Key Points
miR-155 regulates the RB/E2F axis in DLBCL.
SMAD5 plays a dominant role in transducing TGF-β effects in B lymphocytes.
Abstract
MicroRNA-155 (miR-155) plays pleiotropic roles in the biology of normal and malignant B lymphocytes, including the modulation of the transforming growth factor β (TGF-β) pathway via the targeting of SMAD5. However, the extent of the miR-155–mediated disruption of the TGF-β1/SMAD5 axis remains to be elucidated. To address this issue, we used the miR-155 knockout (KO) mouse and diffuse large B-cell lymphoma (DLBCL) cell lines ectopically expressing miR-155. In the DLBCL models, expression of miR-155 blocked TGF-β1–mediated activation of the retinoblastoma protein (RB), decreasing the abundance of the inhibitory pRB-E2F1 complex and limiting G0/G1 arrest. Genetic knockdown of SMAD5, p15, or p21 recapitulated these effects, establishing a circuitry whereby the targeting of SMAD5 by miR-155 blunts the TGF-β1–induced transcription of p15 and p21, thus sustaining RB phosphorylation and inactivity. Next, we demonstrated that SMAD5 levels are elevated in mature B lymphocytes from the miR-155 KO mice, which display a heightened sensitivity to TGF-β1 characterized by suppression of RB phosphorylation and more pronounced G0/G1 cell cycle arrest. Our findings suggest that a miR-155-mediated perturbation of the RB/E2F axis may play a role in DLBCL pathogenesis, and contribute to the reduced number of germinal center B cells and impaired T cell–dependent antibody response found in the miR-155 KO mice.
Introduction
MicroRNAs (miRNAs) are important regulators of the human transcriptome, and their role in multiple physiological processes has been well established. Likewise, the participation of these small noncoding RNAs in various pathological states, including cancer, is now fully recognized.1 Nonetheless, in many instances, the breadth of the dysfunction caused by miRNA deregulation is not entirely known. In part, this reflects their pleiotropic activities because a single miRNA can directly inhibit the expression of dozens, if not hundreds, of genes.2 However, this may also indicate our limited understanding of the full complement of downstream effects that follow a specific miRNA:target gene interaction.
The transforming growth factor β (TGF-β) pathway plays important roles in embryonic development as well as in tissue homeostasis in adult organisms.3 Thus, deregulation of TGF-β signaling is associated with a variety of human disorders, including cancer.4 In epithelial malignancies, where the role of TGF-β has been more extensively investigated, the current evidence suggests the presence of an initial tumor suppressive activity, which is followed by an oncogenic profile characterized by epithelial-to-mesenchymal transition and metastasis.4 In contrast, TGF-β signals in normal and malignant B lymphocytes appear to be predominantly suppressive, indicating that deregulation of this pathway may contribute to the pathogenesis of B-cell malignancies and interfere with the developmental regulation of normal B cells.5,6
We recently described the direct targeting of the transcription factor SMAD5 by microRNA-155 (miR-155).7 In that report, we showed that miR-155, which is often overexpressed in aggressive B-cell lymphomas, contributes to lymphomagenesis by abrogating the cytostatic effects of the TGF-β pathway toward B lymphocytes. Importantly, although SMAD5 is an integral component of the bone morphogenic protein (BMP) signaling module, we showed that the noncanonical TGF-β1–mediated activation of SMAD5 was present in B-cell lymphomas. Consequently, miR-155 targeting of SMAD5 had broader implications than initially appreciated because it disrupted both BMP and TGF-β signaling.
Herein, we examined the downstream effects of the TGF-β1–induced activation of SMAD5 in normal and malignant B lymphocytes, in the context of gain or loss of miR-155 function. TGF-β1–mediated phosphorylation of SMAD5 led to the transcriptional induction of p21 and p15, a process originally ascribed exclusively to the TGF-β1–SMAD2/3 interplay,3 and decreased the levels of the retinoblastoma protein (RB) phosphorylation. Genetic ablation of SMAD5, p21, or p15 confirmed the relevance of the noncanonical TGF-β1–SMAD5 engagement in B lymphocytes. Overexpression of miR-155 in diffuse large B-cell lymphomas (DLBCLs) limited TGF-β1–mediated p21 and p15 induction, resulting in sustained levels of RB hyperphosphorylation and decreased formation of the RB/E2F1 complex. These data suggest that miR-155 can disrupt the RB/E2F axis in DLBCL, a recently recognized event of pathogenetic relevance in this disease.8,9 Further, we found that the TGF-β1–mediated phosphorylation of SMAD5 is not restricted to B-cell lymphoma cell lines but is also active in normal mature B lymphocytes. Examining mature B cells from the miR-155 knockout (KO) mice, we detected an elevated expression of SMAD5 and heightened sensitivity to TGF-β1. In particular, we found an enrichment of hypophosphorylated (active) RB (hypo-pRB) and excessive G0/G1 cell cycle arrest in miR-155 null mature B cells, which may contribute to the reduced number of germinal center (GC) B cells and impaired T cell–dependent antibody response reported in these mice.10
Methods
Cell lines
DLBCL cell lines OCI-Ly7, OCI-Ly18, and SU-DHL5 were grown with RPMI 1640 medium (Invitrogen) containing 10% fetal bovine serum (FBS), penicillin (100 units/mL), streptomycin (100 μg/mL), N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) buffer (10 mmol/L), and l-glutamine (600 μg/mL) at 37°C in 5% CO2. HEK-293 cells were maintained in Dulbecco’s modified Eagle medium (Mediatech) with 10% FBS and sodium pyruvate (1 mmol/L). The identity of the DLBCL cell lines was confirmed by variable number tandem repeat analysis (AmpflSTR kit; ABI) and verified online at the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (German Collection of Microorganisms and Cell Cultures) cell bank (http://www.dsmz.de).
Isolation of mouse spleen B lymphocytes
Isolation of murine spleen B cells was performed as previously described.11 Briefly, spleens were harvested from multiple pairs of miR-155 wild-type (WT) and KO mice, and mature B lymphocytes purified using the Mouse B-Cell Enrichment Kit in the RoboSep Automated Cell Separator (Stem Cell Technologies). Subsequently, the purity and degree of enrichment was determined by fluorescence-activated cell sorter (FACS)-based measurement of CD19-positive cells, in pre- and postseparation aliquots. Consistently, the fraction of CD19-positive cells was >95%. These primary B cells were cultured in full “B cell media” (RPMI 1640 supplemented with 20% FBS, 100 μM β-mercaptoethanol, 10 mM HEPES, 2 μM l-glutamine, 0.1% lipopolysaccharides penicillin/streptomycin, 20 μg/mL LPS, and 2.5 ng/mL murine interleukin 4) for 48 hours. All animal studies were approved by the local institutional animal care and use committee.
TGF-β1 and BMP4 stimulation of DLBCL cell lines and mouse spleen B lymphocytes
DLBCL cells (1-2 × 106/mL) or murine B cells (2-4 × 106/mL) were grown in 2% FBS RPMI 1640 medium overnight prior to exposure to mouse recombinant TGF-β1 (5 ng/mL; Pepro Tech) or recombinant human BMP4 (50 ng/mL; R&D Systems) for various time points. Murine B cells were also exposed to TGF-β1 (5 ng/mL) and BMP4 (100 ng/mL) immediately following animal harvesting and mature B-lymphocyte isolation, in serum-deprived conditions.
Cell cycle analysis
Cell cycle profile was determined by propidium iodide staining, followed by FACS analyses, as we described.7 In brief, DLBCL cell lines were exposed to vehicle or 5 ng/mL of TGF-β1 in 2% FBS RPMI medium for multiple time points, and murine B cells were grown with full “B-cell media” containing TGF-β1 (5 ng/mL) or vehicle control for 48 hours. The DLBCL cell lines or murine mature B cells were harvested, washed twice in cold phosphate-buffered saline (PBS), and fixed with 70% ethanol at 4°C overnight. After additional washes with PBS, the cells were resuspended into 300 to 500 µL of a freshly prepared staining solution containing 50 µg/mL of propidium iodide with 0.1% Trition X-100 and 100 µg/mL RNase A (Sigma) and incubated at 37°C for 1 hour.
Genetic modulation of miR-155, SMAD5, p15, and p21 expression in DLBCL cell lines
The generation of a MSCV–miR-155–eGFP bicistronic retrovirus construct, virus production, DLBCL cell line transduction, and FACS-based enrichment were performed as we reported before.7 Downregulation of SMAD5, p15, and p21 expression in DLBCL cell lines was achieved with an RNA interference (RNAi)-based strategy; for each target gene, 2 unique short interfering RNA (siRNA) duplexes and a control scrambled sequence (Sigma) were electroporated in OCI-Ly7, OCI-Ly18, and SU-DHL5 cell lines. In brief, 5 × 106 cells were washed twice with cold PBS and then resuspended into 400 μL of Opti-MEM medium containing 200 nM of siRNA duplex. Electroporation was performed using a Bio-Rad Gene Pulser MX CELL (Bio-Rad Laboratories) with parameter set up at 250 V, 975 μF, and ∞ resistance. The transfected cells were kept on ice for 10 minutes and then resuspended into the 20% FBS RPMI 1640 medium for 48 hours, followed by TGF-β1 activation as described previously. Subsequently, RNA was isolated for real-time reverse-transcription polymerase chain reaction (RT-PCR), or cell lysate was extracted for western blot analysis to confirm the downregulation of the target gene. The SMAD5 RNAi sequences were also cloned into the pLTR-H1-eGPP retrovirus vector, and stable SMAD5 short hairpin RNA–based knockdown (KD) established as previously described.7 The siRNAs sequences are as follows: Smad5 #1: 5′-AGUCUUACCUCCAGUAUUA-3′; Smad5 #3: 5′-GAUUCACAGAUCCUUCAAA-3′; p15 #2: 5′-AACUCAGUGCAAACGCCUAGA-3′; p15 #3: 5′-GGUCAGACUAAGAAAUAUU-3′; p21 #1: 5′-UGUCAGAACCGGCUGGGGAUU-3′; and p21 #2: 5′-GACCAUGUGGACCUGUCAC-3′.
Whole cell lysate extraction, subcellular fractionation, and western blot analysis
Whole cell lysate was extracted with NP-40 lysis buffer containing 50 mM tris(hydroxymethyl)aminomethane (pH 8.0), 150 mM sodium chloride, 1% NP-40, and 1× proteinase inhibitor as previously described.12 For fractionations, cell pellet was resuspended in 5-pellet volumes of cytoplasmic extraction buffer (10 mM HEPES, 10 mM KCl, 1 mM EDTA, 0.075% [vol/vol] NP-40, 1 mM dithiothreitol, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) and incubated on ice for 10 minutes. The cytoplasmic fraction was collected by centrifugation at 13 000 rpm for 5 minutes at 4°C. The remaining, nuclear, pellet was resuspended in 2-pellet volumes of nuclear extraction buffer (20 mM tris(hydroxymethyl)aminomethane-HCl, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM PMSF, and 25% [vol/vol] glycerol) and incubated on ice for 30 minutes with vortexing at every 10 minutes. The nuclear extract was collected by centrifugation at 13 000 rpm for 20 minutes. The relevant lysate was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. The filters were immunoblotted with antibodies against underphosphorylated pRB (G99-549; BD Pharmingen), RB (G3-245; BD Pharmingen), E2F1 (c-20, sc-193; Santa Cruz), SMAD1 (R&D Systems), SMAD2 (Cell Signaling), SMAD3 (Cell Signaling), SMAD4 (B-8. sc-7966; Santa Cruz), SMAD5 (Cell Signaling), p-SMAD2 (Cell Signaling; S465/467), p-SMAD1/5/8 (Cell Signaling), ppRB Ser780 (Cell Signaling), p21 (Santa Cruz; F-5, sc-6246), and β-actin (Sigma).
Coimmunoprecipitation assay
DLBCL cell lines ectopically expressing MSCV–miR-155 or an empty murine stem cell virus (MSCV) vector were stimulated with TGF-β1 (5 ng/mL) or vehicle for 4 hours. Next, the nuclear extract was isolated and diluted fivefold with IP buffer (50 mM HEPES, 100 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 10% glycerol, 0.1% NP-40, 0.1 mM PMSF, and 2 mM dithiothreitol) and precleared with protein G Plus/Protein A agarose beads (Calbiochem) for 1 hour at 4°C. After centrifugation, the supernatant was incubated with 1 µg of underphosphorylated RB antibody, or mouse IgG control, and protein A/G beads at 4°C overnight. The IP was washed 5 times with IP buffer containing 200 mM NaCl, eluted with 2× Laemmili buffer at 95°C for 5 minutes. The IP supernatant, obtained after pelleting beads containing the underphosphorylated RB immunocomplexes, was also harvested for western blot analysis of free E2F1 (ie, E2F1 that is not bound to underphosphorylated RB). The immunoprecipitates and supernatant were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and detected by western blot using anti-underphosphorylated pRB and anti-E2F1 antibodies.
Real-time RT-PCR
RNA isolation and complementary DNA generation were performed as described previously.13 Real-time RT-PCR was used to quantify the expression of P15 and P21 in DLBCL cell lines ectopically expressing miR-155 or following transient transfection of p15 and p21 siRNA oligonucleotides. The expression of target genes was normalized to that of housekeeping control gene TBP (TATA-binding protein), relative quantification was achieved by calculating ΔΔCT, and expression was defined as 2–ΔΔCT, where either vehicle-treated samples or siRNA controls represented the baseline. Primers for real-time PCR are as follows: TBP: 5′-TATAATCCCAAGCGGTTTGCTGCG-3′ (F) and 5′-AATTGTTGGTGGGTGAGCACAAGG-3′(R); p15: 5′-CTAGTGGAGAAGGTGCGACA-3′ (F) and 5′-TCATCATGACCTGGATCGCG-3′(R); and p21: 5′-TGGAGACTCTCAGGGTCGAAA-3′ (F) and 5′-GGCGTTTGGAGTGGTAGAAAT-3′(R).
Statistics
Analyses were performed using a 2-tailed Student t test. P < .05 was considered significant. Data analyses were performed with Prism software (version 5.0; GraphPad) and Excel software (Microsoft).
Results
TGF-β1 activation of SMAD5 is a feature of both normal and malignant mature B lymphocytes
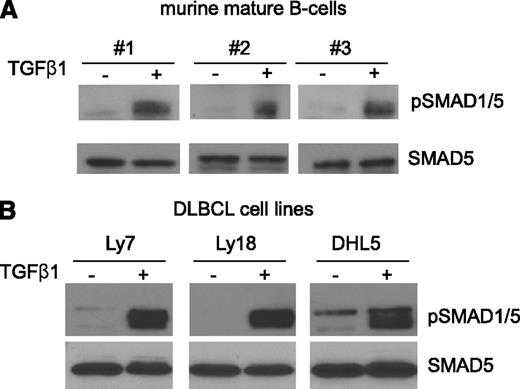
We previously showed that in DLBCL cell lines TGF-β1 could, unexpectedly, phosphorylate the “BMP-SMAD” SMAD5.7 However, no information is currently available as to whether this noncanonical interplay is also present in nontransformed mature B lymphocytes. To address this knowledge gap, we isolated mature B lymphocytes from the spleens of 3 WT C57BL/6 mice and cultured them in serum-deprived conditions (2% serum) in the presence of vehicle or TGF-β1 (5 ng/mL) for 4 hours. Thereafter, nuclear extract was used for western blot examination of phospho-SMAD1/5 levels. TGF-β1 readily phosphorylated SMAD5 in mature B cells, suggesting that this unique signaling module may also play a role in normal B-cell physiology (Figure 1A). To expand on our earlier observations,7 we also tested the ability of TGF-β1 to activate SMAD5 in a new panel of DLBCL cell lines. Herein, the OCI-Ly7, OCI-Ly18, and SU-DHL5 cell lines were exposed to TGF-β1 (5 ng/mL) for 1 hour, and prompt SMAD5 phosphorylation was detected (Figure 1B). Interestingly, although BMP is the central activator of SMAD1/5, not all DLBCL cell lines in our panel responded to BMP activation (supplemental Figure 1; see the Blood Web site), as previously noted,14 further reinforcing the relevance of the TGF-β1–mediated activation of SMAD5.
TGF-β1 phosphorylates SMAD5 in normal and malignant mature B cells. (A) Mature B cells were purified from the spleens of 3 WT C57BL/6 mice and exposed to TGF-β1 (5 ng/mL) or vehicle control for 4 hours. Western blot analysis demonstrates prompt induction of SMAD1/5 phosphorylation in these cells. (B) TGF-β1 (5 ng/mL, 1 hour) also induced marked activation of SMAD1/5 in DLBCL cell lines. In both instances, the western blotting was carried out with nuclear extracts. Equal loading is verified with immunoblotting to total SMAD5. The assays shown in panel A represent 3 biological replicates (B cells from 3 mice), and the assays shown in panel B were confirmed in multiple (more than 3) biological replicates.
TGF-β1 phosphorylates SMAD5 in normal and malignant mature B cells. (A) Mature B cells were purified from the spleens of 3 WT C57BL/6 mice and exposed to TGF-β1 (5 ng/mL) or vehicle control for 4 hours. Western blot analysis demonstrates prompt induction of SMAD1/5 phosphorylation in these cells. (B) TGF-β1 (5 ng/mL, 1 hour) also induced marked activation of SMAD1/5 in DLBCL cell lines. In both instances, the western blotting was carried out with nuclear extracts. Equal loading is verified with immunoblotting to total SMAD5. The assays shown in panel A represent 3 biological replicates (B cells from 3 mice), and the assays shown in panel B were confirmed in multiple (more than 3) biological replicates.
miR-155 controls SMAD5 expression and TGF-β1–mediated induction of p15 and p21 in DLBCL
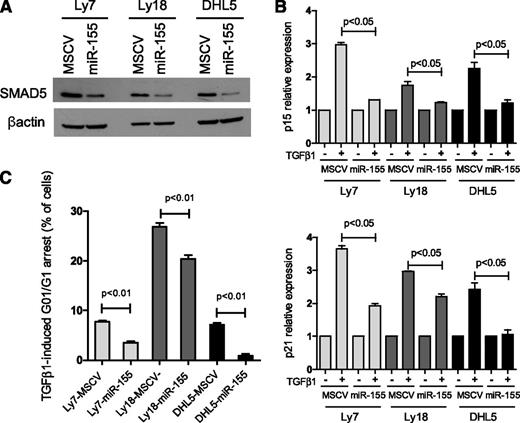
The noncanonical activation of SMAD5 by TGF-β1 in the DLBCL cell lines OCI-Ly7, OCI-Ly18, and SU-DHL5 (Figure 1B) suggested that these cells would be good models to examine how the previously described miR-155/SMAD57 axis contributes to lymphomagenesis. Toward this end, we used a retrovirus system to stably express miR-155, or an empty control vector, in these cell lines. Confirming the ability of miR-155 to directly regulate SMAD5,7 we readily detected lower SMAD5 levels in cells expressing miR-155 when compared with their empty-vector isogenic controls (Figure 2A; supplemental Figure 2A). Importantly, in this model, miR-155 expression did not modify the levels of other TGF-β1–responsive SMAD proteins (SMAD1, SMAD2, SMAD3, or SMAD4; supplemental Figure 2B), suggesting that miR-155 influence on TGF-β1–mediated effects is likely to derive predominantly from the modulation of SMAD5. TGF-β1 cytostatic activities include upregulation of the cyclin-dependent kinase inhibitors (CDKIs) P15 (CDKN2B) and P21 (CDKN1A) and subsequent G0/G1 arrest.3,4 Although these events are primarily associated with SMAD2/3 activation,15,16 we showed previously that ectopic expression of miR-155 could limit the TGF-β1–induced G0/G1 arrest in DLBCL in a SMAD5-dependent manner.7 Herein, we examined whether the miR-155/SMAD5 interplay affected the TGF-β1–mediated induction of P15 and P21. In 3 independent isogenic cell line models, stable expression of miR-155 significantly limited TGF-β1–dependent induction of both P15 and P21 (Figure 2B), which was accompanied by an impaired G0/G1 arrest (Figure 2C; supplemental Figure 3). These findings agree with our previous observations completed in another set of DLBCL cell lines,7 suggesting that malignant mature B cells remain sensitive to TGF-β1 signals.
miR-155 limits the TGF-β1–mediated induction of P15 and P21 and cell cycle arrest in DLBCL. (A) Ectopic expression of miR-155 in 3 DLBCL cell lines decreased the expression of its target SMAD5. (B) DLBCL cell lines stably expressing an empty vector (MSCV) or miR-155 were exposed to 5 ng/mL of TGF-β1 or vehicle control (-) for 1 hour. Quantitative real-time RT-PCR measurements of P15 (upper panel) and P21 (bottom panel) showed a significantly impaired induction of these genes in miR-155–expressing cells than in their isogeneic controls (P < .05, Student t test). Data shown are mean ± standard deviation of cells exposed to TGF-β1 normalized by vehicle-treated cells of a representative assay performed in triplicate; 2 to 4 biological replicates of this experiment were completed. (C) Expression of miR-155 rendered 3 DLBCL cell lines significantly less sensitive to TGF-β1–induced cell cycle arrest than their isogenic controls (P < .01, Student t test). Data shown are the percentage of cells that were arrested in G0/G1 following exposure to TGF-β1 for 48 hours in a representative assay performed in triplicate. Specifically, the display shows the percentage of TGF-β1–exposed minus vehicle-exposed cells in G0/G1. The complete cell cycle profile is shown in supplemental Figure 3. The results of this assay were confirmed in at least 3 biological replicates.
miR-155 limits the TGF-β1–mediated induction of P15 and P21 and cell cycle arrest in DLBCL. (A) Ectopic expression of miR-155 in 3 DLBCL cell lines decreased the expression of its target SMAD5. (B) DLBCL cell lines stably expressing an empty vector (MSCV) or miR-155 were exposed to 5 ng/mL of TGF-β1 or vehicle control (-) for 1 hour. Quantitative real-time RT-PCR measurements of P15 (upper panel) and P21 (bottom panel) showed a significantly impaired induction of these genes in miR-155–expressing cells than in their isogeneic controls (P < .05, Student t test). Data shown are mean ± standard deviation of cells exposed to TGF-β1 normalized by vehicle-treated cells of a representative assay performed in triplicate; 2 to 4 biological replicates of this experiment were completed. (C) Expression of miR-155 rendered 3 DLBCL cell lines significantly less sensitive to TGF-β1–induced cell cycle arrest than their isogenic controls (P < .01, Student t test). Data shown are the percentage of cells that were arrested in G0/G1 following exposure to TGF-β1 for 48 hours in a representative assay performed in triplicate. Specifically, the display shows the percentage of TGF-β1–exposed minus vehicle-exposed cells in G0/G1. The complete cell cycle profile is shown in supplemental Figure 3. The results of this assay were confirmed in at least 3 biological replicates.
Heightened RB phosphorylation and increased abundance of free E2F1 in miR-155–expressing DLBCL
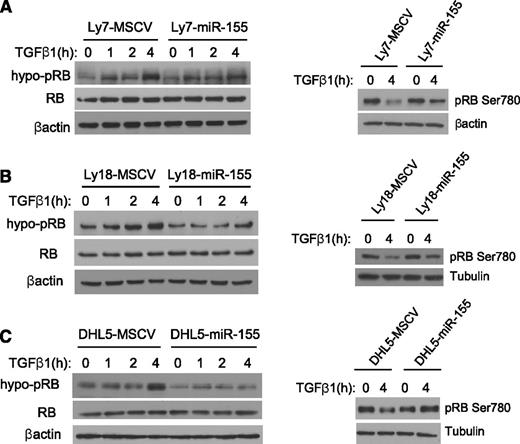
Control of RB phosphorylation levels plays an important role in cell cycle regulation.17 In the hypophosphorylated state, pRB is active and blocks cell cycle progression, at least in part by binding to and inhibiting transcription factors of the E2F family of transcriptional regulators.18 Phosphorylation of RB by cyclin/cyclin-dependent kinase complexes allows E2F to dissociate, effectively inactivating RB and promoting cell cycle progression. Conversely, TGF-β1–inducible CDKIs such as p15 and p21 bind to and inhibit the activity of cyclin/cyclin-dependent kinase complexes, eventually enriching for hypo-pRB and promoting cell cycle arrest.19 To define whether miR-155 could modulate the TGF-β1 effects toward RB phosphorylation, we exposed the 3 DLBCL models expressing an empty vector or miR-155 to vehicle control or 5 ng/mL of TGF-β1 (for 1, 2, or 4 hours). Subsequently, the nuclear fraction was isolated, and the levels of hypo-pRB were determined by western blot. In all DLBCL cell lines expressing an empty vector, TGF-β1 increased the abundance of hypo-pRB (active RB), whereas this effect was significantly reduced in the isogenic cells ectopically expressing miR-155 (Figure 3A-C, left panels). The impact of miR-155 on TGF-β1–mediated RB regulation was also readily detectable by directly measuring the phosphorylation levels of RB’s Ser780 residue (Figure 3A-C, right panels). In these instances, exposure to TGF-β1 markedly decreased the levels of phospho-Ser780 in DLBCL cell lines expressing an empty vector, whereas only a modest change was noticeable in isogenic cells expressing miR-155. Together, these data show that miR-155 significantly blunts the TGF-β1–mediated decrease in RB phosphorylation, consequently limiting this protein’s activity. Of relevance, miR-155–expressing cells displayed similarly reduced sensitivity to BMP4-mediated effects toward RB phosphorylation (supplemental Figure 4), further suggesting the central involvement of a “BMP-SMAD” in this process (ie, SMAD5) and highlighting the broader role of miR-155 in regulating the TGF-β pathway. To determine the downstream consequences of the heightened RB phosphorylation found in miR-155–expressing cells, we performed coimmunoprecipitation experiments and quantified the levels of RB-bound E2F1. In these assays, following TGF-β1 exposure, we consistently found an enhanced pRB-E2F1 complex formation in control cells when compared with the counterparts ectopically expressing miR-155. In agreement with these data, DLBCL cell lines expressing miR-155 displayed a markedly higher level of free E2F1 (supplemental Figure 5).
miR-155 impairs TGF-β1–mediated RB activation in DLBCL. Western blot analysis of hypo-pRB (left panels) or phospho-Ser780 residue (right panels) was performed in the DLBCL cell lines Ly7 (A), Ly18 (B), and DHL5 (C), genetically modified to express an empty vector (MSCV) or miR-155. Upon TGF-β1 exposure (5 ng/mL), an increase in the abundance of hypo-pRB (active RB) is detected in the MSCV-expressing cell lines and, to a much lesser degree, in their isogenic counterparts expressing miR-155 (left panels). In agreement with this observation, the phosphorylation levels of RB’s Ser780 residue was markedly suppressed by TGF-β1 in MSCV-expressing cells, but not in their isogenic miR-155 counterparts (right panels). Immunoblotting for total RB confirms that miR-155 does not modify its expression level, and equal loading is also verified with β-actin or tubulin. The data shown in this figure were confirmed in 2 to 4 biological replicates.
miR-155 impairs TGF-β1–mediated RB activation in DLBCL. Western blot analysis of hypo-pRB (left panels) or phospho-Ser780 residue (right panels) was performed in the DLBCL cell lines Ly7 (A), Ly18 (B), and DHL5 (C), genetically modified to express an empty vector (MSCV) or miR-155. Upon TGF-β1 exposure (5 ng/mL), an increase in the abundance of hypo-pRB (active RB) is detected in the MSCV-expressing cell lines and, to a much lesser degree, in their isogenic counterparts expressing miR-155 (left panels). In agreement with this observation, the phosphorylation levels of RB’s Ser780 residue was markedly suppressed by TGF-β1 in MSCV-expressing cells, but not in their isogenic miR-155 counterparts (right panels). Immunoblotting for total RB confirms that miR-155 does not modify its expression level, and equal loading is also verified with β-actin or tubulin. The data shown in this figure were confirmed in 2 to 4 biological replicates.
Essential role of SMAD5 in TGF-β1–mediated hypophosphorylation of RB
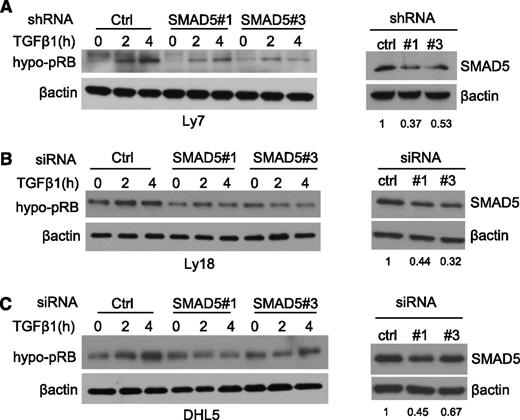
miR-155 directly targets SMAD5 and suppresses its expression. The data presented previously, and our earlier studies,7 suggest that this interaction is critical for miR-155–mediated blockade of the TGF-β1 signals, including its effects on RB phosphorylation. To confirm the important role of SMAD5 on TGF-β1–induced hypophosphorylation of RB, we used an RNAi strategy. Two independent sequences that specifically target SMAD5 as well as a scrambled control RNAi sequence were stably or transiently expressed in our DLBCL cell line models. Subsequently, SMAD5-specific KD was confirmed by western blot, and the cells were exposed to TGF-β1 at multiple time points. In all 3 cell models explored, the abundance of the hypo-pRB was markedly increased in the RNAi control cells, whereas these effects were blunted by SMAD5 KD (Figure 4A-C). Importantly, these RNAi sequences did not modify the expression of other TGF-β1–responsive SMADs (supplemental Figure 6), further illustrating the hitherto unappreciated role of SMAD5 in mediating TGF-β1 activities in malignant B cells. Together, these data show that in the context of TGF-β1 signaling, the SMAD5 KD phenocopies miR-155 expression in DLBCL.
SMAD5 KD phenocopies miR-155 effects on TGF-β1-mediated RB phosphorylation. Western blot analysis of hypo-pRB (left panels) was performed in the DLBCL cell lines Ly7 (A), Ly18 (B), and DHL5 (C) subjected to RNAi-mediated SMAD5 suppression. Upon TGF-β1 exposure (5 ng/mL), the abundance of the hypo-pRB (active RB) isoform increases in the control RNAi cells, but not, or to a lesser degree, in their isogenic counterparts transiently (Ly18 and DHL5) or stably (Ly7) expressing 2 independent RNAi sequences directed at SMAD5 (left panels). In the right panels, western blots depict the effectiveness of the SMAD5 KD; densitometric quantification of the SMAD5 suppression is shown under each panel. β-actin imunoblotting was used to confirm proper loading. The data shown in this figure were confirmed in 2 to 4 biological replicates.
SMAD5 KD phenocopies miR-155 effects on TGF-β1-mediated RB phosphorylation. Western blot analysis of hypo-pRB (left panels) was performed in the DLBCL cell lines Ly7 (A), Ly18 (B), and DHL5 (C) subjected to RNAi-mediated SMAD5 suppression. Upon TGF-β1 exposure (5 ng/mL), the abundance of the hypo-pRB (active RB) isoform increases in the control RNAi cells, but not, or to a lesser degree, in their isogenic counterparts transiently (Ly18 and DHL5) or stably (Ly7) expressing 2 independent RNAi sequences directed at SMAD5 (left panels). In the right panels, western blots depict the effectiveness of the SMAD5 KD; densitometric quantification of the SMAD5 suppression is shown under each panel. β-actin imunoblotting was used to confirm proper loading. The data shown in this figure were confirmed in 2 to 4 biological replicates.
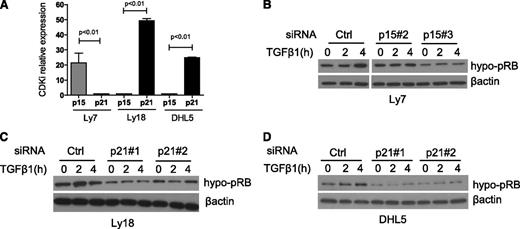
Downstream to the miR-155/SMAD5 interplay, p15 and p21 control TGF-β1 effects on RB phosphorylation
We showed that DLBCL cell lines ectopically expressing miR-155 display a defective induction of P15 and P21 in response to TGF-β1 (Figure 2B). To examine the individual contribution of these CDKIs in the miR-155 modulation of RB phosphorylation and activity, we used an siRNA approach. First, we determined the relative expression of P15 and P21 in our DLBCL models; whereas P15 was found to be the dominant product in the OCI-Ly7 cell line, P21 expression was significantly higher in both OCI-Ly18 and SU-DHL5 (Figure 5A). Next, using 2 unique siRNA sequences for each gene, we knocked down p15 expression in the OCI-Ly7 cell line and p21 in OCI-Ly18 and SU-DHL5 (supplemental Figure 7). Subsequently, these DLBCL cell line models were exposed to TGF-β1, and the levels of hypo-pRB (active RB) were defined by western blot. In the 3 cell lines, TGF-β1 induced the accumulation of the hypo-pRB in the siRNA control cells, whereas these effects were reduced by p15 or p21 downregulation (Figure 5B-D). Together, these results support a model whereby in a TGF-β1–dependent context miR-155 impinges on RB phosphorylation and activity via inhibition of SMAD5 expression and blockade of the transcriptional activation of P15 and P21. Consequently, miR-155 overexpressing DLBCL is less sensitive to TGF-β1 growth inhibitory cues.
p15 and p21 inhibition recapitulates miR-155 suppression of TGF-β1 signals in DLBCL. (A) Real-time RT-PCR quantification of P15 and P21 in 3 DLBCL cell lines shows a dominant expression of P15 in Ly7 and P21 in Ly18 and DHL5 (P < .01, Student t test). (B) Western blot analysis of hypo-pRB was performed in the DLBCL cell line Ly7 transiently expressing an siRNA control or 2 sequences specific for p15. Upon TGF-β1 exposure (5 ng/mL), the abundance of the hypo-pRB (active RB) isoform increases in the control siRNA Ly7 cells, but not in their p15 KD isogenic counterparts. (C-D) Western blot analyses of hypo-pRB were performed in the DLBCL cell lines Ly18 and DHL5 transiently expressing an siRNA control or 2 sequences specific for p21. Upon TGF-β1 exposure (5 ng/mL), the abundance of the hypo-pRB (active RB) isoform increases in the control siRNA cells, but not, or to a lesser extent, in p21 KD cells. The effectiveness of the siRNA-mediated p15 and p21 suppression is shown in supplemental Figure 7. β-actin imunoblotting was used to confirm proper loading. The data shown in this figure were confirmed in 1 to 3 biological replicates.
p15 and p21 inhibition recapitulates miR-155 suppression of TGF-β1 signals in DLBCL. (A) Real-time RT-PCR quantification of P15 and P21 in 3 DLBCL cell lines shows a dominant expression of P15 in Ly7 and P21 in Ly18 and DHL5 (P < .01, Student t test). (B) Western blot analysis of hypo-pRB was performed in the DLBCL cell line Ly7 transiently expressing an siRNA control or 2 sequences specific for p15. Upon TGF-β1 exposure (5 ng/mL), the abundance of the hypo-pRB (active RB) isoform increases in the control siRNA Ly7 cells, but not in their p15 KD isogenic counterparts. (C-D) Western blot analyses of hypo-pRB were performed in the DLBCL cell lines Ly18 and DHL5 transiently expressing an siRNA control or 2 sequences specific for p21. Upon TGF-β1 exposure (5 ng/mL), the abundance of the hypo-pRB (active RB) isoform increases in the control siRNA cells, but not, or to a lesser extent, in p21 KD cells. The effectiveness of the siRNA-mediated p15 and p21 suppression is shown in supplemental Figure 7. β-actin imunoblotting was used to confirm proper loading. The data shown in this figure were confirmed in 1 to 3 biological replicates.
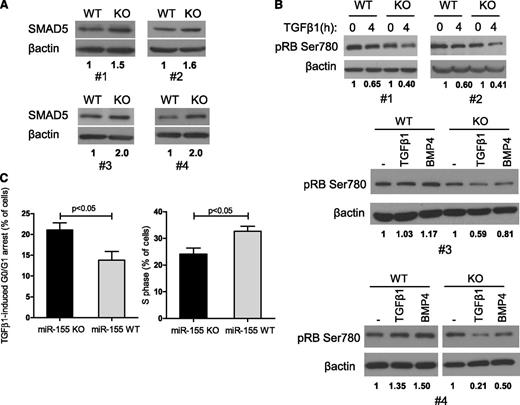
High SMAD5 expression and augmented response to TGF-β1 in miR-155 −/− mature B cells results in suppression of RB's pSer780 and cell cycle arrest
We showed previously that the TGF-β1/SMAD5 axis is active in normal B lymphocytes (Figure 1A) and that ectopic expression of miR-155 in DLBCL cell lines attenuated the TGF-β1–mediated RB activation (Figure 3). To define whether this interplay is also present in normal mature B cells, we used the miR-155 KO mice. Spleens were harvested from miR-155 WT or KO mice matched for age and sex, and mature B cells purified and exposed to TGF-β1 or BMP4. Examination of 4 mice pairs showed a higher expression of SMAD5 in cells from miR-155 KO mice when compared with their WT controls (Figure 6A). Similarly to the DLBCL cell line data, differential miR-155 expression in these nonmalignant B cells altered TGF-β1 effects toward RB phosphorylation. Comparing multiple pairs of WT and KO mice, TGF-β1–mediated suppression of phospho-RB (Ser780) was consistently more pronounced in miR-155−/− B cells than in their WT counterparts (Figure 6B), presumably increasing RB activity. In agreement with this concept, TGF-β1 exposure induced a significantly more pronounced G0/G1 arrest in mature B cells from the miR-155 KO mice than from their WT counterparts (percentage of cells arrested after TGF-β1 exposure KO vs WT, mean 21% ± 3.5 vs 13.8% ± 4.1, P < .05, n = 8 mice), and consequently, a significant decrease in the percentage of cells in S phase was detected in the miR-155 null B cells (KO vs WT, mean 24.1 ± 4.6 vs 32.6 ± 3.7, P < .05, n = 8) (Figure 6C). Of note, in the absence of TGF-β1, there was no significant difference in cell cycle profile of mature B cells from miR-155 WT and KO mice (supplemental Figure 8). This observation highlights the relevance of miR-155 in controlling the growth-suppressing TGF-β1 signals and suggests that this interplay may help explain the characteristic decrease in the number of GC B cells found in miR-155 KO mice.10
miR-155 controls TGF-β1–mediated regulation of RB and cell cycle progression in normal mature B lymphocytes. (A) Western blot analysis in mature B lymphocytes isolated from 4 pairs of WT/miR-155 KO mice shows a consistently higher SMAD5 expression in miR-155 null cells. (B) Western blot–based examination of the phosphorylation levels of RB’s Ser780 following TGF-β1 (4 pairs of mice) or BMP4 (2 pairs of mice) exposure depicts a more pronounced decrease in this residue’s phosphorylation, and consequently RB activation, in miR-155 null cells. β-actin immunoblotting was used as loading control. Densitometric quantification of the SMAD5 (A) or pRB Ser780 (B) is shown under each panel. (C) Homozygous deletion of miR-155 rendered mature B lymphocytes significantly more sensitive to TGF-β1–induced cell cycle arrest (left panel), with consequent decrease in cells in the S phase (right panel) (P < .05, Student t test). On the left, data shown are the mean ± standard deviation of the percentage of B lymphocytes that were arrested in G0/G1 following exposure to TGF-β1 for 48 hours, in 4 pairs of WT vs miR-155 KO mice. On the right, the percentage of cells in S phase after TGF-β1 exposure is shown. The data displayed in this figure represent 4 biological replicates, that is, the comparison between 8 animals, 4 miR-155 WT and 4 KO mice. The complete cell cycle profile is shown in supplemental Figure 8.
miR-155 controls TGF-β1–mediated regulation of RB and cell cycle progression in normal mature B lymphocytes. (A) Western blot analysis in mature B lymphocytes isolated from 4 pairs of WT/miR-155 KO mice shows a consistently higher SMAD5 expression in miR-155 null cells. (B) Western blot–based examination of the phosphorylation levels of RB’s Ser780 following TGF-β1 (4 pairs of mice) or BMP4 (2 pairs of mice) exposure depicts a more pronounced decrease in this residue’s phosphorylation, and consequently RB activation, in miR-155 null cells. β-actin immunoblotting was used as loading control. Densitometric quantification of the SMAD5 (A) or pRB Ser780 (B) is shown under each panel. (C) Homozygous deletion of miR-155 rendered mature B lymphocytes significantly more sensitive to TGF-β1–induced cell cycle arrest (left panel), with consequent decrease in cells in the S phase (right panel) (P < .05, Student t test). On the left, data shown are the mean ± standard deviation of the percentage of B lymphocytes that were arrested in G0/G1 following exposure to TGF-β1 for 48 hours, in 4 pairs of WT vs miR-155 KO mice. On the right, the percentage of cells in S phase after TGF-β1 exposure is shown. The data displayed in this figure represent 4 biological replicates, that is, the comparison between 8 animals, 4 miR-155 WT and 4 KO mice. The complete cell cycle profile is shown in supplemental Figure 8.
Discussion
This report brings to fore 2 important issues: (1) the unexpectedly dominant role of SMAD5 in TGF-β1–mediated growth inhibition and (2) the hitherto unrecognized impact of miR-155 expression on RB phosphorylation/activity, secondary to the noncanonical TGF-β1/SMAD5–mediated modulation of p15 and p21 (summarized in supplemental Figure 9).
Recently, our understanding of the activation of SMAD5 has been expanded to show that this “BMP-SMAD” could also be phosphorylated by TGF-β1. We reported earlier that this phenomenon, initially described in epithelial and endothelial cells,20-22 was also present in B-cell lymphomas.7 In the current report, we built on these observations to demonstrate that SMAD5 is also phosphorylated by TGF-β1 in normal murine mature B cells. Interestingly, in epithelial cell lines, the TGF-β1–mediated activation of SMAD1/5 appears to primarily serve the purpose of blunting the transcriptional responses downstream of BMP,23 via the formation of SMAD1/5-SMAD3 complexes. Conversely, the data presented here, as well as the findings in our earlier report,7 suggest that in B lymphocytes the TGF-β1/SMAD5 engagement induces a response not dissimilar from that of the canonical TGF-β1–SMAD2/3 activation (eg, P15 and P21 mRNA induction and consequent control of RB phosphorylation). In agreement with this concept, we showed that miR-155 overexpression (with consequent SMAD5 downregulation) or a targeted and specific siRNA-mediated SMAD5 KD both limited the effects of TGF-β1 toward p15/p21 and RB phosphorylation levels. Certainly, rescuing our DLBCL models of miR-155 overexpression with a SMAD5 construct lacking miR-155 binding sites would further validate our observations. However, DLBCL ectopically overexpressing SMAD5 consistently lost viability while in culture, and numerous attempts to generate these models were unsuccessful, thus further supporting the tumor-suppressive activities of SMAD5 in these tumors.
The outcome of the TGF-β1/SMAD5 engagement in DLBCL suggested that modulation of SMAD5 expression could impact the responses to TGF-β1 signals during B-cell development. The targeting of SMAD5 by miR-155, and the availability of the miR-155 KO mice, offered a good model system to address this issue. Indeed, we showed that SMAD5 is consistently expressed at higher levels in mature B cells from miR-155 KO mice than from their WT controls. Mice with ablation of miR-155 have a defective B-cell development, characterized by a reduced fraction of GC B cells and impaired T cell–dependent antibody response. In earlier studies, these defects were attributed, at least in part, to aberrant cytokine production by miR-155 null activated B cells and T cells.10 Interestingly, analyses of lipopolysaccharides B-cell proliferation in response to LPS, cytosine guanine dinucleotide, and anti-IgM yielded no difference between miR-155 null and control B cells.10 However, herein we showed that mature B cells from the miR-155 KO mice have higher sensitivity to TGF-β1 and display an accentuated suppression of RB phosphorylation (Ser780) and more pronounced G0/G1 cell cycle arrest, which is accompanied by a significant reduction in the number of cells in S phase. Thus, uncovering SMAD5 as a target of miR-155, and elucidating the relevance of the noncanonical TGF-β1/SMAD5 axis in B cells, led us to propose that the TGF-β1 secreted in the secondary lymphoid organs milieu may contribute to the B-lymphocyte deficiency found in miR-155 null mice. These findings also highlight a novel role for miR-155 in fine-tuning the role of TGF-β1 in maintaining the immune system’s homeostasis.6 We cannot currently exclude the possibility that other components of the TGF-β pathway may contribute to this observation, but our data in B-cell lymphoma support the concept that SMAD5 is the main mediator of these events.
The importance of a deregulated RB/E2F pathway in DLBCL has been recently highlighted.8,9 Typically, copy number loss at the CDKN2A, RB1, and TP53 loci and amplification of CCND3 and CDK2/4/6 are found in these lymphomas, resulting in unrestrained cell cycle progression and consistently poor outcome. Interestingly, the adverse outcome associated with a deregulated RB/E2F axis appears to be independent of the International Prognostic Index and the cell-of-origin categorization of DLBCL.8 Our finding that the targeting of SMAD5 by miR-155 results in sustained phosphorylation (and inactivation) of RB adds to the list of mechanisms by which the RB/E2F axis is perturbed in DLBCL. Perhaps not coincidently, higher expression of miR-155 has been associated with adverse outcome in DLBCL.24-27 Interestingly, the preliminary evidence that DLBCL with cell cycle deregulation is uniquely sensitive to CDKIs8 suggests that miR-155 could function as a biomarker for clinical response to these agents. Finally, considering the pleotropic nature of miRNA function, it should be kept in mind that the putative lymphomagenic activities of miR-155 likely derive from the targeting of multiple genes and modulation of various pathways.
Together, our findings emphasize the relevance of the TGF-β1–mediated activation of SMAD5 in B-cell biology, shed light on the contribution of miR-155 to mature B-cell development, and highlight a novel lymphomagenic property for this miRNA, the ability to deregulate the RB/E2F axis.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Klaus Rajewsky for the miR-155 KO mouse and Hakim Bouamar and Long Wang for technical help.
This work was supported by a grant from the National Institutes of Health National Cancer Institute (R01-CA138747) (R.C.T.A), a Young Investigator Award from the Voelcker Fund (R.C.T.A), and a National Institutes of Health National Cancer Institute Cancer Center Support Grant (P30 CA054174).
Authorship
Contribution: D.J. designed and conducted experiments, read the manuscript, and agreed with its contents; and R.C.T.A. conceived the project, designed experiments, analyzed data, wrote the manuscript, and read and agreed on the contents of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ricardo Aguiar, Department of Medicine, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr, San Antonio, TX, 78229; e-mail: aguiarr@uthscsa.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal