Key Points
Adding sirolimus to tacrolimus/methotrexate GVHD prophylaxis decreased grade 2-4 aGVHD but did not improve survival in pediatric ALL.
The addition of sirolimus to tacrolimus/methotrexate increased rates of VOD and TMA compared with tacrolimus/methotrexate alone.
Abstract
Sirolimus has activity against acute lymphoblastic leukemia (ALL) in xenograft models and efficacy in preventing acute graft-versus-host disease (aGVHD). We tested whether addition of sirolimus to GVHD prophylaxis of children with ALL would decrease aGVHD and relapse. Patients were randomized to tacrolimus/methotrexate (standard) or tacrolimus/methotrexate/sirolimus (experimental). The study met futility rules for survival after enrolling 146 of 259 patients. Rate of Grade 2-4 aGVHD was 31% vs 18% (standard vs experimental, P = .04), however, grade 3-4 aGVHD was not different (13% vs 10%, P = .28). Rates of veno-occlusive disease (VOD) and thrombotic microangiopathy (TMA) were lower in the nonsirolimus arm (9% vs 21% VOD, P = .05; 1% vs 10% TMA, P = .06). At 2 years, event free survival (EFS) and overall survival (OS) were 56% vs 46%, and 65% vs 55% (standard vs experimental), respectively (P = .28 and .23). Multivariate analysis showed increased relapse risk in children with ≥0.1% minimal residual disease (MRD) pretransplant, and decreased risk in patients with grades 1-3 aGVHD (P = .04). Grades 1-3 aGVHD were associated with improved EFS (P = .02), whereas grade 4 aGVHD and extramedullary disease at diagnosis led to inferior OS. Although addition of sirolimus decreased aGVHD, survival was not improved. This study is registered with ClinicalTrials.gov as #NCT00382109.
Introduction
Cure rates using multiagent chemotherapy exceed 80% for children with acute lymphoblastic leukemia (ALL), but there remains a group of patients with high-risk features for whom chemotherapy alone results in poor outcomes. These children may benefit from allogeneic hematopoietic cell transplantation (HCT).1-3 Although recent advances in HCT have resulted in less transplant-related mortality (TRM) and improved survival,4,5 rates of relapse have remained high, making it the most common cause of failure after HCT.6 The development of novel approaches that decrease relapse in children with ALL undergoing allogeneic HCT is a key study priority.
Mammalian target of rapamycin (mTOR) inhibitors have been noted in xenograft models to have significant activity against human ALL as a single agent7,8 and in synergistic combinations with chemotherapy.9 Sirolimus (siro), an oral mTOR inhibitor, given in combination with tacrolimus and methotrexate (tac/mtx), has been shown to have strong activity in prevention of acute graft-versus-host disease (aGVHD),10 and can be given safely and effectively in children with ALL undergoing allogeneic HCT.11 Compelling preclinical activity of mTOR inhibitors in ALL, combined with data suggesting less relapse using siro in adults with lymphoma undergoing reduced intensity allogeneic HCT,12 led investigators from the Children’s Oncology Group (COG) and the Pediatric Blood and Marrow Transplant Consortium (PBMTC) to hypothesize that the use of siro-based GVHD prophylaxis could result in decreased relapse and less GVHD after HCT. We tested this hypothesis by performing a randomized phase 3 trial comparing standard GVHD prophylaxis with tac/mtx vs tac/mtx plus siro (tac/mtx/siro) in children undergoing total body irradiation (TBI)-based allogeneic HCT for ALL.
Patients and methods
Protocol COG ASCT0431 (PBMTC ONC051) was available to member institutions of the COG and PBMTC from March 2007 to May 2011. The study was approved by the National Cancer Institute Central Institutional Review Board as well as local institutional review boards as applicable.
Patient eligibility
High-risk ALL patients were eligible if they were aged 1 to 21 years and in a morphological complete remission (<5% bone marrow [BM] blasts, normal cerebrospinal fluid) tested within 14 days of initiating the preparative regimen. Three risk categories of patients were allowed: high-risk complete remission 1 (CR1) (Philadelphia chromosome positive [Ph+] ALL, extreme hypodiploidy [<44 chromosomes], or primary induction failure [PIF; either >20% blasts of BM at day +29 or 5-20% blasts or minimal residual disease [MRD] >1% at day +43]); high-risk CR2 (B-cell BM relapse <36 months from diagnosis, T-cell or Ph+ BM relapse at any time, T-cell isolated extramedullary [IEM] relapse <18 months from diagnosis); and intermediate-risk CR2 (B-cell BM relapse ≥36 months from diagnosis, B-cell IEM <18 months from diagnosis). Patients were required to have a stem cell donor who was an HLA-matched sibling (intermediate- and high-risk groups), or a 7-8/8 allele-level HLA-matched related or unrelated donor or a 4-6/6 matched single cord blood unit with a minimal prethaw dose of 3 × 107 nucleated cells per kg (high-risk CR1 and CR2 only).
Patients with late IEM relapse (≥18 monthsfrom diagnosis), CR3, failure to obtain remission, Down syndrome, or previous transplant, or women who were pregnant or lactating as well as those with poor performance (Karnofsky or Lansky Score <60), uncontrolled infection, or inadequate organ function for a TBI-based preparative regimen were excluded.
Study procedures
Patients received a preparative regimen of TBI at a dose of 1200 cGy given in 6 fractions over 3 days followed by thiotepa 5 mg/kg per day for 2 days and cyclophosphamide 60 mg/kg per day for 2 days. Etoposide1500 mg/m2 could be substituted for thiotepa (6% of patients), and centers were allowed to omit thiotepa or etoposide and instead give 1320 cGy TBI in 8 doses in addition to cyclophosphamide (9% of patients). GVHD prophylaxis for the standard arm consisted of tac starting day −2 and mtx 5 mg/m2 IV on days +1, +3, +6 for all stem cell sources and day +11 for unrelated BM or peripheral blood stem cells (PBSCs). Patients on the experimental arm received tac/mtx as in the standard arm with the addition of siro by mouth starting on day +0 at a dose of 4 mg/m2. Tac trough levels were kept between 5 and 12 ng/mL for standard arm patients. Target levels on the experimental arm for siro were 3 to 12 ng/mL and tac 5 to 12 ng/mL, with a combined total of tac and siro levels between 12 and 16 ng/mL. Recipients on the experimental arm were scheduled to receive siro for 6 months followed by a 1-month taper. Recipients of sibling donors on both arms underwent tapering of tac between days +42 and 96, whereas recipients of unrelated BM/PBSC and cord blood tapered tac between days +100 and +180. GVHD treatment was at the discretion of the local center. Dose modification guidelines for siro included holding for cytopenias and veno-occlusive disease (VOD), and holding tac for thrombotic microangiopathy (TMA).
Statistical design
At enrollment, patients were stratified by donor type, risk group (HR CR1, HR CR2, IR CR2), and stem cell source. The study was designed to detect an increase in the historical 2-year event-free survival (EFS) rate of 16% (from 40% to 56%) with 80% power (α = 0.05, 1-sided) based on the log-rank test. Interim analyses for efficacy and futility were planned at 5 time points. For efficacy, stopping boundaries were based on the O’Brien-Fleming spending function,13 whereas for futility, lower boundaries were based on testing the alternative hypothesis at the 0.005 level.14 The study plan called for a maximum of 259 patients accrued over 4.4 years. Data current through October 2012 (5.5 and 1.4 years after first and last enrollment, respectively) were used in this analysis.
MRD was measured on BM aspirates using 6-color flow cytometry.15 Samples were stained with 2 different 6-color antibody combinations CD20-FITC/CD10-PE/CD38-PerCPCy5.5/CD58-APC/CD19-PECy7/CD45-APCH7, and CD9/CD13+33/CD34/CD10/CD19/CD45. A third tube contained SYTO-16 to identify all nucleated cells using a method previously described by Dworzak.16 CD19 in this tube was used to express B cells as a percentage of all nucleated cells; MRD identified in either of the 2 test tubes was expressed as a percentage of B cells and the third tube used to calculate MRD as a percentage of nucleated cells. Finally, mononuclear cells were estimated on a display of CD45/SSC to exclude granulocytes, and MRD ultimately expressed as a percent of mononuclear cells.17
Events for EFS calculation included relapse and TRM. TRM was defined as death in a patient who had not relapsed after transplant. Time to event was the time from transplant to occurrence of an event (relapse or TRM). Patients who did not experience an event were censored at the time of last contact. EFS and overall survival (OS) were estimated by the Kaplan-Meier method.18 The log-rank statistic was used to compare EFS and OS between standard and experimental treatment groups. The hazard ratios (HRs), cumulative incidences (CIs), and tests of significance associated with patient characteristics were assessed using proportional hazards regression modeling.19 Cause-specific outcome probabilities of relapse or TRM over time that account for each as a competing risk were estimated using the nonparametric methods of Aalen and Johansen.20 Both relapse and TRM were accounted for as competing risks in the estimation of GVHD probabilities.
Multivariate analysis
A multivariate analysis was performed to define risk factors associated with relapse, TRM, and survival. Disease risk (HR CR1, HR CR2, IR CR2) was based on immunophenotype, recency, and location of relapse, and Philadelphia chromosome status (see patient eligibility). Donor type was restricted by design within the disease risk categories (related donors only for intermediate risk, all for high risk) so that artifactual correlations were induced between these factors. Thus, the multivariate analysis was focused on identification of associations with factors other than the components of disease risk and donor type, after control for disease risk and donor type. The potential risk factors considered were cytomegalovirus (CMV) status, the presence of extramedullary disease at relapse, the presence of extramedullary disease at initial diagnosis, the presence of MRD at the time of transplant, aGVHD status (each grade of GVHD assessed independently, grades 1-3 overlapped in effect and were combined, grade IV differed in effect and was analyzed separately), and chronic GVHD (cGVHD) status. The GVHD variables were included as time-dependent variables in the models. CMV status, the presence of extramedullary disease at relapse, and cGVHD status were not associated with any of the outcomes and so were excluded from the analytic model.
Results
Patient accrual and random assignment
The study was closed to accrual after meeting the stopping criteria for futility in achieving the primary aim of improving survival. One hundred forty-six patients were enrolled from 64 COG-accredited transplant centers (Figure 1). Two patients were found to be ineligible because they did not meet the criteria for complete remission prior to transplant. One patient died after randomization but before transplant. This patient was excluded from the analysis because the intervention took place after transplant. Of the 143 remaining patients, 70 were assigned to the standard and 73 to the experimental arm. Actuarial median follow-up of the cohorts is 26 months (interquartile range, 23-38 months). Table 1 shows the patient and disease characteristics by treatment arm. No significant differences were noted between the standard and experimental arms for important donor and recipient characteristics.
Patient, donor, and disease characteristics by treatment arm
| . | tac/mtx, n = 70 . | siro/tac/mtx, n = 73 . | P . | |||
|---|---|---|---|---|---|---|
| N . | % . | N . | % . | |||
| Age at transplant, y | .37 | |||||
| 1-4 | 15 | 21 | 20 | 27 | ||
| 5-9 | 17 | 24 | 24 | 33 | ||
| 10-14 | 21 | 30 | 14 | 19 | ||
| 15+ | 17 | 24 | 15 | 21 | ||
| Sex | ||||||
| Female | 29 | 41 | 30 | 41 | .97 | |
| Male | 41 | 59 | 43 | 59 | ||
| Immunophenotype | ||||||
| B-precursor | 55 | 79 | 58 | 79 | ||
| T cell | 13 | 19 | 14 | 19 | .96 | |
| Mixed lineage (MLL) | 2 | 2.9 | 1 | 1.4 | ||
| Philadelphia chromosome positive | ||||||
| No | 65 | 93 | 64 | 88 | .30 | |
| Yes | 5 | 7 | 9 | 12 | ||
| Extramedullary disease at diagnosis | ||||||
| No | 59 | 84 | 60 | 82 | .73 | |
| Central nervous system | 10 | 14 | 12 | 16 | ||
| Other site | 1 | 1 | 1 | 1 | ||
| Extramedullary disease in CR2 patients, N = 94 | ||||||
| Diagnosis | Relapse | |||||
| No | No | 27 | 59 | 29 | 60 | .76 |
| No | Yes | 11 | 24 | 10 | 21 | |
| Yes | No | 7 | 15 | 6 | 13 | |
| Yes | Yes | 1 | 2 | 3 | 6 | |
| Cranial radiation at initial treatment | ||||||
| No | 57 | 81 | 61 | 84 | .74 | |
| Yes | 13 | 19 | 12 | 16 | ||
| Total dose of radiation therapy, cGy: median (range) | 1800 (1200-1800) | 1200 (600-2400) | ||||
| Relapse risk group assessment | ||||||
| High-risk CR1 | 24 | 34 | 25 | 34 | .99 | |
| High-risk CR2 | 34 | 49 | 35 | 48 | ||
| Intermediate-risk CR2 | 12 | 17 | 13 | 18 | ||
| Stem cell source | ||||||
| Matched sibling | 38 | 54 | 40 | 55 | .99 | |
| Other related donor | 2 | 3 | 2 | 3 | ||
| Unrelated donor: BM or PBSC | 16 | 23 | 18 | 25 | ||
| Unrelated donor: cord blood | 14 | 20 | 13 | 18 | ||
| CMV status | ||||||
| Donor | Recipient | |||||
| Negative | Negative | 31 | 48 | 27 | 39 | .17 |
| Negative | Positive | 15 | 23 | 17 | 25 | |
| Positive | Negative | 3 | 5 | 11 | 16 | |
| Positive | Positive | 16 | 25 | 14 | 20 | |
| MRD status pretransplant | ||||||
| Negative | 38 | 54 | 37 | 51 | .39 | |
| Positive, <0.1% | 7 | 10 | 6 | 8 | ||
| Positive, 0.1%+ | 5 | 7 | 12 | 16 | ||
| Not available* | 20 | 29 | 18 | 25 | ||
| . | tac/mtx, n = 70 . | siro/tac/mtx, n = 73 . | P . | |||
|---|---|---|---|---|---|---|
| N . | % . | N . | % . | |||
| Age at transplant, y | .37 | |||||
| 1-4 | 15 | 21 | 20 | 27 | ||
| 5-9 | 17 | 24 | 24 | 33 | ||
| 10-14 | 21 | 30 | 14 | 19 | ||
| 15+ | 17 | 24 | 15 | 21 | ||
| Sex | ||||||
| Female | 29 | 41 | 30 | 41 | .97 | |
| Male | 41 | 59 | 43 | 59 | ||
| Immunophenotype | ||||||
| B-precursor | 55 | 79 | 58 | 79 | ||
| T cell | 13 | 19 | 14 | 19 | .96 | |
| Mixed lineage (MLL) | 2 | 2.9 | 1 | 1.4 | ||
| Philadelphia chromosome positive | ||||||
| No | 65 | 93 | 64 | 88 | .30 | |
| Yes | 5 | 7 | 9 | 12 | ||
| Extramedullary disease at diagnosis | ||||||
| No | 59 | 84 | 60 | 82 | .73 | |
| Central nervous system | 10 | 14 | 12 | 16 | ||
| Other site | 1 | 1 | 1 | 1 | ||
| Extramedullary disease in CR2 patients, N = 94 | ||||||
| Diagnosis | Relapse | |||||
| No | No | 27 | 59 | 29 | 60 | .76 |
| No | Yes | 11 | 24 | 10 | 21 | |
| Yes | No | 7 | 15 | 6 | 13 | |
| Yes | Yes | 1 | 2 | 3 | 6 | |
| Cranial radiation at initial treatment | ||||||
| No | 57 | 81 | 61 | 84 | .74 | |
| Yes | 13 | 19 | 12 | 16 | ||
| Total dose of radiation therapy, cGy: median (range) | 1800 (1200-1800) | 1200 (600-2400) | ||||
| Relapse risk group assessment | ||||||
| High-risk CR1 | 24 | 34 | 25 | 34 | .99 | |
| High-risk CR2 | 34 | 49 | 35 | 48 | ||
| Intermediate-risk CR2 | 12 | 17 | 13 | 18 | ||
| Stem cell source | ||||||
| Matched sibling | 38 | 54 | 40 | 55 | .99 | |
| Other related donor | 2 | 3 | 2 | 3 | ||
| Unrelated donor: BM or PBSC | 16 | 23 | 18 | 25 | ||
| Unrelated donor: cord blood | 14 | 20 | 13 | 18 | ||
| CMV status | ||||||
| Donor | Recipient | |||||
| Negative | Negative | 31 | 48 | 27 | 39 | .17 |
| Negative | Positive | 15 | 23 | 17 | 25 | |
| Positive | Negative | 3 | 5 | 11 | 16 | |
| Positive | Positive | 16 | 25 | 14 | 20 | |
| MRD status pretransplant | ||||||
| Negative | 38 | 54 | 37 | 51 | .39 | |
| Positive, <0.1% | 7 | 10 | 6 | 8 | ||
| Positive, 0.1%+ | 5 | 7 | 12 | 16 | ||
| Not available* | 20 | 29 | 18 | 25 | ||
A portion of pre-HCT MRD samples were not available due to patient/parent refusal of biology studies (rare) or more commonly consent being obtained after pre-HCT BM was performed locally and patient/parent refusal of a second “optional” research BM sample.
Engraftment and toxicities
Table 2 shows engraftment characteristics and key measures of toxicity. The portion of patients with neutrophil engraftment was 96% with no difference between standard and experimental arms. The portion with platelet engraftment, however, was lower on the experimental arm (75% vs 90%, experimental vs standard; P = .03). Median times to engraftment were similar for neutrophils and platelets on the standard and experimental arms in BM recipients; in contrast, the median day of platelet engraftment was delayed by nearly 2 months in cord recipients on the experimental arm (91 vs 40 days; P = .03).
Engraftment and key toxicity rates by treatment group
| . | Engraftment . | VOD . | TMA . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil . | Platelet . | Occurrences . | Deaths . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total, n = 143 | 137 | 96 | 118 | 83 | 21 | 15 | 9 | 6 | 8 | 6 |
| Standard, tac/mtx, n = 70 | 67 | 96 | 63 | 90 | 6 | 9 | 3 | 4 | 1 | 1 |
| Experimental, siro/tac/mtx, n = 73 | 70 | 96 | 55 | 75 | 15 | 21 | 6 | 8 | 7 | 10 |
| P | 1.00 | .03 | .06 | .37 | .06 | |||||
| . | Engraftment . | VOD . | TMA . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil . | Platelet . | Occurrences . | Deaths . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total, n = 143 | 137 | 96 | 118 | 83 | 21 | 15 | 9 | 6 | 8 | 6 |
| Standard, tac/mtx, n = 70 | 67 | 96 | 63 | 90 | 6 | 9 | 3 | 4 | 1 | 1 |
| Experimental, siro/tac/mtx, n = 73 | 70 | 96 | 55 | 75 | 15 | 21 | 6 | 8 | 7 | 10 |
| P | 1.00 | .03 | .06 | .37 | .06 | |||||
There was no statistical difference in TRM between standard and experimental arms (12% standard vs 19% experimental, P = .43). TMA occurred in 1% and 10% (P = .06) and VOD occurred in 9% and 21% (P = .06) of patients on the standard vs experimental arm, confirming increased risk in children receiving siro as has been noted in adults.21,22 Much of the VOD in the experimental arm was mild or moderate, however, severe VOD associated with fatality occurred in 4.3% and 8.2% of patients on the standard and experimental arms, respectively (P = .49).
Analysis of GVHD and survival
Table 3 shows rates of acute GVHD in the standard and experimental arms. Rates of any grade of acute GVHD and grades II-IV acute GVHD were decreased by 20% and 13% in the experimental relative to the standard arm (P = .02 for any acute GVHD and P = .04 for grade II-IV acute GVHD), however, rates of grades III-IV aGVHD were not statistically different in the 2 arms (13% vs 10%, P = .28). Among patients with acute GVHD, there was no evidence of a difference in the distribution of grade between the treatment groups (P = .90). In addition, there was no statistical difference in overall and extensive chronic GVHD between the study arms (30% vs 24% overall, P = .59; 6% vs 12% extensive, P = .24 standard vs experimental).
GVHD by treatment group
| . | Any aGVHD . | Acute GVHD grades . | cGVHD . | |||||
|---|---|---|---|---|---|---|---|---|
| 2-4 . | 3-4 . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total, n = 143 | 55 | 38 | 35 | 24 | 16 | 11 | 36 | 25 |
| Standard, tac/mtx, n = 70 | 34 | 49 | 22 | 31 | 9 | 13 | 20 | 29 |
| Experimental, siro/tac/mtx, n = 73 | 21 | 29 | 13 | 18 | 7 | 10 | 16 | 22 |
| P | .02 | .04 | .28 | .44 | ||||
| . | Any aGVHD . | Acute GVHD grades . | cGVHD . | |||||
|---|---|---|---|---|---|---|---|---|
| 2-4 . | 3-4 . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Total, n = 143 | 55 | 38 | 35 | 24 | 16 | 11 | 36 | 25 |
| Standard, tac/mtx, n = 70 | 34 | 49 | 22 | 31 | 9 | 13 | 20 | 29 |
| Experimental, siro/tac/mtx, n = 73 | 21 | 29 | 13 | 18 | 7 | 10 | 16 | 22 |
| P | .02 | .04 | .28 | .44 | ||||
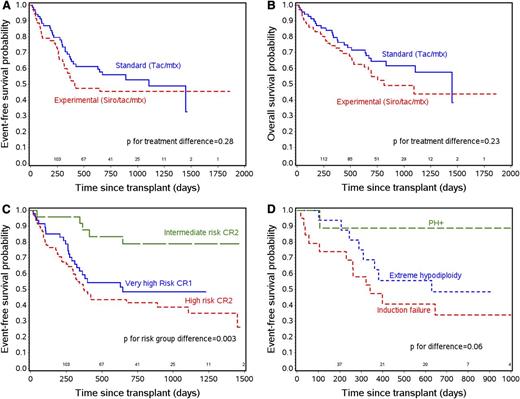
There were no statistically significant differences in EFS and OS between the 2 arms (Figure 2A-B). At 2 years, the estimated EFS probability was 56% and 46% in the standard and experimental arms (P = .28), whereas OS was 65% and 55% (P = .23). Relapse was not statistically different between the 2 arms (P = .45) with estimated 2-year CI of 32% and 36% in standard and experimental arms. Further analysis showed no statistical difference in survival outcomes of the standard vs experimental arms in each of the major risk subgroups of patients.
Survival outcomes. (A) EFS by treatment arm. (B) OS by treatment arm. (C) EFS by risk category. (D) EFS by subgroups of very high-risk CR1 patients.
Survival outcomes. (A) EFS by treatment arm. (B) OS by treatment arm. (C) EFS by risk category. (D) EFS by subgroups of very high-risk CR1 patients.
Of note, the major risk categories themselves had statistically different outcomes compared with each other. Superior outcome was noted for patients in the intermediate-risk group, with 2-year EFS of 79% compared with high-risk CR1 and CR2 patients, with 2-year EFS of 48% and 42%, respectively (Figure 2C, P = .003). The 9 CR1 Ph+ patients had a superior outcome compared with the hypodiploidy and CR1 PIF patients with 2-year EFS of 89%, 49%, and 34%, respectively (P = .06; Figure 2D). High-risk CR2 patients who were B-lineage or T-lineage had survival that was not statistically different (2-year EFS of 45% and 33%, respectively [P = .40]).
Effect of siro dose modifications on GVHD/survival
Major dose modifications were necessary for 23 of 73 (32%) of patients on the siro arm, and 5 (7%) on the siro arm also required major tac modifications. No patients in the standard arm required major modifications in administration of tac/mtx. The CI of aGVHD was markedly lower in patients who received siro without dose modification vs standard (24% vs 48%, P = .009). Those who required dose modification had aGVHD rates closer to the standard arm (39%), providing further evidence of the activity of siro in decreasing aGVHD. In spite of the clear decrease in aGVHD in the cohort receiving siro without dose modification, 2-year relapse probability (36% vs 32%, P = .43), EFS (47% vs 56%, P = .39), and OS (53% vs 65%, P = .36) were not significantly different between patients who received siro without dose modification compared with the standard arm.
Multivariate analysis: risk factors associated with relapse, TRM, EFS, and OS
Because donor type was restricted by design within the disease risk categories (related donors only for intermediate risk, all for high risk), comparisons of donor types across disease risk categories was not possible. Within the HR CR2 risk category, where sufficient numbers of sibling, unrelated donor BM/PBSC, and unrelated cord blood recipients were included, univariate analysis showed no difference in relapse, TRM, or survival based upon stem cell source.
After controlling for disease risk and donor type, significant risk factors for poor outcomes shown by the multivariate model included the presence of extramedullary disease at initial diagnosis, high MRD level at the time of transplant, and lack of development of aGVHD (Table 4). The presence of MRD of ≥0.1% pre-HCT was associated with higher relapse risk (HR, 3.3; P = .01), whereas acute GVHD grades I-III was associated with lower relapse risk (HR, 0.4; P = .04). Any effect of grade IV aGVHD in decreasing relapse risk was obscured by a marked increase in TRM (HR, 6.4; P = .003), whereas grades 1-3 aGVHD had no statistically detectable effect on TRM (HR, 0.6; P = .42).
HRs (95% CIs) for extramedullary disease at diagnosis, MRD level at time of transplant, and aGVHD status in a Cox regression model controlling for disease risk and donor type
| . | Relapse . | TRM . | EFS . | OS . |
|---|---|---|---|---|
| Extramedullary disease at diagnosis | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.82 (0.85-3.91) | 2.51 (0.79-7.96) | 1.98 (1.05-3.72) | 2.22 (1.11-4.44) |
| P | .12 | .12 | .03 | .02 |
| MRD status pretransplant | ||||
| Negative | 1 | 1 | 1 | 1 |
| Positive, <0.1% | 1.59 (0.52-4.82) | 2.30 (0.43-12.4) | 1.66 (0.67-4.13) | 1.63 (0.59-4.44) |
| Positive, ≥0.1% | 3.30 (1.32-8.22) | 1.31 (0.33-5.24) | 2.23 (1.06-4.70) | 1.73 (0.75-4.00) |
| P | .04 | .62 | .09 | .35 |
| aGVHD* | ||||
| None | 1 | 1 | 1 | 1 |
| Grade 1-3 | 0.44 (0.20-0.95) | 0.64 (0.22-1.90) | 0.46 (0.25-0.87) | 0.55 (0.28-1.09) |
| P | .04 | .42 | .02 | .08 |
| Grade 4 | 0 (–) | 6.39 (1.85-22.1) | 2.56 (0.97-6.75) | 2.96 (1.09-8.07) |
| P | .99 | .003 | .06 | .03 |
| . | Relapse . | TRM . | EFS . | OS . |
|---|---|---|---|---|
| Extramedullary disease at diagnosis | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.82 (0.85-3.91) | 2.51 (0.79-7.96) | 1.98 (1.05-3.72) | 2.22 (1.11-4.44) |
| P | .12 | .12 | .03 | .02 |
| MRD status pretransplant | ||||
| Negative | 1 | 1 | 1 | 1 |
| Positive, <0.1% | 1.59 (0.52-4.82) | 2.30 (0.43-12.4) | 1.66 (0.67-4.13) | 1.63 (0.59-4.44) |
| Positive, ≥0.1% | 3.30 (1.32-8.22) | 1.31 (0.33-5.24) | 2.23 (1.06-4.70) | 1.73 (0.75-4.00) |
| P | .04 | .62 | .09 | .35 |
| aGVHD* | ||||
| None | 1 | 1 | 1 | 1 |
| Grade 1-3 | 0.44 (0.20-0.95) | 0.64 (0.22-1.90) | 0.46 (0.25-0.87) | 0.55 (0.28-1.09) |
| P | .04 | .42 | .02 | .08 |
| Grade 4 | 0 (–) | 6.39 (1.85-22.1) | 2.56 (0.97-6.75) | 2.96 (1.09-8.07) |
| P | .99 | .003 | .06 | .03 |
Independent variables worsening EFS included the presence of extramedullary disease at diagnosis (HR, 2.0; P = .03) and pre-HCT MRD ≥ 0.1% (HR, 2.2; P = .04). The occurrence of grades 1-3 aGVHD improved EFS (HR, 0.5; P = .02), whereas grade 4 aGVHD decreased EFS (HR, 2.6; P = .06). Two independent predictors of mortality were the presence of extramedullary disease at initial diagnosis (HR, 2.2; P = .02) and the occurrence of grade 4 aGVHD (HR, 3.0; P = .03).
Discussion
Earlier phase 2 studies in adults suggested less acute GHVD, but no change in chronic GVHD in patients receiving siro for GVHD prophylaxis compared with historical controls.10,23 In this randomized phase 3 trial, however, although siro decreased overall and grades 2-4 aGVHD, it did not change serious aGVHD (grades 3-4) rates, and the addition of siro did not result in a survival advantage. Our results argue against adoption of siro as a new standard, at least in combination with a calcineurin inhibitor and mtx. First, rates of TMA and VOD were increased with the addition of siro. Second, approximately one-third of individuals on the experimental arm required major dose modifications (stopping or holding for an extended period) of siro due to toxicities. The most common reasons included VOD, TMA, fluid retention (ascites, pleural effusions), or cytopenias. Although the occurrence of each of these complications was rare, in total they resulted in a sizable portion of patients who were unable to tolerate siro therapy (32%). Those with modifications of siro therapy experienced aGVHD at rates similar to the standard arm. Finally, although siro decreased mild aGVHD, the occurrence of grades 1-3 aGVHD showed a trend toward decreased relapse and improved EFS, suggesting that eliminating this mild/moderate GVHD may not have been beneficial.
Our result seems to differ from the earlier observation by Armand et al of a survival benefit with the addition of siro to patients undergoing allogeneic HCT for lymphoma.12 The most critical difference between the studies was likely that 96% and 89% of standard and siro patients received PBSC in the Armand study, leading to cGVHD in 48% and 63%, respectively. PBSC use was allowed only if donors refused to donate marrow, and only 6 patients (4%) received this stem cell source from unrelated or nonsibling family members in our trial. Of note, the advantage seen by Armand et al was only apparent when reduced intensity conditioning (RIC) regimens were used. In the context of a RIC regimen, where the graft vs leukemia (GVL) effect is important and rates of cGVHD using PBSC are very high, it is possible that the siro effect noted by Armand was not due to direct antilymphoma cytotoxicity, but rather, modulation of cGVHD morbidity in a RIC setting. Both studies show no survival advantage with lymphoid malignancies when siro is used for GVHD prophylaxis in a myeloablative setting.
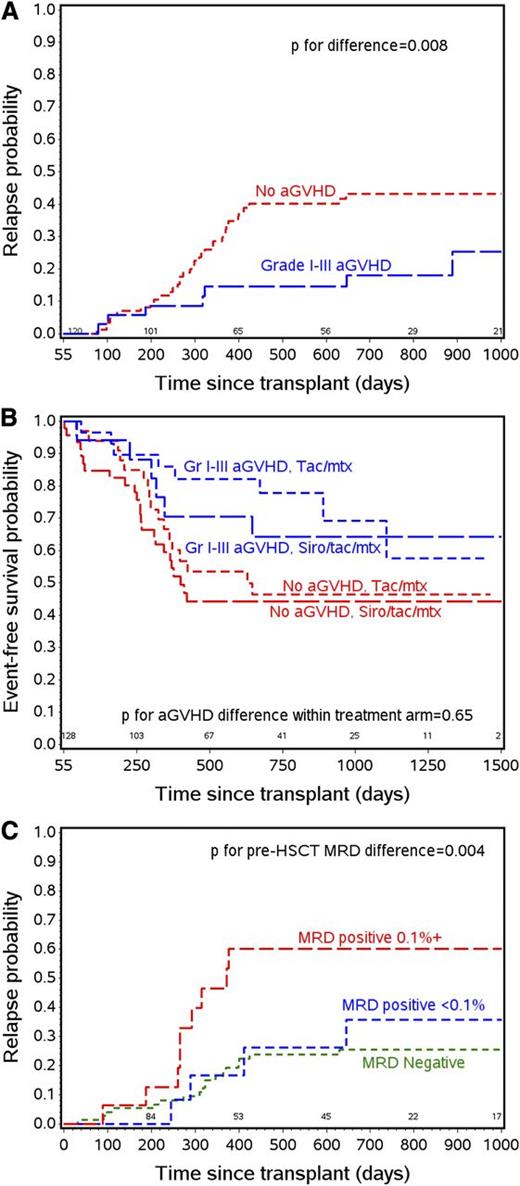
The presence of a GVL effect in ALL is controversial. Observations of a poor response to donor lymphocyte infusions given for ALL relapsed after HCT,24,25 and high relapse rates when patients go to transplant with active disease26 suggest a relatively weak effect. However, a preponderance of studies over the past 20 years,27-32 including studies in pediatric and young adult patients,33-35 suggest an effect of both acute and chronic GVHD in decreasing relapse. This study provides further evidence supporting this observation, showing that in a prospective, randomized cohort, grades 1-3 aGVHD has an independent effect of decreasing relapse (Figure 3A) and improving EFS regardless of treatment arm (Figure 3B), disease risk, T- vs B-cell disease, and the presence of MRD at the time of transplant. As noted in the multivariate analysis section, cGVHD by itself did not have an effect on outcomes. In a subanalysis, however, cGVHD occurring after acute GVHD was noted to provide further protection against relapse (HR = 0.44 for aGVHD [P = .04] compared with no aGVHD; aGVHD plus cGVHD HR = 0.14 [P = .05]). In contrast, de novo chronic GVHD had no effect on relapse or survival, however, patient numbers in the de novo group were small. With these observations in mind, future trials aimed at prevention of relapse will be more likely to succeed if they complement, rather than interfere with, the GVL effect.
The effect of aGVHD and MRD on relapse and survival. (A) Relapse probability curves for patients with grade 1-3 aGVHD by day 55 and with no aGVHD by day 55. (B) EFS probability by aGVHD status and treatment arm. (C) Relapse probability by pre-HCT MRD status.
The effect of aGVHD and MRD on relapse and survival. (A) Relapse probability curves for patients with grade 1-3 aGVHD by day 55 and with no aGVHD by day 55. (B) EFS probability by aGVHD status and treatment arm. (C) Relapse probability by pre-HCT MRD status.
A major association with outcome in our study was the presence of MRD prior to HCT. Earlier studies have shown that the presence of molecularly measured MRD just prior to HCT predicts relapse risk of ALL in children.36 More recent studies confirm that detection of ALL MRD by flow cytometry pre-HCT can also predict relapse,37-39 although the effect on outcome varies markedly between the reports. We showed a profound effect (Figure 3C) in this prospective study with well-defined conditioning regimens, with relapse rates tripling in patients with MRD levels ≥0.1% by flow cytometry prior to HCT. In fact, only the later occurrence of aGVHD in patients entering transplant with this level of MRD altered their risk of relapse and poor survival. A secondary analysis of this cohort looking at the effect of aGVHD in the context of MRD detected pre- and post-HCT is under way.
Many of the other risk factors noted in multivariate analysis were expected, but new observations were noted in the current study. First, although EFS is lower in T-cell vs B-cell cases in univariate analysis, the outcomes are not significantly different by multivariate analysis, and survival of CR2 high-risk T- and B-cell patients is similar. In addition, high-risk CR1 patients had outcomes closer to high-risk CR2 patients, rather than intermediate-risk CR2 patients as has been noted in previous studies. This is likely due to very strict inclusion criteria for CR1 patients. Of note, Ph+ patients did particularly well (8 of 9 patients survived) compared with the other 2 groups. This is likely because pretransplant tyrosine kinase therapy led to deep remissions in this group (5 of 6 with data available were MRD− at HCT). Of the other indications, persistent MRD pre-HCT may explain the poor outcomes in patients with PIF (9 of 19 with data available were MRD+ pre-HCT), but it does not explain the poor outcomes with hypodiploid patients (3 of 14 with data available were MRD+). Larger numbers are needed to draw more firm conclusions about these risk groups.
An additional significant risk factor we noted for EFS and OS is the presence of CNS disease at initial diagnosis. It may be that relapse occurring after the increased intensity of therapy associated with CNS disease at diagnosis portends poor outcome, but further study is required to validate this observation as a risk factor for poor outcome after HCT.
In summary, this trial showed a decrease in aGVHD when siro was added to tac and mtx–based GVHD prophylaxis. In spite of this decrease, due to increases in key toxicities and the overall beneficial effect of lower-grade aGVHD, any anti-ALL activity of siro does not appear to be sufficient to improve survival in this population, and is not recommended. Because of the significant impact of the GVL effect in ALL, investigators seeking to add novel agents in the context of or after allogeneic HCT for ALL to decrease relapse should use caution when considering agents that may decrease GVL.
Presented in part at the American Society of Hematology annual meeting, San Diego, CA, December 10-13, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grants (National Heart, Lung, and Blood Institute) N01 HC-45220/HHSN268200425220C, COG Chair’s grant U10 CA098543, and (National Cancer Institute) R01CA1116660. PBMTC activities were supported by (National Heart, Lung and Blood Institute) 2U01HL069254 and the St. Baldrick’s Foundation.
Authorship
Contribution: M.A.P., D.A.W., K.R.S., N.B., D.T.T., and S.A.G. designed and wrote the study, enrolled patients, participated in study analysis, and wrote the manuscript; B.L. performed data analysis and wrote the manuscript; W.L.C., E.R., S.G., J.M.G.-F., D.H., R.K.G., J.G.D., M.B., Y.B., and C.T. participated in study design, data analysis, and the writing of the manuscript; and J.M.G.-F., M.B., and S.A.G. coordinated study biology and ran MRD and other biology studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Pulsipher, Division of Hematology and Hematological Malignancies, University of Utah School of Medicine/Huntsman Cancer Institute, 30 North 1900 East, Room 5C402, Salt Lake City, UT 84132; e-mail: michael.pulsipher@hsc.utah.edu.