacetate succinimidyl ester (CFSE)bright, and specific killing was determined using the following equation at the effector to target ratio indicated: (dead YAC-1 cells with NK cells) − (dead YAC-1 cells with no NK cells). (B-D) NK cells were identified as NKp46+CD3−, doublet events were excluded by FSC-H vs FSC-A gating, and a viability dye was included to exclude dead cells from the analysis. (B) Degranulation of NK cells from control Npc1+/+ and Npc1−/− animals in response to stimuli in the presence of monensin was calculated according to the following equation: (% of CD107a+ NK cells with stimuli) − (% of CD107a+ unstimulated NK cells). (C) Intracellular IFN-γ production by NK cells from control Npc1+/+ and Npc1−/− animals in response to stimuli was calculated according to the following equation: (% of IFN-γ+ NK cells with stimuli) − (% of IFN-γ+ unstimulated NK cells). (D) Representative traces showing intracellular [Ca2+] changes monitored in single fluo-4–loaded NK cell, normalized to initial fluorescence (F/F0). Lysosomal calcium content was assessed upon addition of 50 μM GPN which lyses cathepsin-containing acidic intracellular calcium stores. The addition of ionomycin at the end of each experiment was used to confirm viability of the cells. (E) Maximal peak fluorescence changes were determined as the difference between basal and the maximum fluorescence, Δ(F/F0), upon addition of 50 μM GPN. Data are presented as the mean ± SEM, n = 3 animals for each group; 158 Npc1+/+ cells and 125 Npc1−/− cells. Clear bars represent control Npc1+/+ animals and solid bars represent affected Npc1−/− animals. (F) Degranulation of NK cells from control Npc1+/+ and Npc1−/− animals in response to PMA/iono in the presence or absence of monensin was determined as described for panel B. Data are presented as mean ± SEM, n = 4 to 6 animals. *P < .05, **P < .01, and ***P < .001, calculated by an unpaired t test using GraphPad Prism v4.
Functional assays and calcium levels in NK cells derived from mouse spleen. (A) Killing of YAC-1 cells by NK cells prepared from control Npc1+/+ or affected Npc1−/− mouse splenocytes. YAC-1 cells were identified as carboxyfluorescein diacetate succinimidyl ester (CFSE)bright, and specific killing was determined using the following equation at the effector to target ratio indicated: (dead YAC-1 cells with NK cells) − (dead YAC-1 cells with no NK cells). (B-D) NK cells were identified as NKp46+CD3−, doublet events were excluded by FSC-H vs FSC-A gating, and a viability dye was included to exclude dead cells from the analysis. (B) Degranulation of NK cells from control Npc1+/+ and Npc1−/− animals in response to stimuli in the presence of monensin was calculated according to the following equation: (% of CD107a+ NK cells with stimuli) − (% of CD107a+ unstimulated NK cells). (C) Intracellular IFN-γ production by NK cells from control Npc1+/+ and Npc1−/− animals in response to stimuli was calculated according to the following equation: (% of IFN-γ+ NK cells with stimuli) − (% of IFN-γ+ unstimulated NK cells). (D) Representative traces showing intracellular [Ca2+] changes monitored in single fluo-4–loaded NK cell, normalized to initial fluorescence (F/F0). Lysosomal calcium content was assessed upon addition of 50 μM GPN which lyses cathepsin-containing acidic intracellular calcium stores. The addition of ionomycin at the end of each experiment was used to confirm viability of the cells. (E) Maximal peak fluorescence changes were determined as the difference between basal and the maximum fluorescence, Δ(F/F0), upon addition of 50 μM GPN. Data are presented as the mean ± SEM, n = 3 animals for each group; 158 Npc1+/+ cells and 125 Npc1−/− cells. Clear bars represent control Npc1+/+ animals and solid bars represent affected Npc1−/− animals. (F) Degranulation of NK cells from control Npc1+/+ and Npc1−/− animals in response to PMA/iono in the presence or absence of monensin was determined as described for panel B. Data are presented as mean ± SEM, n = 4 to 6 animals. *P < .05, **P < .01, and ***P < .001, calculated by an unpaired t test using GraphPad Prism v4.
