Key Points
Increased levels of sCD19 protein in the CSF are associated with CNS disease in DLBCL and BL patients at risk of CNS lymphoma.
Presence of lymphoma cells by FCM and/or increased CSF sCD19 levels are related with a poorer EFS and/or OS in DLBCL and BL patients.
Abstract
Flow cytometry (FCM) is more sensitive than conventional cytology for detection of occult leptomeningeal lymphoma; however, some FCM-negative patients show central nervous system (CNS) recurrence. Here, we evaluated the cerebrospinal fluid (CSF) levels of 13 B-cell–associated markers and their contribution to the diagnosis of CNS lymphoma in 91 diffuse large B-cell lymphomas (DLBCL) and 22 Burkitt lymphomas (BLs). From all markers tested, CD19 was the most informative. Thus, higher soluble CD19 (sCD19) levels were associated with a greater frequency of neurological symptoms in DLBCL and BL and with parenchymal CNS lymphoma in DLBCL; sCD19 emerged as a powerful predictor of event-free and overall survival in DLBCL and BL, particularly when combined with FCM detection of CNS disease. These results support the utility of combined FCM detection of lymphoma cells and assessment of sCD19 levels in CSF, for more accurate identification of CNS disease in DLBCL and BL patients.
Introduction
Primary or secondary central nervous system (CNS) involvement by B-cell non-Hodgkin lymphoma (B-NHL) is a relatively rare condition associated with a very poor prognosis.1,2 Currently, it is well established that flow cytometry (FCM) has a greater sensitivity than conventional cytology (CC) for the detection of cerebrospinal fluid (CSF) infiltration in Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL) patients at risk of CNS disease.3-7 However, still a significant proportion of FCM−/CC− cases (≈10%) present with highly suspicious neurological symptoms and/or parenchymal CNS disease.3,5,7 Moreover, recent data indicate that some FCM-negative cases may develop overt CNS disease early after diagnosis, particularly when CNS-directed prophylaxis had not been administrated (Wyndham H. Wilson, Jacoline E.C. Bromberg, Maryalice Stetler-Stevenson, Seth M. Steinberg, L.M.-M., C.M., J.-M.S., M.D.C., Marjan A. Davidis, Rik A. Brooimans, B.S.-G., A.S., E.G.-B., J.M.R., Margaret Shovlin, Armando Filie, Kieron Dunleavy, Thomas Mehrling, Michele Spina, and A.O., manuscript submitted December 2013).
Altogether, these findings support the notion that parenchymal involvement of CNS by aggressive B-NHL could go undetected by both CC and FCM because, in the absence of leptomeningeal disease, lymphoma cells would not reach the CSF in detectable numbers.8 Although recirculation of tumor cells through CSF might still exist in such cases,9 it could be hypothesized that if an increased turnover of lymphoma cells occurs inside the brain, tumor cell products (eg, proteins, RNA, DNA, and microvesicles) would be released at abnormally high rates into the extracellular medium. Under such circumstances, the lymphoma cell components could potentially reach the CSF. Actually, higher levels of specific microRNAs (miR) (eg, miR-19b, miR-21, and miR-92a) and proteins (eg, CD27) have been reported in CSF from CNS lymphoma vs CNS lymphoma-free patients.10-13 However, in these studies, no B-cell–specific markers, expressed by every lymphoma case, have been investigated.
Herein, we analyzed the CSF levels of a large panel of B-cell–associated proteins in 113 B-NHL (DLBCL and BL) at risk of CNS disease to determine their potential utility as surrogate markers for CNS lymphoma.
Study design
Patients and samples
A total of 113 patients diagnosed with DLBCL (n = 91) and BL (n = 22) using the World Health Organization (WHO) criteria, at the hospitals of the Spanish CNS Lymphoma Study Group (Spain), were studied. Only BL patients plus aggressive DLBCL who met ≥1 of the following criteria entered the study: infiltration of extranodal sites, elevated serum lactate dehydrogenase (LDH), and/or neurological symptoms. The study was approved by the ethics committees of the participating centers, following the Declaration of Helsinki protocol.
Patients were treated according to their institutional standards with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimens including rituximab for DLBCL and standard regimens for BL. All but 6 DLBCL patients received intrathecal prophylaxis (n = 82) or active treatment (n = 25). Median follow-up was of 30 months (range, 1-54 months); 21 patients had recurrent disease (18 DLBCL and 3 BL) from which 2 DLBCL had CNS relapse.
CSF analyses
Conventional cytomorphological analysis of CSF was performed on fresh samples at each center, while FCM was done in Transfix-stabilized CSF samples (<24 hours) at the University Hospital of Salamanca (Spain), as described elsewhere3,14 (supplemental Methods, available on the Blood Web site).
Quantification of soluble CD19, CD21, CD22, CD24, CD38, CD44, CD72, free light chain κ (FLC-κ), FLC-λ, immunoglobulin A (IgA), IgG, IgM, and β2-microglobulin was performed in 100 µL of thawed, freshly-frozen CSF supernatants by standard enzyme-linked immunosorbent assay, FLC, and β2-microglobulin assays (supplemental Methods).
Statistical methods
Receiver operating curve analysis was performed in a subgroup of 40 CSF samples to establish the most accurate cutoff values for each CSF marker, including soluble CD19 (sCD19) (≥1.18 ng/mL); sCD19 was further evaluated in another 73 CSF samples using the same (most informative) cutoff (n = 113). Event-free survival (EFS) and overall survival (OS) were determined by the Kaplan-Meier method and compared by the log-rank test. Multivariate Cox proportional hazard models (stepwise regression) were developed to explore the independent effect of different parameters on EFS and OS.
Results and discussion
First, we analyzed the CSF levels of a panel of 13 B-cell–associated markers in 40 B-NHL patients at risk of CNS disease, classified according to the presence vs absence of CNS disease by FCM and/or presence of highly suspicious neurological symptoms. From all markers investigated in these 40 cases, only sCD19 (≥1.18 ng/mL) and to a lesser extent also β2-microglobulin (≥2.56 ng/mL), showed a significant association (P < .05) with CNS disease, with an accuracy of 88% and 78%, respectively (vs <70% for all the other markers) (supplemental Table 1, supplemental Figure 1). Of note, CD19 was the only protein investigated which is both a pan-B-cell marker and a B-cell–specific protein.15 All other markers display either a pattern of expression restricted to specific B-cell maturation stages and to some subtypes of B-NHL, including BL and DLBCL, where they are expressed at variable percentages (eg, CD21 and CD24),16-18 and/or they are also expressed by cells other than B cells from brain tissues (eg, neurons, astrocytes, glial, and glioma cells).19-21 Overall, this could contribute to explain why sCD19 emerged as the marker with the highest degree of association with CNS lymphoma. However, the biological significance of sCD19 remains to be fully elucidated.
Further analysis of sCD19 in the whole cohort of 113 patients (Table 1) showed that higher sCD19 CSF levels (≥1.18 ng/mL) were more frequently found among those DLBCL cases displaying overt (FCM+/CC+; P = .05) and occult (FCM+/CC−; P < .001) leptomeningeal disease, as well as among DLBCL and BL patients who presented with highly suspicious neurological symptoms (P < .001 and P < .02, respectively) and DLBCL cases with parenchymal lymphoma by magnetic resonance imaging (P = .007). DLBCL cases with higher sCD19 levels also showed older age (P = .04), higher International Prognostic Index (P = .05), and poorer performance status (P = .04). These results indicate that sCD19 could be a sensitive marker for the detection of CNS involvement in patients with BL and DLBCL at risk of CNS disease in routine diagnostics, particularly if combined with FCM. At the same time, they suggest that sCD19 CSF levels in DLBCL and BL are not directly impacted by systemic disease because no association (P > .05) of sCD19 with peripheral blood, lymph node, and bone marrow involvement was observed. Then, we further investigated the potential impact of sCD19 CSF levels on patient survival.
sCD19 protein levels in CSF samples from DLBCL and BL patients screened for CNS disease: association with other prognostic factors of the disease
| . | . | DLBCL . | BL . | ||||
|---|---|---|---|---|---|---|---|
| Disease features . | Total cases (%) n = 113 . | sCD19 <1.18 ng/mL (%) n = 59 . | sCD19 ≥1.18 ng/mL (%) n = 32 . | P . | sCD19 <1.18 ng/mL (%) n = 13 . | sCD19 ≥1.18 ng/mL (%) n = 9 . | P . |
| Sex, male/female | 66/34 | 68/32 | 50/50 | NS | 85/15 | 78/22 | NS |
| Age at diagnosis ≥60 y | 43 | 41 | 63 | .04 | 8 | 44 | .07 |
| Stage III/IV | 74 | 66 | 78 | NS | 77 | 100 | NS |
| IPI ≥2 | 75 | 66 | 84 | .05 | 77 | 100 | NS |
| ECOG ≥2 | 49 | 41 | 63 | .04 | 38 | 67 | NS |
| Neurological symptoms* | 21 | 5 | 41 | <.001 | 15 | 67 | .02 |
| Adenopathies | 75 | 80 | 81 | NS | 62 | 44 | NS |
| Hepato and or splenomegaly | 30 | 36 | 28 | NS | 23 | 11 | NS |
| Extranodal involvement | 83 | 80 | 81 | NS | 92 | 100 | NS |
| Extranodal sites involved ≥2 | 38 | 27 | 44 | NS | 38 | 89 | .03 |
| PB involvement | 6 | 2 | 7 | NS | 23 | 13 | NS |
| BM involvement | 34 | 27 | 25 | NS | 54 | 67 | NS |
| Parenchymal CNS disease | 8 | 2 | 19 | .007 | 8 | 11 | NS |
| Hemoglobin <100 g/L | 25 | 22 | 19 | NS | 38 | 44 | NS |
| No. of platelets <100 × 109/L | 12 | 5 | 19 | .05 | 23 | 11 | NS |
| No. of lymphocytes >5 × 109/L | 19 | 10 | 20 | NS | 33 | 56 | NS |
| Total proteins <70 g/L | 73 | 73 | 62 | NS | 85 | 87 | NS |
| Serum LDH ≥450 IU/L | 62 | 61 | 50 | NS | 69 | 88 | NS |
| CRP >0.5 mg/dL | 81 | 86 | 73 | NS | 69 | 88 | NS |
| Serum β2-M ≥3mg/L | 48 | 50 | 43 | NS | 30 | 83 | .06 |
| Immune suppression | 14 | 14 | 16 | NS | 15 | 11 | NS |
| HIV+ | 9 | 10 | 3 | NS | 15 | 11 | NS |
| Intrathecal prophylaxis | 73 | 88 | 56† | .001 | 77 | 22 | .02 |
| Active intrathecal treatment | 22 | 5† | 38 | <.001 | 23 | 78 | .02 |
| CSF parameters | |||||||
| Glucose >120 mg/dL | 4 | 2 | 4 | NS | 0 | 29 | NS |
| Total proteins ≥0.45 g/L | 29 | 25 | 26 | NS | 33 | 57 | NS |
| FCM+/CC+ | 8 | 2 | 13 | .05 | 8 | 33 | NS |
| FCM+/CC− | 13 | 0 | 41 | <.001 | 8 | 17 | NS |
| . | . | DLBCL . | BL . | ||||
|---|---|---|---|---|---|---|---|
| Disease features . | Total cases (%) n = 113 . | sCD19 <1.18 ng/mL (%) n = 59 . | sCD19 ≥1.18 ng/mL (%) n = 32 . | P . | sCD19 <1.18 ng/mL (%) n = 13 . | sCD19 ≥1.18 ng/mL (%) n = 9 . | P . |
| Sex, male/female | 66/34 | 68/32 | 50/50 | NS | 85/15 | 78/22 | NS |
| Age at diagnosis ≥60 y | 43 | 41 | 63 | .04 | 8 | 44 | .07 |
| Stage III/IV | 74 | 66 | 78 | NS | 77 | 100 | NS |
| IPI ≥2 | 75 | 66 | 84 | .05 | 77 | 100 | NS |
| ECOG ≥2 | 49 | 41 | 63 | .04 | 38 | 67 | NS |
| Neurological symptoms* | 21 | 5 | 41 | <.001 | 15 | 67 | .02 |
| Adenopathies | 75 | 80 | 81 | NS | 62 | 44 | NS |
| Hepato and or splenomegaly | 30 | 36 | 28 | NS | 23 | 11 | NS |
| Extranodal involvement | 83 | 80 | 81 | NS | 92 | 100 | NS |
| Extranodal sites involved ≥2 | 38 | 27 | 44 | NS | 38 | 89 | .03 |
| PB involvement | 6 | 2 | 7 | NS | 23 | 13 | NS |
| BM involvement | 34 | 27 | 25 | NS | 54 | 67 | NS |
| Parenchymal CNS disease | 8 | 2 | 19 | .007 | 8 | 11 | NS |
| Hemoglobin <100 g/L | 25 | 22 | 19 | NS | 38 | 44 | NS |
| No. of platelets <100 × 109/L | 12 | 5 | 19 | .05 | 23 | 11 | NS |
| No. of lymphocytes >5 × 109/L | 19 | 10 | 20 | NS | 33 | 56 | NS |
| Total proteins <70 g/L | 73 | 73 | 62 | NS | 85 | 87 | NS |
| Serum LDH ≥450 IU/L | 62 | 61 | 50 | NS | 69 | 88 | NS |
| CRP >0.5 mg/dL | 81 | 86 | 73 | NS | 69 | 88 | NS |
| Serum β2-M ≥3mg/L | 48 | 50 | 43 | NS | 30 | 83 | .06 |
| Immune suppression | 14 | 14 | 16 | NS | 15 | 11 | NS |
| HIV+ | 9 | 10 | 3 | NS | 15 | 11 | NS |
| Intrathecal prophylaxis | 73 | 88 | 56† | .001 | 77 | 22 | .02 |
| Active intrathecal treatment | 22 | 5† | 38 | <.001 | 23 | 78 | .02 |
| CSF parameters | |||||||
| Glucose >120 mg/dL | 4 | 2 | 4 | NS | 0 | 29 | NS |
| Total proteins ≥0.45 g/L | 29 | 25 | 26 | NS | 33 | 57 | NS |
| FCM+/CC+ | 8 | 2 | 13 | .05 | 8 | 33 | NS |
| FCM+/CC− | 13 | 0 | 41 | <.001 | 8 | 17 | NS |
β2-M, β2-microglobulin; BM, bone marrow; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; NS, not statistically significant; PB, peripheral blood.
Fifteen of 24 (63%) patients with neurological symptoms received active intrathecal therapy.
Cases with CNS relapse.
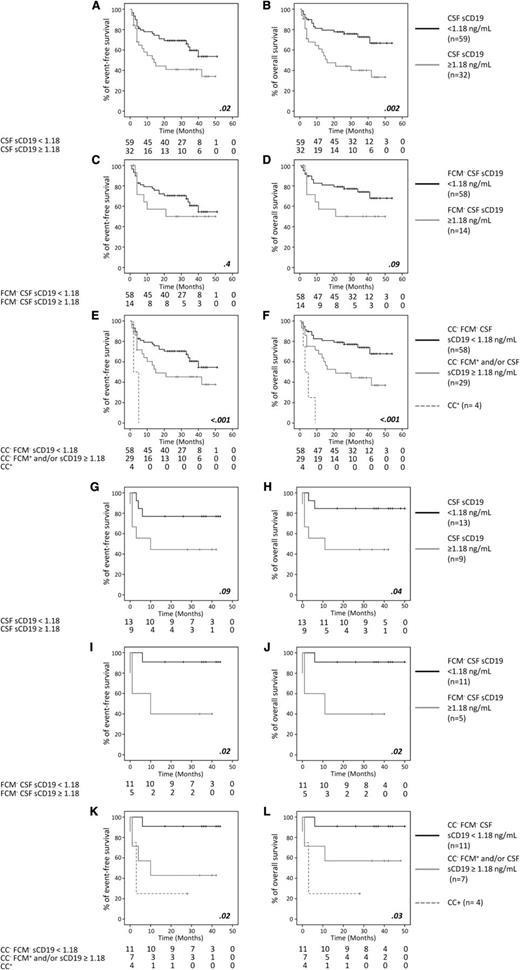
Several studies have shown a clear association between occult leptomeningeal disease detected by FCM and a shorter CNS relapse-free survival.22 Despite a similar frequency of occult leptomeningeal disease found in these and our series, only 2 of our patients had CNS recurrence, which is probably due to the administration of active therapy to most (71%) of our FCM+ cases. Consequently, it was not possible to establish here the impact of sCD19 on CNS relapse-free survival. However, previous studies have also recurrently shown that occult leptomeningeal disease by FCM has a clear impact on OS, of both DLBCL and BL.6,22 In this regard, we also found an association between higher sCD19 CSF levels and a shorter EFS and OS among DLBCL (P = .02 and P = .002, respectively; Figure 1A-B) and BL patients (P = .09 and P = .04, respectively; Figure 1G-H). Even more, once FCM-negative cases were specifically considered, FCM-negative BL patients with higher sCD19 showed a shorter EFS (P = .02) and OS (P = .02) than FCM−/sCD19low cases, the survival rates of FCM−/CC−/sCD19high cases being similar (P > .05) to those of FCM+/CC− BL patients (Figure 1I-J); a similar tendency (P > .05) was also observed for DLBCL (Figure 1C-D). The greater impact of sCD19 than FCM on BL patient survival supports the notion that in high proliferative tumors with increased apoptosis such as BL,23,24 the measurement of sCD19 could be particularly informative. Further studies are required to validate our findings and determine whether sCD19 CSF measurements should become routine in BL and high- as well as low-risk DLBCL. In turn, our results suggest that despite the absence of CNS recurrence, minimal CNS involvement (eg, CC− but FCM+ and/or sCD19high) may contribute to systemic disease recurrence, particularly when no CNS-directed therapy is used. Most interestingly, sCD19 CSF levels, when combined with FCM, emerged as a powerful independent CSF-associated prognostic factor for OS (P = .007) in DLBCL and for both EFS (P = .03) and OS (P = .05) in BL (supplemental Tables 2-3).
Prognostic impact of sCD19 levels, CC, FCM, and their combination in CSF samples from DLBCL and BL patients screened for CNS disease. EFS (A,C,E,G,I,K) and OS (B,D,F,H,J,L) curves are separately shown for DLBCL (A-F) and BL (G-L) cases, classified according to their CSF status as defined by sCD19 levels in both the whole patient group (A-B,G-H) and among only FCM− cases (C-D,I-J), and by CC and FCM in combination with sCD19 (E-F,K-L).
Prognostic impact of sCD19 levels, CC, FCM, and their combination in CSF samples from DLBCL and BL patients screened for CNS disease. EFS (A,C,E,G,I,K) and OS (B,D,F,H,J,L) curves are separately shown for DLBCL (A-F) and BL (G-L) cases, classified according to their CSF status as defined by sCD19 levels in both the whole patient group (A-B,G-H) and among only FCM− cases (C-D,I-J), and by CC and FCM in combination with sCD19 (E-F,K-L).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants RD06/0020/0035 and RD12/0036/0048 from Redes Telemáticas de Investigación Cooperativa en Salud (Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Madrid, Spain and Fondo Europeo de Desarrollo Regional) and an unrestricted grant from Mundipharma Pharmaceuticals SL.
Authorship
Contribution: C.M. and L.M.-M. performed experiments, analyzed results, made figures, and contributed to the writing of the manuscript; A.L. and J.A. analyzed results; A.O. designed the research, interpreted data, and wrote the manuscript; B.S.-G., A.S., J.-M.S., J.M.R., C.H., F.J.P., M.G., E.G.-B., N.A., B.N., T.O., F.S., E. Conde, J.A.M., E. Cabezudo, A.C., M.G.-M., and M.D.C. enrolled patients and collected clinical data; and all authors read and agreed with the contents of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Spanish Group for the Study of CNS Disease in NHL appears in “Appendix.”
Correspondence: Alberto Orfao, Centro de Investigación del Cáncer, Paseo de la Universidad de Coimbra s/n, Campus Miguel de Unamuno, 37007 Salamanca, Spain; e-mail: orfao@usal.es.
Appendix
The members of the Spanish Group for the Study of CNS Disease in NHL are: Alberto Orfao, Lourdes Martín-Martín, Carmen Muñiz, Antonio López, Blanca Sánchez-González, Antonio Salar, Julia Almeida, Juan-Manuel Sancho, José María Ribera, Cecilia Heras, Francisco Javier Peñalver, Marta Gómez, Eva González-Barca, Natalia Alonso, Belén Navarro, Teresa Olave, Francisco Sala, Eulogio Conde, José Antonio Márquez, Elena Cabezudo, Antonia Cladera, María García-Malo, María Dolores Caballero, Ana Fernández-Teijeiro, Teresa Giménez, Elena Pérez Ceballos, Santiago Mercadal, Jorge Sánchez, Pilar Martínez Barranco, Eva Domingo, Lucía Villalón, Adolfo De la Fuente, José Antonio Queizán, Purificación García Miguel, Carmen Toledo, Irene Peláez, Montserrat Arnan, Carlota Calvo, Rodolfo Álvarez, Alberto Cantalapiedra, Garazi Letamendi, Jordi Bruna, Cristina Quero, Miguel De la Cruz, Alfons Serrano, Carlos Grande, Mª Carmen Mateos Rodríguez, Guillermo Ortí, María José García Pérez, María Calbacho, Mª Paz Garrastazul, Carlos Panizo, Isabel Sánchez Ortega, Roser Velasco, Mónica Lizuain, Ana Rosell, José Manuel Calvo Villas, Raquel Del Campo, Mª Paz Queipo, Pilar Rabasa, Antonia Rodríguez Izquierdo, Almudena De la Iglesia, Ana López García, Mª Jesús Peñarrubia, Ana Carboné, Pilar Martínez Sánchez, Deborah Moreno, Lina Abenoza, Mª Luz Amigo, Ricardo López Almaraz, José Ángel Hernández Rivas, Oliver Gutiérrez Pérez, Diana García, José Mª Hernández Martín, Belén Navarro, Jesús Mª Ojanguren, Joan Bargay, Mª José Berruezo, Pilar Bravo, José Antonio García Vela, Pilar Herrera and Breno Moreno de Gusmao.
References
Author notes
C.M. and L.M.-M. have equally contributed to this work and the two should be considered as first authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal