Key Points
Use of native E coli asparaginase in induction leads to high hypersensitivity rates to PEGasparaginase in intensification.
Switching to Erwinia asparaginase leads to effective asparaginase activity levels in most patients who experienced an allergy to PEGasparaginase.
Abstract
This study prospectively analyzed the efficacy of very prolonged courses of pegylated Escherichia coli asparaginase (PEGasparaginase) and Erwinia asparaginase in pediatric acute lymphoblastic leukemia (ALL) patients. Patients received 15 PEGasparaginase infusions (2500 IU/m2 every 2 weeks) in intensification after receiving native E coli asparaginase in induction. In case of allergy to or silent inactivation of PEGasparaginase, Erwinia asparaginase (20 000 IU/m2 2-3 times weekly) was given. Eighty-nine patients were enrolled in the PEGasparaginase study. Twenty (22%) of the PEGasparaginase–treated patients developed an allergy; 7 (8%) showed silent inactivation. The PEGasparaginase level was 0 in all allergic patients (grade 1-4). Patients without hypersensitivity to PEGasparaginase had serum mean trough levels of 899 U/L. Fifty-nine patients were included in the Erwinia asparaginase study; 2 (3%) developed an allergy and none silent inactivation. Ninety-six percent had at least 1 trough level ≥100 U/L. The serum asparagine level was not always completely depleted with Erwinia asparaginase in contrast to PEGasparaginase. The presence of asparaginase antibodies was related to allergies and silent inactivation, but with low specificity (64%). Use of native E coli asparaginase in induction leads to high hypersensitivity rates to PEGasparaginase in intensification. Therefore, PEGasparaginase should be used upfront in induction, and we suggest that the dose could be lowered. Switching to Erwinia asparaginase leads to effective asparaginase levels in most patients. Therapeutic drug monitoring has been added to our ALL-11 protocol to individualize asparaginase therapy.
Introduction
Asparaginase is an enzymatic drug and an essential component of the combination chemotherapy of childhood acute lymphoblastic leukemia (ALL).1 This drug depletes asparagine in the blood, and the malignant lymphoid cells that depend on extracellular asparagine will thus go into apoptosis.2,3 Currently, several asparaginase agents are available on the market. Either these are derived from Escherichia coli in its native form (native E coli asparaginase) or as a pegylated enzyme (PEGasparaginase). Otherwise, asparaginase is extracted from Erwinia chrysanthemi (Erwinia asparaginase).
It has been shown that intensified use of asparaginase increases event-free survival (EFS) for children with ALL by 10% to 15%.4-7 Administration of asparaginase can be limited by the occurrence of hypersensitivity reactions to asparaginase, like allergic or anaphylactic reactions.8 Patients with these reactions are switched to another asparaginase product to ensure that they are exposed to asparaginase according to the treatment plan and to ensure an optimal EFS.9 Clinical allergy is associated with inactivation of asparaginase by antibodies.10,11 Formation of asparaginase antibodies (AAAs) can also neutralize asparaginase without any clinical signs of hypersensitivity, so-called silent inactivation. Panosyan et al and Vrooman et al showed that children with silent inactivation of native E coli asparaginase had a poorer outcome because they were not switched to alternative asparaginase agents, whereas those with clinically overt allergy were switched and had no poorer outcome.8,12 In most protocols, asparaginase is given during the induction course, followed by asparaginase-free consolidation courses, and after that asparaginase is again given during the intensification/reinduction course. The majority of hypersensitivity reactions occur during the intensification phase.
The Dutch Childhood Oncology Group (DCOG) ALL-10 protocol used native E coli asparaginase in induction and the less immunogenic PEGasparaginase in the intensification phase in an attempt to prevent hypersensitivity reactions.13 In case of either hypersensitivity to PEGasparaginase or silent inactivation, children were switched to Erwinia asparaginase as a second-line agent in intensification. Only a few studies have been performed on silent inactivation using intensive PEGasparaginase14 or intensive Erwinia asparaginase.15,16
The aim of this prospective drug-monitoring study was to analyze the efficacy of very prolonged use of PEGasparaginase and Erwinia asparaginase by assessing asparaginase activity, asparagine, glutamine levels, and AAAs.
Methods
Patients
Children between 1 and 18 years of age with newly diagnosed ALL and stratified as medium-risk patients were included in the prospective PEGasparaginase study from May 2009 until October 2012 in 2 pediatric oncology centers. Patients were assigned to the medium-risk group based on a prednisone good response at day 8, cytomorphologic complete remission at day 33, and minimal residual disease positivity at day 33 and/or day 79 (before the start of protocol M), but minimal residual disease level at day 79 <10−3 and no presence of the t(4;11)(q11;q23) translocation or the corresponding fusion gene MLL/AF4 in the leukemia cells at diagnosis. Children who had an allergy to PEGasparaginase or silent inactivation were switched to Erwinia asparaginase and included in the prospective Erwinia asparaginase study. Because of the expected low number of allergic reactions to PEGasparaginase, the latter study was carried out in all 7 pediatric oncology centers in the same study period.
The Institutional Review Board approved this study before patient enrollment. Informed consent was obtained from parents or guardians and from patients ≥12 years of age. This study was in accordance with the Declaration of Helsinki.
DCOG ALL-10 treatment protocol
Patients were stratified into 3 risk groups after induction treatment: standard risk, medium risk, and high risk.17 The treatment scheme of the ALL-10 protocol and the intensification phase of the medium-risk patients in greater detail are given in supplemental Figure 1 (available on the Blood Web site).
All patients received 8 doses of native E coli asparaginase (5000 IU/m2 per dose) every 3 days in induction. If a patient was stratified as medium risk, PEGasparaginase as a first-line agent (2500 IU/m2 per dose every other week) was given for a total of 15 doses during the first 30 weeks of intensification. In case of an allergy to or silent inactivation of PEGasparaginase, the drug was replaced by Erwinia asparaginase as a second-line agent (20 000 IU/m2 per dose) 3 times per week to complete 30 weeks of asparaginase therapy. All asparaginase agents were administered intravenously.
Study design
In the PEGasparaginase study, the PEGasparaginase activity trough levels and AAAs were measured in serum at the start of intensification (week 0); in weeks 2, 4, 6, 8, 10, 14, 16, 24, 26, and 28; and also 1 week after administration in weeks 3, 9, 15, and 25. Serum asparagine, aspartic acid, glutamine, and glutamic acid levels were measured at weeks 0, 2, 4, 14, and 24 during PEGasparaginase therapy.
Children who had an allergy to PEGasparaginase or silent inactivation were included in the Erwinia asparaginase study. For this study, 6 blood samples of Erwinia asparaginase activity levels during the first 2 weeks of therapy were obtained from children who received Erwinia asparaginase (Monday, Wednesday, and Friday). In case of high activity levels (72-hour levels ≥100 U/L), the frequency of administration of Erwinia asparaginase was reduced to 2 times per week. Thereafter, every 4 weeks a blood sample was assessed.
Allergy was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Silent inactivation was defined as serum PEGasparaginase activity level <100 U/L at day 7 ± 1 or <20 U/L at day 14 ± 1 after administration in a patient without clinical symptoms of allergy. Silent inactivation of Erwinia asparaginase was defined as serum asparaginase activity level <20 U/L at day 2 after administration of Erwinia asparaginase in patients without clinical allergy.
Laboratory measurements
Serum asparaginase activity and amino acid levels were processed and assessed as described earlier.18,19 Antibodies against native E coli asparaginase (Asparaginase medac) (Coli-AAAs), against PEGasparaginase (Oncaspar) (PEG-AAAs), and against Erwinia asparaginase (Erwinase) (Erwinia-AAAs) were measured by enzyme-linked immunosorbent assays (see supplemental Methods). All AAAs were expressed as optical density (OD) readings. Samples were defined as positive for AAAs if the Coli-AAA OD was >0.13, if the PEG-AAA OD was >0.25, and if the Erwinia-AAA OD was >1.96 standard deviations above the negative control processed mean (using Westgard rules).20
To evaluate if Coli-AAAs in induction predict an allergy or silent inactivation in intensification, we measured Coli-AAAs after induction in a single center (Rotterdam).21
Statistical analysis
The data were analyzed with the software package SPSS, version 20.0.0.1 (SPSS, Chicago, IL). Repeated measurements of the asparaginase activity levels were evaluated using mixed models analysis of variance (see supplemental Methods). The antibody levels were compared using nonparametric tests. The role of Coli-AAAs at day 79 (at the start of consolidation; protocol M) was analyzed to predict an allergy or silent inactivation at day 140 (start of intensification); for this, sensitivity and specificity were given. Sensitivity and specificity were calculated with the Fisher’s exact test for 2-by-2 contingency tables.
The occurrence of allergy to or silent inactivation of PEGasparaginase related to age and gender was investigated with the χ2 test/ Fisher’s exact tests where appropriate. A 2-sided P value < .05 was considered statistically significant. Data are presented as mean ± standard error of the mean (SEM) or specified otherwise.
Results
PEGasparaginase study
Supplemental Table 1 displays the characteristics of 89 patients included in the PEGasparaginase study. Twenty of the 89 (22%) patients had clinical allergic reactions to PEGasparaginase (6 grade 1, 7 grade 2, and 7 grade 3), and 7 of 89 (8%) patients showed silent inactivation. Eighteen of the 20 (90%) allergic reactions occurred on the second PEGasparaginase dose. Age, gender, and ALL immunophenotype did not differ between patients with or without allergy/silent inactivation of PEGasparaginase.
Serum PEGasparaginase activity levels were measured in 592 samples of 89 children (Figure 1A). PEGasparaginase activity levels were not related to age (<10 years or ≥10 years) or gender (both P = .2). Patients without allergy to and without silent inactivation of PEGasparaginase had a mean trough activity level of 899 U/L (see also supplemental Figure 2). All 20/89 (22%) allergic patients (including grade 1-4 Common Terminology Criteria for Adverse Events) showed PEGasparaginase activity levels of 0. This was not attributable to the fact that the infusion was stopped because 18 patients showed their allergic reactions at the second dose; the serum asparaginase activity level after the first full dose already appeared to be 0 in all 18 cases. Moreover, in 4 cases the second full PEGasparaginase dose was given with clemastine and hydrocortisone, also resulting in unmeasurable serum activity levels of PEGasparaginase after the second dose. All allergic patients were switched to Erwinia asparaginase. Two of the 7 children with silent inactivation were switched to Erwinia asparaginase. The other 5 patients continued with PEGasparaginase because real-time asparaginase measurements were not available at that moment. Those were excluded from further analysis.
Pharmacokinetics of very prolonged PEGasparaginase and Erwinia asparaginase use. (A) Serum PEGasparaginase activity levels (mean ± SEM) of children with or without allergy to or silent inactivation of PEGasparaginase (2500 IU/m2 every other week) (n = 62). (B) Serum Erwinia asparaginase activity levels (mean ± SEM) of children without allergy to Erwinia asparaginase (20 000 IU/m2 2-3 times per week) (n = 57). In panel B, 3 curves are shown in the first 2 weeks of Erwinia asparaginase therapy. The upper curve (white open circles) shows 19 children who had high Erwinia asparaginase levels (72-hour levels ≥100 U/L) and were switched to infusions 2 times per week after the first 2 weeks of Erwinia asparaginase therapy. The lower curve (gray blocks) shows 38 children who had low Erwinia asparaginase levels (72-hour levels <100 U/L) and continued infusions 3 times per week after the first 2 weeks of Erwinia asparaginase therapy. The middle curve (closed circles) shows analysis of variance estimates of all 57 children receiving Erwinia asparaginase 3 times per week in the first 2 weeks.
Pharmacokinetics of very prolonged PEGasparaginase and Erwinia asparaginase use. (A) Serum PEGasparaginase activity levels (mean ± SEM) of children with or without allergy to or silent inactivation of PEGasparaginase (2500 IU/m2 every other week) (n = 62). (B) Serum Erwinia asparaginase activity levels (mean ± SEM) of children without allergy to Erwinia asparaginase (20 000 IU/m2 2-3 times per week) (n = 57). In panel B, 3 curves are shown in the first 2 weeks of Erwinia asparaginase therapy. The upper curve (white open circles) shows 19 children who had high Erwinia asparaginase levels (72-hour levels ≥100 U/L) and were switched to infusions 2 times per week after the first 2 weeks of Erwinia asparaginase therapy. The lower curve (gray blocks) shows 38 children who had low Erwinia asparaginase levels (72-hour levels <100 U/L) and continued infusions 3 times per week after the first 2 weeks of Erwinia asparaginase therapy. The middle curve (closed circles) shows analysis of variance estimates of all 57 children receiving Erwinia asparaginase 3 times per week in the first 2 weeks.
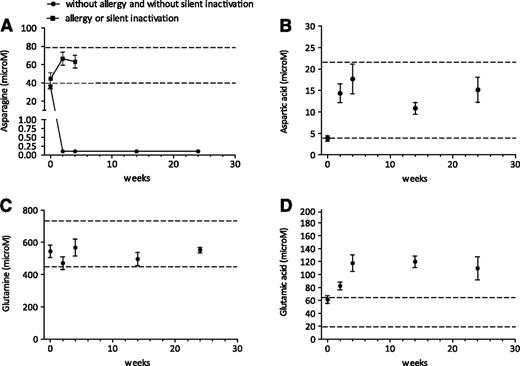
At the start of the intensification phase, the asparagine levels were normal in almost all patients (normal range 40-80 μM). All children without an allergy to PEGasparaginase and without silent inactivation had complete asparagine depletion over time with a lower level of quantification (LLQ) of 0.2 μM (Figure 2A). Children with an allergy to PEGasparaginase or silent inactivation showed no asparagine depletion (Figure 2A). Supplemental Figure 3A shows PEGasparaginase activity levels in relation to the asparagine levels. Figure 2B-D displays aspartic acid, glutamine, and glutamic acid levels. Aspartic acid levels increased after 1 PEGasparaginase infusion in children without allergy and without silent inactivation in line with the asparagine depletion. Thereafter, no changes were seen. No glutamine depletion was seen during PEGasparaginase therapy. Glutamic acid levels increased after the first 2 PEGasparaginase infusions in children without allergy and without silent inactivation; no changes were seen thereafter.
Pharmacodynamics of very intensified PEGasparaginase courses. Serum asparagine (A), aspartic acid (B), glutamine (C), and glutamic acid (D) levels (mean ± SEM) during PEGasparaginase therapy (2500 IU/m2) in children without allergy to PEGasparaginase and without silent inactivation. Dashed lines show normal values of asparagine (40-80 μM), aspartic acid (4-22 μM), glutamine (457-738 μM), and glutamic acid (18-65 μM). The LLQ of asparagine is 0.2 μM.
Pharmacodynamics of very intensified PEGasparaginase courses. Serum asparagine (A), aspartic acid (B), glutamine (C), and glutamic acid (D) levels (mean ± SEM) during PEGasparaginase therapy (2500 IU/m2) in children without allergy to PEGasparaginase and without silent inactivation. Dashed lines show normal values of asparagine (40-80 μM), aspartic acid (4-22 μM), glutamine (457-738 μM), and glutamic acid (18-65 μM). The LLQ of asparagine is 0.2 μM.
Predictive value of Coli-AAAs in induction for an allergy or silent inactivation in intensification
Coli-AAAs were measured serially in 40 patients; 11/40 (27.5%) had allergy and 4/40 (10%) had silent inactivation, respectively, in intensification. The sensitivity of Coli-AAAs at day 79 to detect allergy or silent inactivation in intensification (day 140) was 87% (95% CI, 60% to 98%), and specificity was 64% (95% CI, 43% to 82%).
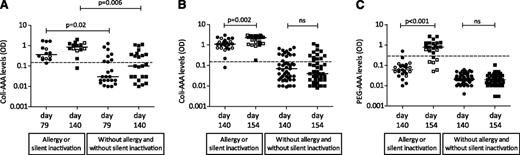
At day 79, the Coli-AAA levels of children with allergy to or silent inactivation of PEGasparaginase in the subsequent intensification phase were significantly higher than those in children without (P = .02). The same was found at day 140 (P = .006) (Figure 3A-B). All children allergic to PEGasparaginase, except 1, had Coli-AAAs at the start of intensification (day 140) (Figure 3B).
Patients with AAAs during the consolidation phase and in the first two weeks of the intensification. Serum AAAs against native E coli asparaginase (Coli-AAA levels) of patients at day 79 (start of the consolidation phase) and at day 140 (start of the intensification) (A), and Coli-AAA levels and serum AAAs against PEGasparaginase (PEG-AAA levels) at day 140 (before first PEGasparaginase dose) and at day 154 (14 days after first PEGasparaginase dose) (B-C). Dashed lines indicate that samples were defined as positive for AAAs if the Coli-AAA OD was >0.13 and if the PEG-AAA OD was >0.25. Closed circles and blocks show cases with allergy or cases without allergy and without silent inactivation; silent inactivation cases are shown by open circles and blocks. Median Coli-AAA and PEG-AAA levels are indicated by bars. NS, not significant.
Patients with AAAs during the consolidation phase and in the first two weeks of the intensification. Serum AAAs against native E coli asparaginase (Coli-AAA levels) of patients at day 79 (start of the consolidation phase) and at day 140 (start of the intensification) (A), and Coli-AAA levels and serum AAAs against PEGasparaginase (PEG-AAA levels) at day 140 (before first PEGasparaginase dose) and at day 154 (14 days after first PEGasparaginase dose) (B-C). Dashed lines indicate that samples were defined as positive for AAAs if the Coli-AAA OD was >0.13 and if the PEG-AAA OD was >0.25. Closed circles and blocks show cases with allergy or cases without allergy and without silent inactivation; silent inactivation cases are shown by open circles and blocks. Median Coli-AAA and PEG-AAA levels are indicated by bars. NS, not significant.
Only 1 patient had PEG-AAAs at day 140 (Figure 3C). The levels of Coli-AAAs and PEG-AAAs increased significantly from day 140 to day 154 after the first PEGasparaginase infusion in allergic patients (P = .002 and P < .001, respectively). All allergic children except 1 developed PEG-AAAs after the first PEGasparaginase infusion. All children with silent inactivation had Coli-AAAs before (day 140) and after (day 154) the first PEGasparaginase infusion. Only 2/7 silent inactivation patients had PEG-AAAs after the first PEGasparaginase infusion (day 154), and patients with silent inactivation had lower PEG-AAAs at day 154 than patients with clinical allergy (P = .001).
Erwinia asparaginase study
Supplemental Table 1 presents the characteristics of the 59 patients with an allergy to or silent inactivation of PEGasparaginase in this 7-center study; 25 patients had already participated in the PEGasparaginase study.
Two of the 59 (3%) patients developed an allergy to Erwinia asparaginase; one showed a grade 3 allergy at the fourth infusion, and the other a grade 2 allergy at the sixth infusion. These 2 patients did not receive further asparaginase therapy. No patients with silent inactivation of Erwinia asparaginase were seen.
Serum Erwinia asparaginase activity levels were measured in 444 samples of 57 patients without allergy to Erwinia asparaginase to complete 30 weeks of exposure (Figure 1B). The 2 children with an allergy to Erwinia asparaginase had asparaginase activity levels of 0. This was not attributable to the fact that the infusion was stopped, because the asparaginase level of the previous dose already appeared to be 0 in both patients. Also, in both cases, the next full Erwinia asparaginase dose was given with clemastine and hydrocortisone, resulting in unmeasurable serum Erwinia asparaginase activity levels.
Table 1 shows the Erwinia asparaginase activity levels during intensification therapy. Erwinia asparaginase activity levels were not related to age (<10 years or ≥10 years) or gender (P = .7 and P = .4, respectively). In the first 2 weeks, all children received 6 doses of Erwinia asparaginase. Of the nonallergic Erwinia-asparaginase patients in the first 2 weeks, 55/57 (96%) had at least 1 Erwinia asparaginase activity level ≥100 U/L and 57/57 (100%) had ≥50 U/L. In 65% and 85% of all patients, all Erwinia asparaginase activity levels in the first 2 weeks were ≥100 U/L and ≥50 U/L, respectively. Median trough levels were 183 U/L at 48 hours and 93 U/L at 72 hours. Nineteen children (33%) were switched to twice-weekly infusions because high activity levels (72-hour levels ≥100 U/L) were measured in the first 2 weeks.
Serum Erwinia asparaginase trough levels ≥20 U/L, ≥50 U/L, or ≥100 U/L
| . | Erwinase activity median (range) . | Samples* . | Patients (n = 57) . | ||||
|---|---|---|---|---|---|---|---|
| ≥20 . | ≥50 . | ≥100 . | ≥20 . | ≥50 . | ≥100 . | ||
| First 2 weeks of Erwinia asparaginase therapy | |||||||
| 3 times per week | 157 (11-913) | 98% | 85% | 65% | 95%† | 60%† | 32%† |
| At least 1 sample above 20, 50, or 100 U/L | 100% | 100% | 96% | ||||
| Week 6 to week 30 of Erwinia asparaginase therapy | |||||||
| 3 times per week, 48-h interval | 182 (22-737) | 100% | 94% | 77% | 100%† | 87%† | 47%† |
| At least 1 sample above 20, 50, or 100 U/L | 100% | 100% | 100% | ||||
| 2 times per week, 72-h interval | 83 (14-908) | 96% | 86% | 34% | 95%† | 68%† | 11%† |
| At least 1 sample above 20, 50, or 100 U/L | 100% | 100% | 68% | ||||
| . | Erwinase activity median (range) . | Samples* . | Patients (n = 57) . | ||||
|---|---|---|---|---|---|---|---|
| ≥20 . | ≥50 . | ≥100 . | ≥20 . | ≥50 . | ≥100 . | ||
| First 2 weeks of Erwinia asparaginase therapy | |||||||
| 3 times per week | 157 (11-913) | 98% | 85% | 65% | 95%† | 60%† | 32%† |
| At least 1 sample above 20, 50, or 100 U/L | 100% | 100% | 96% | ||||
| Week 6 to week 30 of Erwinia asparaginase therapy | |||||||
| 3 times per week, 48-h interval | 182 (22-737) | 100% | 94% | 77% | 100%† | 87%† | 47%† |
| At least 1 sample above 20, 50, or 100 U/L | 100% | 100% | 100% | ||||
| 2 times per week, 72-h interval | 83 (14-908) | 96% | 86% | 34% | 95%† | 68%† | 11%† |
| At least 1 sample above 20, 50, or 100 U/L | 100% | 100% | 68% | ||||
The table is based on 57 patients without allergy to Erwinia asparaginase. The 2 patients with clinical allergy to Erwinia asparaginase were removed from this group. The table shows the data of the period week 6 to week 30 of Erwinia asparaginase therapy of 142 samples of 38 patients who continued infusions 3 times per week after the first 2 weeks of Erwinia asparaginase therapy and the data of 71 samples of 19 patients who were switched to infusions 2 times per week after the first 2 weeks of therapy. h, hour.
For first 2 weeks of Erwinia asparaginase therapy, n = 231 samples; for week 6 to week 30 of Erwinia asparaginase therapy, n = 213 samples.
Number of patients with all samples above 20 U/L, 50 U/L, or 100 U/L.
Children receiving therapy 2 times per week showed a significant decrease of Erwinia asparaginase activity levels at day 37 (week 6) as compared with day 11 (week 2) (P = .002), whereas children continuing Erwinia asparaginase 3 times per week showed a significant increase of activity levels (P = .004) (Figure 1B). The Erwinia asparaginase activity levels remained stable thereafter for all patients. Concerning the nonallergic patients during Erwinia-asparaginase therapy, 57/57 (100%) had at least 1 Erwinia asparaginase activity level ≥100 U/L after the first 2 weeks. Median trough levels were 177 U/L at 48 hours and 86 U/L at 72 hours.
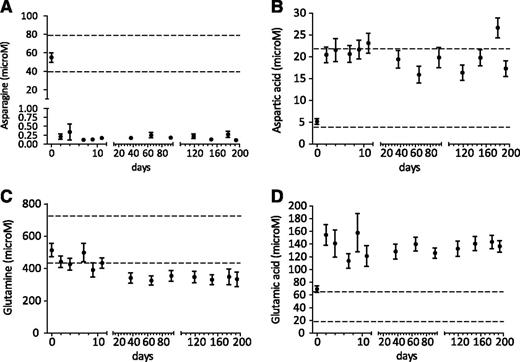
Before the start of Erwinia asparaginase, the asparagine levels were normal (normal range 40-80 μM). During repeated asparagine measurements, these levels were strongly depleted but not always fully depleted over time with an LLQ of 0.2 μM (Figure 4A). The 2 children with an allergy to Erwinia asparaginase showed no asparagine depletion.
Pharmacodynamics of very intensified Erwinia asparaginase courses. Serum asparagine (A), aspartic acid (B), glutamine (C), and glutamic acid (D) levels (mean ± SEM) during Erwinia asparaginase therapy (20 000 IU/m2) in children without allergy to Erwinia asparaginase and without silent inactivation. Dashed lines show normal values of asparagine (40-80 μM), aspartic acid (4-22 μM), glutamine (457-738 μM), and glutamic acid (18-65 μM). The LLQ of asparagine is 0.2 μM.
Pharmacodynamics of very intensified Erwinia asparaginase courses. Serum asparagine (A), aspartic acid (B), glutamine (C), and glutamic acid (D) levels (mean ± SEM) during Erwinia asparaginase therapy (20 000 IU/m2) in children without allergy to Erwinia asparaginase and without silent inactivation. Dashed lines show normal values of asparagine (40-80 μM), aspartic acid (4-22 μM), glutamine (457-738 μM), and glutamic acid (18-65 μM). The LLQ of asparagine is 0.2 μM.
Supplemental Figure 3B shows Erwinia asparaginase activity levels in relation to the asparagine levels, and Figure 4B-D displays aspartic acid, glutamine, and glutamic acid levels. No glutamine depletion was seen during therapy; however, after 6 weeks of Erwinia asparaginase therapy, the glutamine levels were decreased, but not significantly.
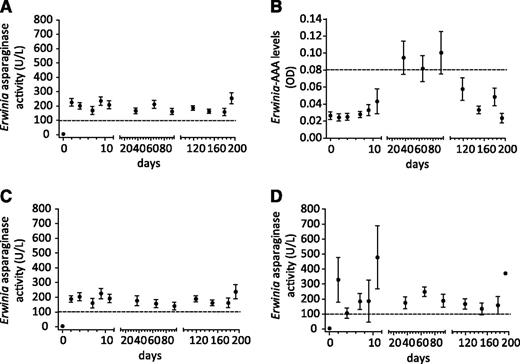
Figure 5 presents the Erwinia-AAA and Erwinia asparaginase activity levels over time. In total, 38% of patients developed Erwinia-AAAs during therapy, all at days 37, 65, and 92. Thereafter, the levels dropped again to baseline values. The presence of Erwinia-AAAs did not influence Erwinia asparaginase activity levels. No silent inactivation was seen during therapy. Both children (3%) with an allergy to Erwinia asparaginase had detectable Erwinia-AAAs.
Pharmacokinetics of very prolonged Erwinia asparaginase use in relation to AAAs. Serum Erwinia asparaginase activity (A) and serum AAAs (B) against Erwinia asparaginase (Erwinia-AAAs) over time. Serum Erwinia asparaginase activity levels of patients without Erwinia-AAAs (C) and with Erwinia-AAAs (D) (mean ± SEM). Dashed lines in panels A, C, and D indicate Erwinia asparaginase activity level of 100 U/L. The dashed line in panel B indicates samples defined as positive for Erwinia-AAAs if the OD was >1.96 standard deviations above the negative control processed mean (using Westgard rules).20
Pharmacokinetics of very prolonged Erwinia asparaginase use in relation to AAAs. Serum Erwinia asparaginase activity (A) and serum AAAs (B) against Erwinia asparaginase (Erwinia-AAAs) over time. Serum Erwinia asparaginase activity levels of patients without Erwinia-AAAs (C) and with Erwinia-AAAs (D) (mean ± SEM). Dashed lines in panels A, C, and D indicate Erwinia asparaginase activity level of 100 U/L. The dashed line in panel B indicates samples defined as positive for Erwinia-AAAs if the OD was >1.96 standard deviations above the negative control processed mean (using Westgard rules).20
Discussion
This prospective drug monitoring of asparaginases has resulted in several important findings. First, if patients show no clinical allergy to or silent inactivation of PEGasparaginase, the serum levels are too high using a schedule of 2500 IU/m2 every other week. Based on this finding, we suggest that the administered PEGasparaginase dose can be lowered. This dose reduction seems feasible because different reinduction protocols used 1000 IU/m2 PEGasparaginase with adequate trough levels of ≥100 U/L in ∼80% of the patients.22,23 Appel et al showed earlier that 1000 IU/m2 PEGasparaginase in induction resulted in asparaginase levels above 100 U/L for at least 2 weeks with complete asparagine depletion in all patients.24 However, this dose reduction of PEGasparaginase should be used and guided by careful monitoring of asparaginase activity levels. There is a need in the future to evaluate this dose reduction with respect to outcome and toxicity data. Of interest, a dose reduction of PEGasparaginase could lead to cost savings; this should also be studied.
Second, we found a high incidence of inactivation of PEGasparaginase (22% clinical allergy and 8% silent inactivation) in the intensification phase because of antibody development against native E coli asparaginase, which was used in induction. This implies that PEGasparaginase should be used upfront during the induction course instead of native E coli asparaginase because this has been shown to result in less antibody formation.16 A similar inactivation rate of PEGasparaginase (36%) was found 1 week after the PEGasparaginase course by Müller et al using the same asparaginase regimen in induction and a single PEGasparaginase in reinduction.25 However, they reported no allergies in reinduction. If a subsequent second PEGasparaginase course was administered, we believe that the allergy rate of PEGasparaginase would be increased. An important finding is that even very mild allergic reactions (grade 1) also necessitate a switch to Erwinia asparaginase. Continuing with PEGasparaginase combined with pretreatment of clemastine and hydrocortisone is not useful because PEGasparaginase activity levels remain 0 (Figure 1A).
Third, drug monitoring is useful because it detects too high levels in some of the patients and, even more important, silent inactivation in other the patients. Detection of silent inactivation is important to prevent useless continuation of an inactive asparaginase product, which may lead to a worse EFS as shown by Panosyan et al and Vrooman et al.8,12 Nevertheless, drug monitoring is the only way to detect cases of silent inactivation of asparaginase agents.
Fourth, in the case of allergy to or silent inactivation of PEGasparaginase, patients can be treated effectively with Erwinia asparaginase. The majority of the patients that switched to Erwinia asparaginase showed effective asparaginase activity levels during the first 2 weeks of Erwinia asparaginase, namely median trough activity level of 183 U/L (48-hour level) and 93 U/L (72-hour level) and asparaginase activity level ≥100 U/L in 100% (48-hour level) and 33% (72-hour level) of the patients. Similar rates were found by Vrooman et al: 83% (48-hour level) and 45% (72-hour level) using a dose of 25 000 IU/m2 3 times per week intravenously.26 These activity levels were lower compared with that of the study by Salzer et al; they found 645 U/L (48-hour level) and 248 U/L (72-hour level) as median trough activity levels and asparaginase activity levels ≥100 U/L in 93% (48-hour level) and 88% (72-hour level) of the patients using intramuscular Erwinia asparaginase in a dose of 25 000 IU/m2 3 times per week.27 The route of Erwinia asparaginase administration might explain the higher median asparaginase activity levels that were found by Salzer et al.27 However, previous studies have shown that no differences in mean asparaginase activity levels, asparagine depletion, and AAAs were found after intravenous or intramuscular administration of Erwinia asparaginase.16,28,29
Only 2/59 (3%) developed an allergy to Erwinia asparaginase in our study. Please note that this study does not allow comparison of allergy rates to E coli asparaginase (including the pegylated form) and Erwinia asparaginase because E coli asparaginases were given in induction and intensification with an asparaginase-free interval of 4 months, whereas Erwinia asparaginase was administered continuously without such interval.
Fifth, the mean asparagine levels for both preparations were below the detection level of 0.5 μM as used by others.14,30-32 We observed that asparagine levels were not always completely depleted with our detection level of 0.2 μM21 in Erwinia asparaginase–treated patients in contrast to PEGasparaginase. This may simply reflect the differences in serum drug levels of these 2 compounds. With our very low LLQ of 0.2 μM,21 19/57 patients (33%) showed no complete asparagine depletion. If the threshold levels from the literature are used (mean of 0.5 μM), 9 of 57 patients (16%) showed incomplete asparagine depletion.14,30-32 Samples with Erwinia asparaginase activity levels >100 U/L and asparagine >0.2 μM were also found in 9 of 57 patients (16%).
Why this difference in asparagine levels during asparaginase therapy exists between Erwinia asparaginase and PEGasparaginase is unclear. Measuring asparaginase activity levels is preferred over measuring asparagine levels to monitor the efficacy of asparaginase therapy because, if not properly handled, asparagine is very rapidly degraded ex vivo in the tube by asparaginase leading to false low asparagine levels.33 It is remarkable that the glutamine levels of Erwinia asparaginase–treated patients were lower as compared with those receiving PEGasparaginase irrespective of the much lower serum activity levels of Erwinia asparaginase. This can be explained by the higher glutaminase activity of Erwinia asparaginase.34 Although glutamine is broken down to glutamic acid by asparaginase, this does not lead to glutamine depletion because this is supplemented from other organ stocks in vivo.
Sixth, our study shows that the presence of AAAs is related to allergy to and silent inactivation of asparaginase as shown by others,10,20 but predicting asparaginase allergy or silent inactivation based on antibody formation is hampered by low specificity (64%) of the test. The specificity of the Coli-AAA test in intensification was higher, 73%, but still not perfect. The levels of PEG-AAAs were above the cutoff in almost all allergic patients after the first PEGasparaginase infusion and negative in children without an allergy. The specificity of the PEG-AAA test is 100% to predict an allergy to or silent inactivation of PEGasparaginase in intensification. However, the 2 patients with an allergy at the first PEGasparaginase infusion had no detectable PEG-AAAs (day 0 of intensification). And Erwinia-AAAs were found in 38% of the patients but were not associated with inactivation of asparaginase. So, measuring asparaginase levels is a more direct and more accurate way of drug monitoring than measuring AAAs. It has been reported that females and toddlers are more prone to develop allergies.35 However, we found no relation between age or gender and allergy or antibody formation.
In conclusion, the use of native E coli asparaginase in induction leads to a significant rate of allergy to and silent inactivation of PEGasparaginase in intensification. Switching to Erwinia asparaginase in case of allergy to or silent inactivation of PEGasparaginase leads to effective asparaginase activity levels in most patients, but close drug monitoring remains necessary to ensure adequate drug levels. The relevance of AAAs in clinical practice appears to be limited. It is more useful to monitor the serum asparaginase activity levels. In the absence of allergy or silent inactivation, PEGasparaginase activity levels are too high with a dose schedule of 2500 IU/m2 every other week.
This study has therefore resulted in significant changes in the use of asparaginase in the DCOG ALL-11 protocol. PEGasparaginase is used instead of native E coli asparaginase upfront in the induction, and the starting dose of PEGasparaginase has been lowered to 1500 IU/m2. Also, a therapeutic drug–monitoring program is now used to individualize the PEGasparaginase dose and to detect silent inactivation. In case of allergy or silent inactivation, patients are switched to Erwinia asparaginase with therapeutic drug monitoring to allow individualized dosing of Erwinia asparaginase.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients and their parents and (research) nurses. We gratefully acknowledge the laboratories of the University Children’s Hospital in Muenster, Germany, Medac GmbH, and St. Jude Children’s Research Hospital in Memphis, TN, for their technical support.
This work was supported by the KiKa foundation, EUSA Pharma, the National Institutes of Health, National Cancer Institute grant CA 21765 (M.V.R.), and the American Lebanese Syrian Associated Charities (M.V.R.).
Authorship
Contribution: W.H.T., R.P., and I.M.v.d.S. designed, analyzed, and interpreted the clinical data; C.L.-K. was responsible for the measurements of asparaginase activities and amino acid levels; M.V.R. was responsible for the Erwinia-AAAs; G.J.L.K., D.M.W.M.t.L., M.B.B., C.v.d.B., W.J.W.K., and W.J.E.T. were responsible for the inclusion of patients in the other centers; W.H.T. and W.C.J.H. did the statistical analysis; R.P. and I.M.v.d.S. supervised this research; W.H.T., R.P., and I.M.v.d.S. were responsible for the manuscript preparation; and all authors revised the article critically for important intellectual content and approved the final version to be published.
Conflict-of-interest disclosure: W.H.T. receives funding for investigator-initiated research from EUSA Pharma. M.V.R. receives funding for investigator-initiated research from Sigma-τ Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Inge M. van der Sluis, Erasmus MC-Sophia Children’s Hospital, Department of Pediatric Oncology/Hematology, Room Na-1607, Dr Molewaterplein 60, 3015 GJ, Rotterdam, The Netherlands; e-mail: i.vandersluis@erasmusmc.nl.