To investigate the basis for the defective cytotoxicity, we measured degranulation and IFN-γ production from the NK cells in response to YAC-1 cells and pharmacologic activation. After activation with YAC-1 cells or phorbol 12-myristate 13-acetate/ionomycin (PMA/iono) there was a significant reduction in the number of CD107a+ NK cells from Npc1−/− mice (Figure 4B), indicating a reduction in the number of cytotoxic granules/secretory lysosomes that have fused with the cell surface. The total level of CD107a was the same in control Npc1+/+ and Npc1−/− NK cells after fixation and permeabilization (data not shown), suggesting that the decreased staining is not associated with a decreased amount of CD107a in Npc1−/− NK cells. There was no reduction in the number of IFN-γ+ NK cells after 3 different activation stimuli (Figure 4C), suggesting that the signaling pathways are intact and instead there is a specific defect with degranulation. In fact, using YAC-1 cells as a target, the Npc1−/− NK cells had a significant increase in intracellular IFN-γ production (Figure 4C).
Because lysosomes, in addition to the endoplasmic reticulum, can be a source of calcium release,25 we sought to determine if the decreased lysosomal calcium known to occur in Npc1−/− cells11 impacts granule exocytosis. In order to specifically assess the lysosomal calcium content of NK cells, we performed single-cell calcium assays on negatively isolated unstimulated NK cells. Lysosomal calcium stores were released by the application of GPN, which osmotically lyses cathepsin-containing lysosomes.26 An example trace from a single NK cell is shown in Figure 4D, where after the addition of GPN there was an increase in the fluorescence of the control Npc1+/+ NK cell indicative of lysosomal calcium release whereas the release was greatly decreased in the Npc1−/− NK cell. In total, 158 NK cells from 3 Npc1+/+ mice and 125 NK cells from 3 Npc1−/− mice were analyzed and demonstrated a significant decrease in the lysosomal calcium release from Npc1−/− NK cells (Figure 4E), consistent with previous studies of other NPC1 disease cell types.11 Finally, to assess the role of lysosomal calcium in NK-cell degranulation, we performed the degranulation experiment in the presence or absence of monensin to alter the intracellular stores that ionomycin can target for calcium release.27 In the absence of monensin, ionomycin can only release calcium from the endoplasmic reticulum and there was no difference in degranulation between Npc1+/+ and Npc1−/− NK cells (Figure 4F). However, in the presence of monensin (the same as for Figure 4B), when ionomycin can release calcium from both the endoplasmic reticulum and lysosomes, there was a large increase in the degranulation of NK cells from Npc1+/+ mice but no increase in Npc1−/− NK cells, resulting in a significant decrease in the number of cells that had degranulated (Figure 4F). These data indicate that lysosomal calcium contributes to the calcium release required for NK-cell cytotoxic granule exocytosis and that this is defective in NPC1 cells due to the decreased calcium content of lysosomes/lysosome-related organelles.
NK-cell frequencies in NPC1 patients and carriers
We then sought to determine whether similar NK-cell changes occur in NPC1 patients. Analysis of healthy controls showed that NK-cell frequencies change with age, in agreement with a previous report.28 Therefore, we stratified the data into birth to 18 years and 18 years and older. The frequency of circulating NK cells as percentage of CD45+ lymphocytes (Figure 5A) or absolute cell counts (Figure 5B) was decreased in NPC1 patients aged over 18 compared with age-matched controls. Interestingly, NK-cell numbers were also decreased in NPC1 heterozygotes (carrier parents aged >18 years) compared with controls older than 18 years (Figure 5A-B). Furthermore, when plotted as a function of age, the NK-cell percentage increased in healthy controls as previously reported,28 whereas NPC1 patients exhibited no expansion in NK-cell number and the regression lines were significantly different from healthy controls (Figure 5C-D). It was not possible to perform the same analysis on NPC1 heterozygotes because we only knew they were older than 18 years. There were no differences in other lymphocyte populations in either the NPC1 patients or NPC1 carriers compared with controls (supplemental Figures 5 and 6).
NK-cell frequencies in the peripheral blood of control individuals, NPC1 patients, and carrier parents. (A-D) NK cells were identified using a CD45+ lymphocyte gate and defined as CD16/56+CD3− using a clinical whole-blood TBNK monitoring assay. (A,C) NK-cell frequency as a percentage of CD45+ lymphocytes is depicted. (B,D) Absolute numbers of NK cells per microliter of blood is depicted. For all panels, each symbol indicates an individual sample with the line at the median. (A-B) *P < .05, **P < .01, and ***P < .001 calculated by a Mann-Whitney U test using GraphPad Prism v4. Circles represent control NPC1+/+, diamonds represent NPC1+/− carriers, and squares represent affected NPC1−/− blood samples. (C-D) Correlation lines were calculated by the Spearman method with the following R values: (C) control 0.3356 and NPC1 0.0523, (D) control 0.1698 and NPC1 −0.1661. het, heterozygous carriers.
NK-cell frequencies in the peripheral blood of control individuals, NPC1 patients, and carrier parents. (A-D) NK cells were identified using a CD45+ lymphocyte gate and defined as CD16/56+CD3− using a clinical whole-blood TBNK monitoring assay. (A,C) NK-cell frequency as a percentage of CD45+ lymphocytes is depicted. (B,D) Absolute numbers of NK cells per microliter of blood is depicted. For all panels, each symbol indicates an individual sample with the line at the median. (A-B) *P < .05, **P < .01, and ***P < .001 calculated by a Mann-Whitney U test using GraphPad Prism v4. Circles represent control NPC1+/+, diamonds represent NPC1+/− carriers, and squares represent affected NPC1−/− blood samples. (C-D) Correlation lines were calculated by the Spearman method with the following R values: (C) control 0.3356 and NPC1 0.0523, (D) control 0.1698 and NPC1 −0.1661. het, heterozygous carriers.
NK-cell phenotype in NPC1 patients and carriers
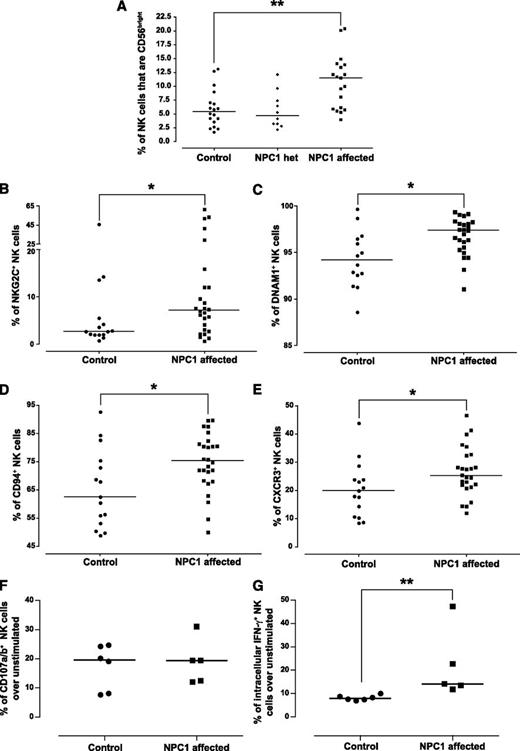
We evaluated a large panel of phenotypic markers combining all NPC1 patients into a single group, because we did not find any influence of age on expression (data not shown). Human NK cells in the blood are either CD56bright or CD56dim with differential capacity for cytokine production, cytotoxicity, and proliferation,29,30 with CD56bright NK cells reported to be a more immature developmental intermediate.31 The percentage of NK cells that were CD56bright was significantly higher in the blood of NPC1-affected patients than in controls (Figure 6A), although it is not possible to determine if this is a relative increase in CD56bright NK cells or a decrease in CD56dim NK cells. We found no difference in the expression of markers including CD25, NKp30, and NKp46 (data not shown). However, there was a significant increase in the percentage of NKG2C+ (Figure 6B), DNAM1+ (Figure 6C), CD94+ (Figure 6D), and CXCR3+ (Figure 6E) NK cells in NPC1 patients compared with controls. Taken together, NK cells in NPC1-affected patients exhibit altered developmental status. We performed functional characterization on a limited number of adult NPC1 patients and controls. There was no difference in degranulation in response to K562 cells relative to controls (Figure 6F). Although this does not reproduce the findings in the mouse model, it should be noted that these were adult NPC1 patients and hence have a milder phenotype. However, there was a significant increase in the percentage of IFN-γ+ NK cells after activation with IL-12 and IL-18 in these patient samples (P = .0043; Figure 6G). This could be linked with the increased percentage of CD56bright NK cells in NPC1 patients, because the CD56bright NK-cell population is the most significant producer of IFN-γ31 (supplemental Figure 7).
Phenotypic and functional characterization of human blood NK cells. (A-E) NK cells were identified using a CD45+ lymphocyte gate, doublets were excluded by FSC-H versus FSC-A gating, and dead cells were excluded via the use of a viability dye. NK cells were defined as CD56+CD3−. (A) The frequency of CD56bright NK cells was determined by plotting CD56 against CD16 and identifying CD56bright CD16dim cells. (B-E) The analysis was performed on total NK cells with no discrimination between CD56bright and CD56dim, with positive gates set using fluorescence minus 1 with isotype controls. (B) Percentage of NKG2C+ NK cells. (C) Percentage of DNAM1+ NK cells. (D) Percentage of CD94+ NK cells. (E) Percentage of CXCR3+ NK cells. (F) Degranulation of NK cells from control NPC1+/+ and affected NPC1−/− blood samples in response to K562 cells in the presence of monensin was calculated according to the following equation: (% of CD107a+ NK cells with stimuli) − (% of CD107a+ unstimulated NK cells). (G) Intracellular production of IFN-γ in NK cells from control NPC1+/+ and affected NPC1−/− blood samples in response to IL-12 and IL-18 simulation was calculated according to the following equation: (% of IFN-γ+ NK cells with stimuli) − (% of IFN-γ+ unstimulated NK cells). Circles represent control NPC1+/+, diamonds represent NPC1+/− carriers, and squares represent affected NPC1−/− blood samples. Each symbol indicates an individual sample with the line at the median; *P < .05 and **P < .01 calculated by the Mann-Whitney U test, using GraphPad Prism v4. het, heterozygous carriers.
Phenotypic and functional characterization of human blood NK cells. (A-E) NK cells were identified using a CD45+ lymphocyte gate, doublets were excluded by FSC-H versus FSC-A gating, and dead cells were excluded via the use of a viability dye. NK cells were defined as CD56+CD3−. (A) The frequency of CD56bright NK cells was determined by plotting CD56 against CD16 and identifying CD56bright CD16dim cells. (B-E) The analysis was performed on total NK cells with no discrimination between CD56bright and CD56dim, with positive gates set using fluorescence minus 1 with isotype controls. (B) Percentage of NKG2C+ NK cells. (C) Percentage of DNAM1+ NK cells. (D) Percentage of CD94+ NK cells. (E) Percentage of CXCR3+ NK cells. (F) Degranulation of NK cells from control NPC1+/+ and affected NPC1−/− blood samples in response to K562 cells in the presence of monensin was calculated according to the following equation: (% of CD107a+ NK cells with stimuli) − (% of CD107a+ unstimulated NK cells). (G) Intracellular production of IFN-γ in NK cells from control NPC1+/+ and affected NPC1−/− blood samples in response to IL-12 and IL-18 simulation was calculated according to the following equation: (% of IFN-γ+ NK cells with stimuli) − (% of IFN-γ+ unstimulated NK cells). Circles represent control NPC1+/+, diamonds represent NPC1+/− carriers, and squares represent affected NPC1−/− blood samples. Each symbol indicates an individual sample with the line at the median; *P < .05 and **P < .01 calculated by the Mann-Whitney U test, using GraphPad Prism v4. het, heterozygous carriers.
Discussion
NK cells are significantly altered in frequency, maturation status, phenotype, and function in NPC1 mice compared with age-matched controls. These observations are clinically relevant, because we observed a similar decrease in the frequency of NK cells in the blood of adult (>18 years) NPC1 patients compared with age-matched controls. We also found that NPC1 heterozygote carriers have decreased NK-cell numbers compared with controls, which is in agreement with previous findings from fibroblasts,32 suggesting that 50% NPC1 function is not sufficient to prevent cellular phenotypes developing.
NK cells isolated from the spleens of NPC1 mice demonstrated a defective ability to kill an NK-sensitive target cell. This defect was associated with an inability to release cytotoxic granules, as demonstrated by decreased CD107a externalization and linked to the decreased lysosomal calcium content that occurs in NPC1 cells, including NK cells. This is, to our knowledge, the first time that lysosomal calcium has been implicated in cytotoxic granule secretion in NK cells, although this is consistent with them being a specialized secretory lysosome.33 In agreement with this, cytotoxic granules in cytotoxic T cells (CTLs) were recently shown to be a source of calcium for their exocytosis.34 When NK cells contact their target, they form an immunologic synapse in an analogous manner to CTLs where the microtubule organizing center and Golgi apparatus become polarized.35 Calcium influx from an extracellular source has been shown to be essential for granule exocytosis from CTLs36 via store-operated calcium entry (SOCE), which requires the calcium-release–activated channel, ORAI1,37 and the intracellular-calcium–depletion sensor, STIM1.38 Recently, it has been demonstrated that SOCE, via ORAI1 and STIM1, is also required for cytotoxic granule secretion from human NK cells39 ; however, the requirements for murine NK cells have not been studied. Interestingly, it has previously been reported that treatment of NK cells with ammonium chloride, which would alter the pH gradient and deplete the lysosomes of calcium, greatly reduces the cytotoxic function of murine NK cells.40 Furthermore, other lysomotropic agents such as chloroquine and dansylcadaverine have also been demonstrated to decrease target-cell killing by both NK cells and CTLs.41 Therefore, the role of lysosomal calcium in cytotoxic granule release from both NK cells and CTLs is a new area of investigation. Functional assays on NK cells from human NPC1 patients and controls were performed on a limited number of donors. Degranulation after activation with K562 cells was not affected, but IFN-γ production was significantly increased in response to stimulation with IL-12 and IL-18 in NPC1 patients. Further investigation, in particular with more severe NPC1 mutations, will be of interest to further explore these findings.
The mechanisms causing the alterations in NK-cell frequencies in NPC1 mice and in NPC1 patients and heterozygotes remain to be fully dissected. They are likely linked to alterations in S1P gradients, although this will require studies in a larger animal model of NPC1, such as the feline.42 This is supported by the fact that at end stage, the NPC1 mouse NK distribution phenocopies that reported in S1P5 knockout mice.16 Furthermore, T- and B-cell frequencies are reduced in the blood of the NPC1 mouse at end stage but are not altered in blood samples from NPC1 patients (A.O.S. and F.M.P., unpublished data; supplemental Figures 5 and 6). A potential reason for this differential response of NK cells compared with T and B cells could be the very severe phenotype of the mouse relative to the patients and/or differential emergence of NK, T, and B cells during human development. In agreement with S1P alterations being a crucial factor, our lipidomic analysis demonstrates the storage of sphingosine and also S1P in the Npc1−/− lymph nodes compared with Npc1+/+ controls. The catabolism of ceramide to produce the sphingosine required for the generation of S1P can occur in several cellular compartments and in the extracellular space by the action of multiple pH-sensitive ceramidases.43 However, because sphingosine kinase is expressed in the cytosol, the majority of S1P is generated intracellularly and requires transport out of the cell to act on extracellular receptors.43 It has recently been shown that Spns2 is an S1P transporter essential for immune system function and that expression of Spns2 in endothelial cells is required for correct lymphocyte trafficking,44,45 but the results on the impact on S1P levels in the lymphatic fluid and plasma are conflicting.45,46 Furthermore, these studies indicate that Spns2 can transport sphingosine in addition to S1P,46 so it could be that the increased sphingosine competes with S1P for transport. It is also possible that a cell-intrinsic S1P signaling pathway could be disrupted.
A developmental defect was also identified in NK cells from both the NPC1 mouse and patients. In the murine model, we assessed the developmental status of NK cells in multiple tissues using a panel of markers and identified that the severity of the developmental abnormality was linked to disease progression. In human NPC1 patients, we identified an increase in the number of NK cells that expressed high levels of CD56; these CD56bright NK cells in the peripheral blood are believed to be more immature compared with their CD56dim counterparts.30 In addition, we also found that more NK cells from NPC1 patients expressed CD94 and CXCR3, markers that have been linked to developmental status and/or specialized subsets of human NK cells.31,47 The increased percentage of NKG2C+ NK cells in the blood of NPC1 patients is unexplained, although we do not know the cytomegalovirus status of our clinical samples. Further studies will be required to understand the causal factors behind the developmental defect of NK cells in NPC1 mice and humans. However, it is probable that many aspects are associated with disease progression/progressive lipid storage and inflammatory changes (F.M.P., unpublished observations).
These findings have important clinical implications, because NK cells in human NPC1 pediatric patients with severe disease may exhibit the same functional defect in cytotoxic granule secretion as occurs in the NPC1 mouse. This may result in an inability to efficiently clear infections, which could be linked to respiratory complications, often contributing to cause of death.48 Because inflammation in the periphery and the CNS are interrelated in neurodegenerative diseases,7,49 alterations to NK cells and inflammation in the periphery could also influence inflammation in the brain.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Claire Smith, Sian Pooley, and Denise Jelfs for technical assistance and Dr Grant Churchill for helpful discussions. They are also indebted to the NPC1 patients and their relatives who participated in this study.
A.O.S. is funded by the MRC (GO700851), D.t.V. by Action Medical Research, D.A.S. by SOAR-NPC, and L.C.D. and A.J.M. by the Wellcome Trust (084102/Z/07/Z). An equipment grant from the Wellcome Trust funded the flow cytometer (084631). F.M.P. is a Royal Society Wolfson Research Merit Award holder. This work was supported in part by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and a Bench to Bedside grant from the Office of Rare Diseases and the National Insitutes of Health Clinical Centre (F.D.P.). N.M.Y. was supported by APMRF and DART. This work was supported by a grant from DART (D.S.O.) and the Washington University Metabolomics Facility.
Authorship
Contribution: A.O.S. designed the study; performed, analyzed, and interpreted the data; performed statistical analysis; and wrote the manuscript; D.t.V., L.C.D., A.J.M., D.A.S., R.S., and H.F. performed research and collected data; N.M.Y., L.S., J.I., E.J., R.H., H.R., E.M., M.B., J.E.W., C.H., R.L., and F.D.P. obtained ethical permission, collected clinical samples, and provided data; C.C., D.S.O., A.G., and E.V. designed the study and interpreted data; and F.M.P. designed the study, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Frances Platt, Department of Pharmacology, University of Oxford, Mansfield Rd, Oxford, OX1 3QT, United Kingdom; e-mail: frances.platt@pharm.ox.ac.uk.


![Figure 4. Functional assays and calcium levels in NK cells derived from mouse spleen. (A) Killing of YAC-1 cells by NK cells prepared from control Npc1+/+ or affected Npc1−/− mouse splenocytes. YAC-1 cells were identified as carboxyfluorescein diacetate succinimidyl ester (CFSE)bright, and specific killing was determined using the following equation at the effector to target ratio indicated: (dead YAC-1 cells with NK cells) − (dead YAC-1 cells with no NK cells). (B-D) NK cells were identified as NKp46+CD3−, doublet events were excluded by FSC-H vs FSC-A gating, and a viability dye was included to exclude dead cells from the analysis. (B) Degranulation of NK cells from control Npc1+/+ and Npc1−/− animals in response to stimuli in the presence of monensin was calculated according to the following equation: (% of CD107a+ NK cells with stimuli) − (% of CD107a+ unstimulated NK cells). (C) Intracellular IFN-γ production by NK cells from control Npc1+/+ and Npc1−/− animals in response to stimuli was calculated according to the following equation: (% of IFN-γ+ NK cells with stimuli) − (% of IFN-γ+ unstimulated NK cells). (D) Representative traces showing intracellular [Ca2+] changes monitored in single fluo-4–loaded NK cell, normalized to initial fluorescence (F/F0). Lysosomal calcium content was assessed upon addition of 50 μM GPN which lyses cathepsin-containing acidic intracellular calcium stores. The addition of ionomycin at the end of each experiment was used to confirm viability of the cells. (E) Maximal peak fluorescence changes were determined as the difference between basal and the maximum fluorescence, Δ(F/F0), upon addition of 50 μM GPN. Data are presented as the mean ± SEM, n = 3 animals for each group; 158 Npc1+/+ cells and 125 Npc1−/− cells. Clear bars represent control Npc1+/+ animals and solid bars represent affected Npc1−/− animals. (F) Degranulation of NK cells from control Npc1+/+ and Npc1−/− animals in response to PMA/iono in the presence or absence of monensin was determined as described for panel B. Data are presented as mean ± SEM, n = 4 to 6 animals. *P < .05, **P < .01, and ***P < .001, calculated by an unpaired t test using GraphPad Prism v4.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/1/10.1182_blood-2013-03-488692/4/m_51f4.jpeg?Expires=1769232882&Signature=rUk6KIqKAuI-1kzxYcmMa2x2pb5JsP25XMsuBCuNla75lLoOW5KUwAsEgO430mHVvTn1bulwICSfGI1pzjP3pARJ5qhStipUSiN3XHHo2iNpaKGZXRTshF3dAH6mlaF1akWU-Z0WJD40alEnz~1Tck15cPOwNu~smSb7kvw5UntkXG4gAkmhvYuXwanUp5Ejlh52NY9vZebrZctOQg5lWLeQMfyNVMWdcEoe7mkR4m-vLVwLBlUSZyVQ2VeYOY3I-DmEwgT-7tQKEmUZKUy2xUOD06FMhnJDSkjZYCo7Fx3892f1xfTvnzwVYVqskT1rDwnme1C3Q0r1ZPFczalibg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal