In this issue of Blood, Risitano et al demonstrate that small-molecule inhibitors of C3 cleavage prevent complement activation on erythrocytes from patients with paroxysmal nocturnal hemoglobinuria (PNH); the authors demonstrate that these agents reach therapeutic concentrations after subcutaneous injection in nonhuman primates.1
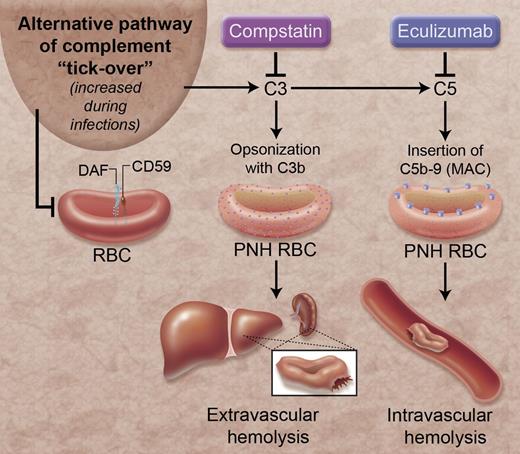
Complement inhibition and hemolysis in PNH. The alternative pathway of complement is continually activated through a process called “tick-over.” Stressors, such as infections, can intensify this process. DAF and CD59 are complement regulatory proteins that are linked to the surface of RBCs by GPI anchors and protect the RBCs from alternative pathway-mediated lysis. However, these proteins are absent from some RBCs in patients with PNH. In these patients, activation of the alternative pathway causes extravascular hemolysis of RBCs by coating the RBC with the C3b fragment, and causes intravascular hemolysis through formation of C5b-9, or the MAC, on the RBC surface. Eculizumab reduces hemolysis by preventing formation of C5b-9. Compstatin prevents the formation of C3b and C5b-9 on PNH RBCs. MAC, membrane attack complex; RBC, red blood cell. Professional illustration by Alice Y. Chen.
Complement inhibition and hemolysis in PNH. The alternative pathway of complement is continually activated through a process called “tick-over.” Stressors, such as infections, can intensify this process. DAF and CD59 are complement regulatory proteins that are linked to the surface of RBCs by GPI anchors and protect the RBCs from alternative pathway-mediated lysis. However, these proteins are absent from some RBCs in patients with PNH. In these patients, activation of the alternative pathway causes extravascular hemolysis of RBCs by coating the RBC with the C3b fragment, and causes intravascular hemolysis through formation of C5b-9, or the MAC, on the RBC surface. Eculizumab reduces hemolysis by preventing formation of C5b-9. Compstatin prevents the formation of C3b and C5b-9 on PNH RBCs. MAC, membrane attack complex; RBC, red blood cell. Professional illustration by Alice Y. Chen.
PNH is a disease in which the complement system causes lysis of erythrocytes. The proteins of the complement cascade are synthesized in the liver and circulate in plasma as inactive zymogens, but there is continuous low-level activation of the alternative pathway of complement through a process called “tick-over.”2 Tick-over of the alternative pathway initiates complement activation on exposed cells unless the cells express complement regulatory proteins that terminate the process. If complement activation is not adequately controlled on the cell surface it generates C5b-9 multimers which form pores in the plasma membrane and allow the flux of ions and water into cells.3 Erythrocytes are continually surrounded by complement proteins in plasma, so they are vulnerable to C5b-9–mediated osmotic lysis. Consequently, erythrocytes are critically dependent upon complement regulatory proteins.
PNH is caused by a somatic mutation in the gene for the protein phosphatidylinositol glycan class A (PIG-A). This defect impairs the synthesis of glycosylphosphatidylinositol (GPI). Decay accelerating factor (DAF; CD55) and CD59 are complement regulatory proteins that are anchored to the surface of erythrocytes via a GPI tail, so a subpopulation of erythrocytes in patients with PNH does not carry these 2 complement regulatory proteins and is susceptible to complement-mediated lysis. Eculizumab, a monoclonal antibody to C5, prevents C5b-9 formation and was approved by the US Food and Drug Administration (FDA) in 2007 for treatment of PNH.4 PNH patients treated with eculizumab require fewer transfusions, have higher quality-of-life indices, and may have a survival benefit.5,6 However, eculizumab does not completely halt hemolysis in PNH. This may be because eculizumab does not prevent opsonization of the PNH erythrocytes with C3b (see figure). Erythrocytes opsonized with C3b undergo extravascular hemolysis in the liver and spleen and have a shortened lifespan.
In the study by Risitano et al, the investigators tested the efficacy of several small-molecule complement inhibitors in hemolysis assays using erythrocytes from patients with PNH. The inhibitors tested are all derivatives of compstatin, an agent that prevents cleavage and activation of the complement protein C3. The rationale for these studies is twofold. First, the compstatin derivatives prevent opsonization of PNH erythrocytes with C3b as well as insertion of C5b-9 in the erythrocytes. Second, although other complement inhibitors have been tested in similar systems, most anti-complement therapeutics are large proteins, and their use is limited by immunogenicity of the molecules and high costs of production. The authors suggest that large-scale production costs for the various forms of compstatin could be as low as several dollars per dose. The authors present results demonstrating that these agents prevent C3 deposition and lysis of erythrocytes from PNH patients. They also performed pharmacokinetic experiments in nonhuman primates treated subcutaneously with one of the compstatins and show that this strategy achieved therapeutic levels of the drug.
One of the interesting findings in this study is that the levels of plasma C3 increased in nonhuman primates treated with IV pegylated-Cp40 (a long-acting compstatin derivative). This effect may be due to decreased turnover of C3, and it raises the possibility that the level of compstatin (and other C3 inhibitors) needed to prevent hemolysis could increase over time. Similarly, C3 is part of the acute-phase response and the dose of drug needed to maintain complement inhibition may increase during acute illness. Although the authors carefully measured the levels of the compound in treated animals, they did not test functional inhibition of the complement cascade, so the data presented do not confirm that the achieved levels are sufficient to fully prevent hemolysis. The benefits of fully inhibiting the complement system in patients with PNH must also be weighed against the increased risk of infection that such treatment entails. Blockade of the complement cascade at the level of C5 could theoretically pose a lower risk of infection than does blocking complement at the level of C3. The current study does not address this issue.
Because PNH is a lifelong disease, the ideal drug for this condition would be safe, inexpensive, easily administered, and nonimmunogenic with repeated exposure. Eculizumab has provided a major advance in the treatment of PNH, but it is expensive and does not completely prevent hemolysis. The compstatin derivatives tested in the current study may overcome some of these shortcomings, although these compounds still need to be evaluated in human patients before their benefits can be fully assessed. PNH is a rare disease, so studies in this patient population are not easy to conduct. Yet, the nature of PNH also makes it advantageous for testing the efficacy of new complement inhibitors in vivo: complement activation is central to the disease pathogenesis and there are clear readouts of disease activity. New drugs that prove effective in PNH will be useful for treating other complement-mediated disorders too, so efforts to develop these new therapies will have benefits beyond PNH.
Conflict-of-interest disclosure: J.M.T. is a consultant for Alexion Pharmaceuticals, Inc.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal