Key Points
In contracted clots and thrombi, erythrocytes are compressed to close-packed polyhedral structures with platelets and fibrin on the surface.
Polyhedrocytes form an impermeable seal to stem bleeding and help prevent vascular obstruction but confer resistance to fibrinolysis.
Abstract
Contraction of blood clots is necessary for hemostasis and wound healing and to restore flow past obstructive thrombi, but little is known about the structure of contracted clots or the role of erythrocytes in contraction. We found that contracted blood clots develop a remarkable structure, with a meshwork of fibrin and platelet aggregates on the exterior of the clot and a close-packed, tessellated array of compressed polyhedral erythrocytes within. The same results were obtained after initiation of clotting with various activators and also with clots from reconstituted human blood and mouse blood. Such close-packed arrays of polyhedral erythrocytes, or polyhedrocytes, were also observed in human arterial thrombi taken from patients. The mechanical nature of this shape change was confirmed by polyhedrocyte formation from the forces of centrifugation of blood without clotting. Platelets (with their cytoskeletal motility proteins) and fibrin(ogen) (as the substrate bridging platelets for contraction) are required to generate the forces necessary to segregate platelets/fibrin from erythrocytes and to compress erythrocytes into a tightly packed array. These results demonstrate how contracted clots form an impermeable barrier important for hemostasis and wound healing and help explain how fibrinolysis is greatly retarded as clots contract.
Introduction
Blood clotting is a necessary part of hemostasis in which platelets aggregate to form a temporary sealant and fibrinogen is converted to a network of fibrin polymers to stem bleeding, yet both of these processes are also linked to thrombosis.1-4 The resulting viscoelastic gel then contracts through the action of cytoplasmic motility proteins inside platelets, such that fluid (serum) is expelled, a process called clot contraction or retraction. Clots made from platelet-rich plasma (PRP) generate a bulk contractile force that begins shortly after the clot is formed and increases over minutes to hours to a maximum of about 1500 to 4500 dyn/cm2.5,6 The function of clot contraction is not fully known, but it appears to reinforce hemostasis by forming a seal, promote wound healing by approximating the edges, and restore blood flow by decreasing the area obstructed by intravascular clots.6-8
Although erythrocytes are a major component of blood clots, little is known about their participation in clot contraction. Historically, the presence of erythrocytes in contracted blood clots has been recognized and sometimes exploited; for example, during the time of medical bloodletting, the size of the contracted clot from blood removed from the patient was used as a measure of erythrocyte mass to determine when the procedure should cease.6 Moreover, erythrocytes are uniquely deformable cells with shapes determined by their geometric and material properties9-11 that regularly undergo extensive, reversible shape changes under the influence of intravascular fluid forces and while passing through vessels in the microcirculation smaller than their diameter. However, the effects on erythrocytes of the forces generated by clot contraction and their impact on hemostasis have not heretofore been investigated.
A new tool utilizing T2 magnetic resonance (T2MR) to follow the dynamic process of clot formation and contraction of whole blood as a function of time (see “Methods”) facilitated the discovery of previously undescribed effects of forces generated during contraction on erythrocytes. Here, we show that the interior of contracted blood clots and thrombi is primarily composed of erythrocytes that are compressed to form a tessellated array of polyhedra, with platelets and fibrin on the exterior.
Methods
Preparation of clots
Blood was obtained from deidentified healthy volunteers not taking aspirin, nonsteroidal anti-inflammatory drugs, or other medications known to inhibit platelet function for least 7 to 10 days, with informed consent in accordance with the Declaration of Helsinki and approval by the University of Pennsylvania Institutional Review Board. We used 11 blood donations for about 65 experiments that form the basis of the studies reported here. Blood was drawn via venipuncture into 3.2% trisodium citrate (9:1) following standard procedures that minimize platelet activation. Samples were kept at room temperature and were studied within 4 hours after the blood draw. A complete blood count was performed on an automated hematology analyzer (HemaVet 950FS, Drew Scientific).
Clotting was initiated by addition of 2 µL of 0.2M CaCl2 (Sigma-Aldrich) solution and 1 µL of kaolin (Haemonetics) solution to 34 μL of blood that was prewarmed for 1 minute at 37°C. In other experiments, thrombin was used instead of kaolin (final concentration, 0.1-2.0 U/mL; Sigma-Aldrich). Blood was also activated with tissue factor by adding 2.4 μL of ex-tem (tissue factor reagent) and 2.4 μL of star-tem (calcium reagent) (Tem Systems, Durham, NC) to prewarmed, citrated blood. Triton X-100–coated tubes were used to ensure the integrity of the clot and to prevent its adhesion to the tube wall, but care was taken to avoid potential lysis of the cells.
To determine the requirements for the observed structures of contracted clots, a reconstituted system was developed so that the components of blood could be varied. A total of 12 mL of blood was centrifuged for 15 minutes at 210g at 25°C to obtain a PRP fraction (top layer) that was transferred to a new tube after the first spin. The residual blood preparation as well as the PRP fraction was further centrifuged at 900g for 10 minutes at 25°C. To prevent platelet aggregation, prostaglandin E1 (final concentration, 5 μM; Sigma-Aldrich) was added to the PRP. The supernatant from the PRP fraction was aspirated, and the pellet was resuspended in platelet-poor plasma (PPP) not containing prostaglandin E1 to yield a concentrated platelet suspension. PPP was obtained by centrifugation of PRP for 5 minutes at 20 000g at 25°C. Packed erythrocytes, composed of the fraction left in the tube after the PPP and the buffy coat were removed, were obtained after the second centrifugation. Reconstituted blood samples were produced by mixing PPP, concentrated platelets, and packed erythrocytes in predesignated ratios. Complete blood counts for these samples were determined using the HemaVet analyzer.
Lightly fixed erythrocytes with increased membrane rigidity were prepared as described previously.12 Normal erythrocytes were washed 3 times in buffered saline containing potassium and glucose (134 mM NaCl, 5 mM KCl, 8.6 mM Na2HPO4, 1.4 mM NaH2PO4, and 11 mM glucose [pH 7.4]) and resuspended to a 5% hematocrit. A 3% stock solution of glutaraldehyde in the same buffer was then added to the erythrocyte suspension to give a final concentration of 0.03% (∼3 μM) or 0.06%. The erythrocyte suspension was incubated for 5 minutes at room temperature, and the cells were washed twice in the same buffer and resuspended in PRP to a final hematocrit of 40%. Treatment at these low concentrations of glutaraldehyde results in increased membrane rigidity in the absence of detectable hemoglobin crosslinking or consequent increases in cytoplasmic viscosity.12
Mouse blood was drawn from the retro-orbital plexus using a protocol approved by the Institutional Animal Care and Use Committee at the Children’s Hospital of Philadelphia into a 50-µL capillary containing 5 µL of 3.8% sodium citrate (final concentration, 12.9 mM). Clots were formed and isolated as described above for human blood.
Coronary artery thrombi
Thrombi were aspirated with a low-profile catheter (Export 6F; Medtronic, Santa Rosa, CA) by the treating cardiologists from patients with ST-elevation myocardial infarction (with a Thrombolysis In Myocardial Infarction flow grade 0 or all patients with a visible thrombus if the flow grade was 1 or more) who were referred to the catheterization laboratory, whenever the anatomy of the coronary artery (curve and size) allowed it.13 The thrombi were immediately washed and prepared for scanning electron microscopy.
Scanning electron microscopy
Clots were washed in 0.05 M sodium cacodylate (pH 7.4), 0.10 M NaCl for several minutes and fixed in 2% glutaraldehyde, dehydrated in ethanol, dried with hexamethyldisilazane, and sputter coated with gold-palladium.14,15 Samples were examined in a Phillip/FEI XL20 scanning electron microscope (FEI, Hillsboro, OR).
Confocal light microscopy
Three-dimensional–image data sets of blood and clots before and at various times during contraction were collected with a Zeiss LSM 710 confocal microscope. For erythrocyte labeling, 1 mL of citrated blood was spun at 190g for 10 minutes to obtain PRP and to remove the buffy coat. Erythrocytes were washed twice with phosphate-buffered saline with 0.5% bovine serum albumin, resuspended in 2 mL of the same buffer, and labeled with 40 µL of Vybrant DiD (Invitrogen) for 15 min at 37°C. After washing twice with 15 mL of phosphate-buffered saline with 0.5% bovine serum albumin, the erythrocytes were spun at 930g and the buffer was discarded. The samples for confocal microscopy were prepared by mixing 60 μL of blood containing calcein AM (final concentration, 2 mM; Invitrogen), Alexa 564–labeled fibrinogen (final concentration, 50 µg/mL; Invitrogen), 20 µL of labeled erythrocytes, and 20 µL of PRP. The flow cytometric analysis of the sample confirmed calcein-labeled platelets and white blood cells and ∼30% of DiD-labeled erythrocytes. Clots were made in an 8-well gasket chamber slide by recalcification and addition of 1 U/mL thrombin as described above. Confocal images were obtained before and during clotting.
T2MR measurement of diffusion into contracted clots
The discovery of unique effects of forces of contraction on erythrocytes and the identification of the appropriate conditions for analysis were facilitated by measuring blood-clot contraction with T2MR. T2MR consists of the use of a portable nuclear magnetic resonance relaxometer to measure T2 (spin-spin) relaxation in small liquid samples. The T2MR instrument (T2 Biosystems) contains a temperature-controlled 0.5 T permanent magnet specialized for T2 relaxation measurements in 20- to 40-μL liquid samples. T2MR signals are measured using the Carr-Purcell-Meiboom-Gill method of determining T2 relaxation16 and an inverse Laplace transform17 to deconvolute multiple T2 signals that exist in heterogeneous samples. Similar methods have been previously applied to extracting multiple T2 relaxation components from gel-transition reactions such as milk syneresis.17 In our study, measuring the T2 signals over time allows monitoring of the T2 signals from changes in water populations from different components of the sample during the course of clot contraction.
Water comprises the majority of hydrogen atoms in blood, so using water as a probe by measuring its T2MR properties over time can be a sensitive and general way to monitor the changes that occur on coagulation. Hydrogen atoms from macromolecules (proteins, nucleic acids, and lipids) have relaxation times that are much shorter than those from water and are excluded from our analysis on that basis. T2MR signals were used for measurements of the diffusion rates of water in contracted and noncontracted clots. Diffusion of D2O into a clotted sample was achieved by diluting the sample 3.8× with D2O/isotonic saline solution and observing the changes in the T2MR spectrum over time. To identify the conditions and time course for clot retraction, T2MR was also used to monitor continuous changes in blood during clotting.18 The T2MR spectra showed discrete populations for serum, fibrin/erythrocyte aggregates, and contracted clots.
Results
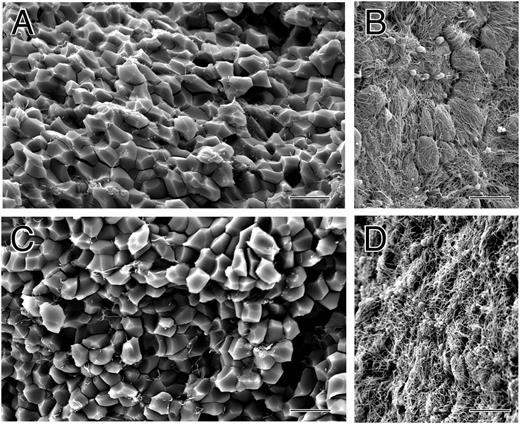
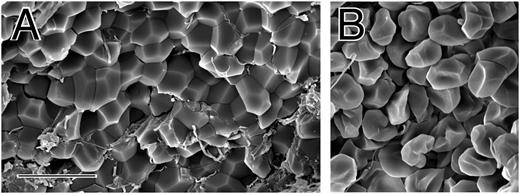
Small volumes (30-40 μL) of whole blood collected into sodium citrate as an anticoagulant were clotted by recalcification and addition of thrombin. After contraction was complete, clots were washed and fixed, and they were fractured so that both the outside and inside could be examined by scanning electron microscopy. The outer surface of the contracted clot was composed primarily of a dense meshwork of fibrin together with platelet aggregates (Figure 1B,D). In some areas on the surface, there were large clumps of biconcave erythrocytes (supplemental Figures 1 and 2, available on the Blood Web site). In contrast, the inside of the contracted clot was composed mostly of closely packed polyhedra, each about 5 μm in diameter, with little fibrin and few platelets (Figure 1A,C).
The structure of contracted human whole blood clots. Scanning electron micrographs of whole-blood clots activated by thrombin following recalcification. (A,C) Inside of contracted clots, revealing close-packed polyhedra. (B,D) Outside of contracted clots, showing a thick meshwork of fibrin and platelet aggregates. Magnification bar = 10 µm.
The structure of contracted human whole blood clots. Scanning electron micrographs of whole-blood clots activated by thrombin following recalcification. (A,C) Inside of contracted clots, revealing close-packed polyhedra. (B,D) Outside of contracted clots, showing a thick meshwork of fibrin and platelet aggregates. Magnification bar = 10 µm.
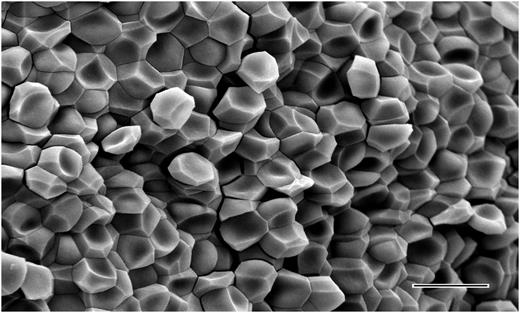
There are several lines of evidence indicating that these polyhedra are compressed erythrocytes, which we call polyhedrocytes based on the naming of other erythrocyte shapes. First, no other component of blood was present in sufficient quantity to account for the abundance of these structures. Second, some partially compressed erythrocytes were seen that were intermediate in shape between the typical biconcave structure and the polyhedra (supplemental Figure 3). Third, clots made with low fibrin(ogen) or at low platelet counts contained normally shaped erythrocytes on the inside and outside of the clot and no polyhedrocytes (supplemental Figure 4), indicating that the force of platelet contraction acting on fibrin is necessary for the compression of erythrocytes into polyhedrocytes. Fourth, pockets within a clot containing normally shaped erythrocytes contiguous with polyhedrocytes were observed on occasion (supplemental Figure 5); we infer that the forces of contraction could not be transmitted to the erythrocytes on the surface of this pocket and hence they retained their normal shape. Fifth, the estimated volume of these polyhedral structures, calculated from their size, approximates that of an erythrocyte. Finally, we demonstrated that these polyhedral structures arise from the forces exerted on erythrocytes by observation of the same close-packed polyhedrocytes after centrifugation of blood without clotting at forces of at least 1000g (Figure 2), which corresponds to minimal required stresses of ∼150 dyn/cm2 (see Figure 2 legend).
The structure of erythrocytes from centrifuged unclotted human blood. Scanning electron micrograph showing the shape change of erythrocytes as a result of the forces of centrifugation at 6000g. Polyhedrocytes were observed with centrifugation of blood at forces of 1000g or greater, but not at lower forces. From the weight of a single erythrocyte of ∼1 pN or 10−7 dyn force applied to half of its surface area, the minimal stress required to induce polyhedrocyte formation and tight packing is estimated to be ∼75 to 150 dyn/cm2, well within the range of stress generated by platelets during contraction. Magnification bar = 10 µm.
The structure of erythrocytes from centrifuged unclotted human blood. Scanning electron micrograph showing the shape change of erythrocytes as a result of the forces of centrifugation at 6000g. Polyhedrocytes were observed with centrifugation of blood at forces of 1000g or greater, but not at lower forces. From the weight of a single erythrocyte of ∼1 pN or 10−7 dyn force applied to half of its surface area, the minimal stress required to induce polyhedrocyte formation and tight packing is estimated to be ∼75 to 150 dyn/cm2, well within the range of stress generated by platelets during contraction. Magnification bar = 10 µm.
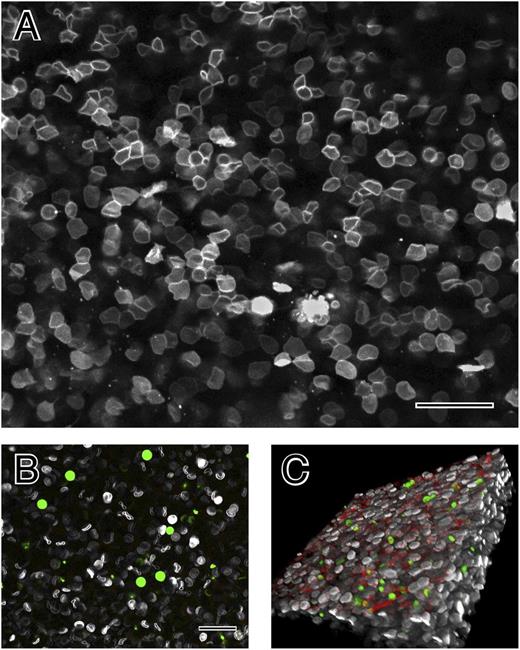
To ensure that these structures were not an artifact of specimen preparation for electron microscopy, fresh hydrated blood clots were also examined by confocal light microscopy with erythrocytes, platelets, and fibrin(ogen), each labeled with a different fluorophore. Shortly after clotting, the erythrocytes were biconcave (Figure 3B), but as contraction occurred, they became polyhedral, in tightly packed arrays with an appearance very similar to that in scanning electron micrographs (Figure 3A). Moreover, fibrin and platelets were primarily on the outside of the contracted clots (Figure 3C).
The structure of contracted human whole blood clots by confocal light microscopy. (A) Confocal light micrograph of contracted whole-blood clot activated by thrombin following recalcification. Erythrocyte membranes of 30% of cells were fluorescently labeled with DiD. (B) Whole blood before clotting. Leukocytes and platelets were labeled with calcein (green); larger cells are leukocytes and smaller ones are platelets. Fibrinogen was labeled with Alexa 564 but is not visible, because it is not polymerized. (C) Three-dimensional reconstruction of contracted whole-blood clot. The outside of the clot is on the top, where there are platelets (green), fibrin (red), and biconcave erythrocytes (white). Erythrocytes deeper in the clot are polyhedral. Magnification bar = 25 µm.
The structure of contracted human whole blood clots by confocal light microscopy. (A) Confocal light micrograph of contracted whole-blood clot activated by thrombin following recalcification. Erythrocyte membranes of 30% of cells were fluorescently labeled with DiD. (B) Whole blood before clotting. Leukocytes and platelets were labeled with calcein (green); larger cells are leukocytes and smaller ones are platelets. Fibrinogen was labeled with Alexa 564 but is not visible, because it is not polymerized. (C) Three-dimensional reconstruction of contracted whole-blood clot. The outside of the clot is on the top, where there are platelets (green), fibrin (red), and biconcave erythrocytes (white). Erythrocytes deeper in the clot are polyhedral. Magnification bar = 25 µm.
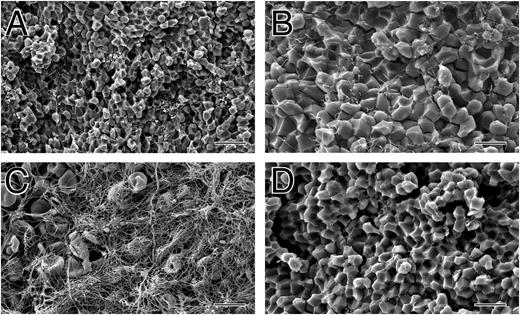
Nearly all earlier published studies on clot contraction were carried out with PRP without erythrocytes. To investigate the involvement of erythrocytes, platelets, and fibrin in the compression of erythrocytes, clots were made using a reconstituted system by adding varying amounts of fibrinogen, washed platelets, and erythrocytes. At normal levels of all components, the exteriors of the resultant contracted clots were composed mainly of fibrin and platelet aggregates and the interiors of polyhedrocytes (Figure 4A-C), a composition and structure very similar to that of natural whole blood clots (Figure 1). In contrast, clots made at low levels of platelets did not contract and contained biconcave erythrocytes, fibrin, and platelets on the interior and exterior surfaces (supplemental Figure 4). Clots were then made with low levels of fibrinogen (0.5 mg/mL) with normal hematocrit and 6 different levels of platelets. At low fibrinogen levels, a higher concentration of platelets was required for polyhedrocytes to form during clot contraction. At a concentration of 300 000/μL platelets, the formation of polyhedrocytes was marginal, while they formed uniformly when platelets were present at 600 000/μL and 900 000/μL. In contrast, at normal concentrations of fibrinogen, polyhedrocytes form at 150 000/μL platelets. Instead of adding thrombin directly, clotting was also initiated by either extrinsic or intrinsic pathways. Clots made upon recalcification of whole blood and addition of kaolin, which activates the intrinsic pathway or contact-phase reactions, underwent contraction and had the same appearance as those generated by thrombin (Figure 4A-C).
The structure of clots made from reconstituted human whole blood activated with kaolin and mouse whole blood. (A-C) Scanning electron micrographs of clots made from reconstituted human whole blood consisting of 2 mg/mL fibrinogen, 167 000 platelets/μL, 37% hematocrit, activated by kaolin following recalcification. (A-B) Inside of these contracted clots, revealing close-packed polyhedra. (C) Outside of contracted clot, showing a thick meshwork of fibrin and platelet aggregates. (D) Scanning electron micrograph of the inside of a mouse whole-blood clot. Magnification bar = 20 µm (A) and 10 µm (B-D).
The structure of clots made from reconstituted human whole blood activated with kaolin and mouse whole blood. (A-C) Scanning electron micrographs of clots made from reconstituted human whole blood consisting of 2 mg/mL fibrinogen, 167 000 platelets/μL, 37% hematocrit, activated by kaolin following recalcification. (A-B) Inside of these contracted clots, revealing close-packed polyhedra. (C) Outside of contracted clot, showing a thick meshwork of fibrin and platelet aggregates. (D) Scanning electron micrograph of the inside of a mouse whole-blood clot. Magnification bar = 20 µm (A) and 10 µm (B-D).
What is markedly different about the polyhedral erythrocyte shape is that it allows closer packing of the compressed cells that is much denser than the rouleaux, or stacks of discoid erythrocytes, that occur in inflammatory conditions, diabetes, and some cancers. To test this assessment, we replaced the water surrounding fibrin clots with a D2O/saline solution and examined the change in the T2MR signals during hydrogen/deuterium exchange. We observed rapid hydrogen/deuterium exchange for noncontracted clots (seconds) and slow exchange for contracted whole-blood clots (hours), consistent with their tightly packed structure (see Table 1). The exchange rate fell as the hematocrit was lowered and was most rapid for contracted clots that formed in the absence of erythrocytes (Table 1). These T2MR results demonstrate that the contracted clots with polyhedrocytes form a barrier only slowly permeable to water, a function that is likely to be important for hemostasis.
Hydrogen/deuterium exchange rates
Approximate time to half the initial signal.
This value is limited by the time necessary to do the experiment; in reality, it is likely to be milliseconds.
Results were variable (sometimes many hours).
Some unusual oval erythrocytes, or ovalocytes, have a cell membrane that is considerably stiffer than that of normal erythrocytes.12 We therefore investigated the effects of the stiffness of the erythrocyte membrane on the observed shape changes. To do so, we lightly fixed erythrocytes so that they had about the same stiffness as ovalocytes, namely about 5% of the deformability of normal erythrocytes. We found that polyhedrocytes were still formed upon contraction of clots made with 2 U/mL thrombin, although fixation affected the surface of erythrocytes, such that the polyhedrocytes had faces that were not as smooth and edges that were not as straight (supplemental Figure 6A). In contracted clots made with 0.05 U/mL of thrombin, most, but not all, of the erythrocytes were polyhedral. At a 2× higher concentration of glutaraldehyde (0.06%)12 in clots made at 2 U/mL of thrombin, most of the cells were polyhedrocytes, but when clots were formed at 0.05 U/mL thrombin, many erythrocytes were not polyhedral (supplemental Figure 6B).
To investigate the presence of these polyhedrocyte structures in other animal species, we also examined clots made from the blood of mice, because this is one of the most commonly used laboratory animal models. We found that contracted mouse clots also show polyhedrocytes (Figure 4D), similar to those in human clots but slightly smaller, consistent with the smaller size of mouse erythrocytes.
To ensure that these structures were not an in vitro artifact, we also examined coronary artery thrombi aspirated by cardiologists via catheter from 10 human patients with ST-elevation myocardial infarction. Closely packed polyhedra, identical to those described above, were found in these coronary artery thrombi (Figure 5), demonstrating that these contracted clot structures do occur in vivo and have the same morphology as those formed in vitro.
Polyhedral erythrocytes in human coronary artery thrombi. (A-B) Scanning electron micrographs of portions of thrombi aspirated from the coronary arteries of patients with ST-elevation myocardial infarction. Erythrocytes make up only about 11% of the volume of these thrombi, but some are polyhedral. Magnification bar = 10 µm.
Polyhedral erythrocytes in human coronary artery thrombi. (A-B) Scanning electron micrographs of portions of thrombi aspirated from the coronary arteries of patients with ST-elevation myocardial infarction. Erythrocytes make up only about 11% of the volume of these thrombi, but some are polyhedral. Magnification bar = 10 µm.
Discussion
The structure and biological properties of contracted blood clots can be understood by considering some aspects of the mechanisms of clot contraction and basic physical principles. Contraction is brought about by cytoskeletal systems similar to those responsible for motility or contraction of other cells. Platelets contain actin and nonmuscle myosin IIA, which are essential for contraction.19 Although there had been controversy over whether contraction involves only platelet-platelet interactions, it is now accepted that fibrin is necessary to provide connections between platelets to allow the transmission of force.20-23 Similarly, polyhedrocytes do not form at low concentrations of platelets, probably because they cannot generate sufficient force. Higher platelet levels are necessary for the formation of polyhedrocytes if the fibrinogen concentration is low, perhaps because some platelets are pulling ineffectively if they cannot find enough fibrin(ogen).
In addition to uniquely shaped erythrocytes, a major unexpected result from these studies was the presence of much fibrin and platelet aggregates on the surface of the contracted clots but little in the interior, which was composed mostly of polyhedrocytes. Prior to contraction, these clotting components were distributed uniformly throughout the clot and the network was isotropic. Some clues to mechanisms of the subsequent spatial separation of the erythrocytes and the fibrin/platelet aggregates during contraction come from published observations. Erythrocytes tend to segregate themselves amid the pores within a fibrin clot.24 Platelet contraction may then force those and adjacent erythrocytes closer together, forming larger clusters, while extruding other erythrocytes to the outside of the network, consistent with our observation that some erythrocytes were not incorporated into the clots. However, to achieve the observed segregation of these components, there must also be remodeling of the fibrin-platelet network and redistribution of the components to the exterior. It has been demonstrated that remodeling of fibrin networks can occur prior to crosslinking by factor XIIIa, because fibrin polymerization is a reversible reaction.25 Such a mechanism could account for the disassembly and reassembly of the network, but the mechanism of the redistribution is as yet unknown, although it is likely that both the forces generated by platelets and their action on a large mass of cells play roles. In addition, there is a fibrinogen-binding adhesion receptor on erythrocytes,26,27 which may be responsible for erythrocyte aggregation in the blood associated with cardiovascular disease and could also be involved in the self-association of erythrocytes within the contracted clot.
Although there have been a plethora of studies of various and often dramatic changes in shape that erythrocytes can undergo under certain pathological and experimental conditions,28 these polyhedral structures have not been reported previously. Erythrocytes can go from normal discocyte to echinocyte (with convex rounded protrusions or spicules) or spheroechinocyte, acanthocyte (with club-shaped spicules of varying lengths) or stomatocyte (with a uniconcave cup shape). Thus, the polyhedrocyte is another physical form of these uniquely deformable cells.
Moreover, the propensity of erythrocytes to undergo such deformation is known to influence disease states such as sickle cell anemia and malaria. Some Malayan aborigines in Papua, New Guinea, have ovalocytes or elliptocytes, erythrocytes that are oval or elliptical. This condition, ovalocytosis, confers resistance to malaria, because these ovalocytes have a stiffer membrane than normal erythrocytes.12 Because light fixation of normal erythrocytes by glutaraldehyde also confers resistance to malaria, we tested if such an increase in rigidity would prevent these erythrocytes from being made into polyhedrocytes by clot contraction. We found that the erythrocytes were still polyhedral unless we went to low thrombin and higher glutaraldehyde concentration, indicating that platelets have considerably more strength than necessary to compress erythrocytes.
The viscoelastic properties of erythrocytes are determined by many factors, including the viscosity of their cytoplasm, the hemoglobin concentration and composition, and their unique cytoskeleton and plasma membranes.9 Each time erythrocytes reach a small vessel, they must stretch into a bullet-like shape to squeeze through and then return to their original discoid shape upon exiting the vessel. Their biconcave-discoid shape provides extra surface, enabling shape change without increasing surface area, and thus requiring significantly smaller forces to make the physical transitions.29 The extent and geometry of these shape changes are determined by the magnitude of the applied forces and the mechanical properties of erythrocytes, which arise in part from the spectrin network underlying the membrane.11 The tight packing of polyhedra in contracted clots minimizes the volume occupied by the mass of erythrocytes.
Why do the erythrocytes become polyhedral when they are compressed? In appearance, the close-packed arrays of polyhedra hardly seem biological. Yet, D’Arcy Thompson, in his classic book On Growth and Form using basic physical principles to examine why living things take the shapes that they do, discussed groups of similar polyhedral cells in certain tissues, such as vegetable parenchyma.30 Lord Kelvin solved the problem of how to partition space into cells such that the total area of interfaces between them was a minimum and demonstrated that certain polyhedra are optimal.31 The physical reason for the existence of any of these tightly packed polyhedra is that this shape minimizes the potential energy of the system.30 Because this is a basic physical principle, it is a likely explanation for the existence of polyhedrocytes as well, probably involving the minimization of membrane-bending energy and stabilization by the membrane skeleton.10 Although the polyhedral shape of erythrocytes is newly discovered, this is an ancient cell shape evolutionarily, because contracted mouse clots also show polyhedrocytes (Figure 4D).
The erythrocytes in contracted clots form a space-filling array of polyhedra, which generate a tessellation of space, forming geometric shapes that pack with no gaps and no overlaps, as in Voronoi cells.32 Such dense-packing problems have been studied at least since the time of Aristotle, but most recent reports have focused on functionally important nonbiological materials.33,34 Furthermore, most research has concentrated on close packing of Platonic and Archimedean solids rather than such irregular polyhedra as these.35 The occurrence of such geometric shapes and packings in a common biological setting such as blood clots may stimulate more research in this area, not as an abstract exercise in mathematics but rather to understand more about their structure and functional properties, just as similar studies of other Voronoi cells have been important for both research and development of nonbiological materials.
The findings reported here may have important functional and clinical implications. Clot contraction is a necessary part of hemostasis, because both human genetic disorders of platelet myosin IIA and megakaryocyte myosin-IIA–knockout mice show a bleeding phenotype.7,36 Our results account for this bleeding phenotype and reinforce the importance of clot contraction for hemostasis. It is likely that the observed structure of contracted clots makes them stiff, rigid structures. We have demonstrated by T2MR that the tightly packed erythrocytes form a nearly impermeable, watertight seal. These features are likely to be important to stem bleeding, meaning that erythrocytes play an important role in hemostasis, as predicted.37 In support of such a function, it has been shown that contracted clots formed under flow in a model system have the same low permeability as the endothelial cell lining of blood vessels.8 In other words, one function of clot contraction may be to replicate the barrier function of the endothelial cell lining after injury.
Contraction of clots within the vasculature may also relieve obstruction of blood vessels and allow recanalization.8 This function is likely to be more significant in the venous system where clots contain large amounts of erythrocytes, but recent studies of the composition of coronary artery thrombi taken from patients after myocardial infarction13 and middle cerebral artery thrombi taken from patients after stroke38 have shown that they too can contain considerable numbers of erythrocytes. In the present study, we observed closely packed polyhedra in coronary arterial thrombi from patients after myocardial infarction (Figure 5), demonstrating that these contracted clot structures occur in vivo.
These results might also account for long-standing clinical observation that fibrinolysis is greatly retarded following clot contraction,39-43 because not all fibrin is on the outside and perfusion or diffusion of lytic enzymes into these tightly packed polyhedral erythrocytes would be very slow.44
In conclusion, clot contraction is an underexplored and underestimated pathophysiological process that could be a potential target for therapeutic intervention. Understanding the mechanisms underlying these sequential changes that occur during clot contraction and lead to impermeability could be used therapeutically to enhance hemostasis after trauma on the one hand, while interference with contraction might represent a new approach to prevent ischemic vascular occlusion.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ed Thayer (T2 Biosystems) for algorithm construction and data analysis support and Slava Papkov (T2 Biosystems) for T2MR measurement training and support. We acknowledge the support of National Institutes of Health, National Heart Lung and Blood Institute grants HL090774 and HL110860.
Authorship
Contribution: D.B.C., T.L., W.M., R.I.L, L.R., T.J.L., and J.W.W. designed experiments and analyzed data; T.L., C.N., V.H., W.M., R.I.L., and L.R. performed experiments; and D.B.C., T.L., W.M., R.I.L., L.R., T.J.L., and J.W.W. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Weisel, Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104-6058; e-mail: weisel@mail.med.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal