In this issue of Blood, Cines and colleagues discover that red blood cells take up an unusual, and hitherto undiscovered, shape that contributes to blood clotting.1
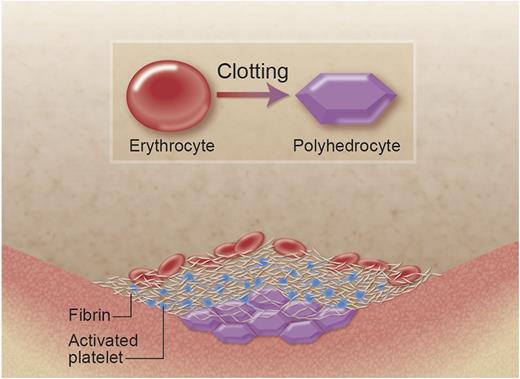
A new shape of the red blood cell helps to seal the clot. The erythrocyte (red blood cell, on the left) loses its typical biconcave shape when it is incorporated into the clot. In particular, at the center of the blood clot, due to the pressures induced by clot contraction (platelets pulling at fibrin fibers), the red blood cell takes up a different, new shape of a polyhedrocyte (right). The polyhedrocyte is characterized by flat surfaces and straight edges, which, when packed closely together, makes an almost perfect seal at the center of the blood clot, reducing blood loss.1 Professional illustration by Alice Y. Chen.
A new shape of the red blood cell helps to seal the clot. The erythrocyte (red blood cell, on the left) loses its typical biconcave shape when it is incorporated into the clot. In particular, at the center of the blood clot, due to the pressures induced by clot contraction (platelets pulling at fibrin fibers), the red blood cell takes up a different, new shape of a polyhedrocyte (right). The polyhedrocyte is characterized by flat surfaces and straight edges, which, when packed closely together, makes an almost perfect seal at the center of the blood clot, reducing blood loss.1 Professional illustration by Alice Y. Chen.
The red cells in the clot produce a polyhedral shape, which as it turns out, leads to an almost perfect seal of the clot, due to maximum cell-cell interaction and minimum interstitial space. The polyhedron is a shape with several flat surfaces and straight edges, which can be packed more tightly in small spaces than other more round shapes of similar volume. In analogy with the nomenclature for other red cell shapes such as macroovalocytes, microcytes, or poikilocytes, the authors call the new red cells polyhedrocytes (see figure). Polyhedrocytes occur particularly at the center of the clot, where the cells are most tightly packed. Toward the outer edges of the clot, the authors largely found normal, biconcave red cells or intermediate shapes and a higher concentration of fibrin fibers. The authors show that the formation of polyhedrocytes requires the presence of both fibrin and platelets, and they conclude that clot retraction due to the pulling of activated platelets on fibrin fibers, and the subsequent compression of red cells at the center of the clot, is the most likely cause for the generation of these types of cells.1
The role of red cells in hemostasis and thrombosis has recently attracted renewed attention. It was previously recognized that increased aggregation of red cells and changes in blood viscosity (which is largely determined by red cells) contribute to the risk of thrombosis. However, the red cell does not appear to be a mere bystander in blood clot formation because other studies have shown that red blood cells influence fibrin network structure and pore size.2 Recent studies have shown that red cells also influence the viscoelastic properties of the clot,3 and slow down fibrinolysis by different mechanisms not only based on reduced penetration of the clot by the fibrinolytic enzymes.4 Using atomic force microscopy, Carvalho and colleagues showed that red blood cells specifically interact with fibrin(ogen) via a β3-dependent integrin receptor.5 It is thought that this receptor interacts with the fibrinogen α-chain between residues 207 and 303.6 In addition, the red cell is able to produce microparticles that activate the coagulation7 and complement pathways.8 Taken together, these studies clearly show that the red blood cell actively contributes to processes involved in the stemming of bleeding and fighting infection.
Many different shapes of red blood cells are known to exist, particularly in pathological conditions associated with anemia. Anemia induced by iron deficiency, for example, leads to the production of microcytes (unusually small red cells due to abnormalities in heme production), whereas anemia induced by folate or vitamin B12 deficiency associates with the formation of macroovalocytes (large oval-shaped red cells due to abnormalities in cell mitosis). Both types of acquired anemia are characterized by anisocytosis (cells of different sizes) and poikilocytosis (cells of varying shape). Hemolytic anemias may be caused by abnormalities in the red cell membrane due to mutations in the cytoskeletal protein spectrin,9 or glucose-6-phosphate dehydrogenase deficiency,10 leading to a reduced red cell lifespan associated with the presence of spherocytes (small and dense cells) or not. Another example of hemolytic anemia is sickle cell disease which is caused by mutations in the β-globin gene leading to hemoglobin aggregation and sickling of the red cell, also called drepanocyte. In view of this nomenclature based on red cell shapes, Cines and colleagues decided to call the new red cell a polyhedrocyte. This new shape of red cell, although it appears to be largely induced by external forces (clot retraction induced by platelets pulling on fibrin fibers), has an obvious functional role in providing a suitable structural seal for the blood clot.
Based on these findings, the notion that the red cell would be an innocent bystander when it comes to hemostasis and thrombosis can no longer be supported. It is perhaps not coincidental that the most abundant anucleate blood cell (the red cell) plays an important role together with the second most abundant and only other anucleate blood cell (the platelet) in the stemming of bleeding. These fundamental discoveries about the role of the red cell in clot formation reported in this issue of Blood may lead to future new developments in the treatment of diseases associated with bleeding or thrombosis.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal