In this issue of Blood, Tong et al have reported that therapeutic drug monitoring (TDM) of asparaginase (ASP) activity levels in plasma may be an important tool for the optimization of its therapeutic effects in pediatric acute lymphoblastic leukemia (ALL).1
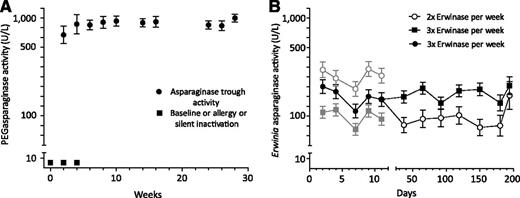
Serum PEGasparaginase activity (A) and serum Erwinia asparaginase activity (B) levels over time (mean ± standard error of the mean). See Figure 1 in the article by Tong et al that begins on page 2026.
Serum PEGasparaginase activity (A) and serum Erwinia asparaginase activity (B) levels over time (mean ± standard error of the mean). See Figure 1 in the article by Tong et al that begins on page 2026.
The main and commendable aim of the study conducted by Tong et al was the improvement of ASP therapeutic effects by preventing the onset of allergic reactions and silent inactivation (SI) in children with ALL. These 2 phenomena are in fact among the most important factors encountered leading to ASP treatment failure.2-4 To achieve this goal (ie, improvement of ASP therapeutic effects), the authors routinely switched their patients to receive pegylated ASP (PEGASP) during consolidation (second exposure), a treatment phase delivered after native Escherichia coli ASP had been given in induction (first exposure). Erwinia chrysanthemi (Erwinia C) ASP was given instead of PEGASP in patients with allergic reactions or SI occurring during its administration.
To better understand this study, we need to go back in time a little. The antileukemic properties of ASP were discovered in the early 1960s, and since then, ASP has been considered an essential component of modern chemotherapeutic regimens for ALL. Its use has in fact been associated with substantial improvements of cure rates in ALL, especially in children. Currently, 3 different types of ASP are available, 2 derived from the bacteria E coli (the native and the PEGASP forms) and another native form derived from Erwinia C. These 3 products have specific half-lives and distinct immunogenic profiles, and these properties do not make them easily interchangeable. The antileukemic effects of ASP depend on its mechanism of action: ASP in fact hydrolyzes asparagine (ASN) into aspartic acid + ammonia, and this leads to a prolonged and sustained depletion of circulating concentrations of the amino acid. Leukemic blasts, unlike healthy cells, are unable to produce ASN individually because they lack the ASN synthetase enzyme; for this reason, when extracellular ASN disappears, these cells have no chance to survive. ASP treatment effects may be monitored by measuring ASN depletion in plasma or, in an even simpler way, by measuring ASP activity levels in plasma by using relatively simple assays. ASP levels are in fact inversely correlated with ASN depletion.5,6 However, resistance to ASP may arise for clinical or biological reasons: among the most frequent clinical reasons are pancreatitis, thrombosis, and allergy. Allergy is by far the most frequent cause of treatment delays, product shift, or treatment discontinuation. Among the biological reasons, one should keep in mind that ASP is a nonhuman protein and its administration in humans may generate an immune response. Antibodies directed against ASP have been associated with the onset of clinical allergy and also with the so-called SI phenomenon (ie, the activity levels in the plasma dramatically decrease and often become undetectable). Because of these events, it can be difficult to predict ASP efficacy, and this can sometimes result in suboptimal treatment. Therefore, a number of important challenges still exist to optimize ASP therapy for individual patients. A basic understanding of the factors influencing the ASP dose-depletion relationship will better equip physicians to identify and adjust therapy in patients that fail to achieve adequate depletion.
From the study by Tong et al, it can be easily inferred that the use of E coli ASP in induction and PEGASP during a later phase in childhood ALL may allow very high PEGASP activity levels in nonhypersensitive patients (70% of the whole cohort), but also that hypersensitivity rates and drug inactivation are found in the remaining 30% of patients (see figure). These findings partially confirm the results of another investigation conducted in the late 1990s, even though it involved a different treatment schedule and setting.7 On this basis, the authors conclude that in order to prevent this detrimental phenomenon it is advisable to use PEGASP upfront in induction. This treatment approach is already largely applied, as demonstrated by several international protocol studies, such as the ongoing Associazione Italiana Ematologia Oncologia Pediatrica-Berlin-Franklin-Munster ALL 2009 trial,8 which have already adopted this strategy and have in place from the beginning of the protocol a thorough TDM aimed at the early discovery of patients with SI.
In the study by Tong et al, patients treated with Erwinia C ASP also underwent a strict TDM. Very few of these patients presented with an allergic reaction, and none displayed SI, thus confirming that the Erwinia C product has very low cross-reactivity with the E coli–derived products. Erwinia C ASP was given with a very tight schedule (every 2-3 days) and at high dosage (20 000 IU/m2); as expected, ASP activity levels were much lower than those observed during PEGASP administration, but higher than 100 IU in the first 2 weeks of treatment especially in patients treated with a schedule of every 2 days (see figure). The levels reported by Tong et al are very similar to those recently reported by Vrooman et al4 ; however, they are clearly lower than those reported by Salzer et al with a fixed schedule of every 2 days, a higher dosage (25 000 IU/m2), and the intramuscular route.9
Additional findings of the study were that antibodies against PEGASP were found in a number of patients, but their specificity was found to be low. Therefore, the usefulness of antibody (both against ASP and/or PEG) detection remains limited and doubtlessly controversial, with several factors influencing its interpretation.10
It is well known that galaxies, elements believed to constitute the universe, are made up of a number of stars, but also of large amounts of gases and dust, elements able to influence each other. There are so many factors influencing ASP activities that this set of elements all together resembles a galaxy. The paper by Tong et al sheds additional light on this “ASP galaxy” and allows scientists to get closer to a better understanding of which elements are the stars, which are the gases, and which are the dust.
Conflict-of-interest disclosure: C.R. has received institutional research grants for studies on ASP pharmacology.