Key Points
Repeated doses of agonist antibodies targeting the costimulatory receptors GITR and OX40 result in anaphylaxis in mice.
Anaphylaxis caused by the GITR agonist antibody DTA-1 is dependent on GITR, IL-4, basophils, and platelet-activating factor.
Abstract
Immunotherapy for cancer using antibodies to enhance T-cell function has been successful in recent clinical trials. Many molecules that improve activation and effector function of T cells have been investigated as potential new targets for immunomodulatory antibodies, including the tumor necrosis factor receptor superfamily members GITR and OX40. Antibodies engaging GITR or OX40 result in significant tumor protection in preclinical models. In this study, we observed that the GITR agonist antibody DTA-1 causes anaphylaxis in mice upon repeated intraperitoneal dosing. DTA-1–induced anaphylaxis requires GITR, CD4+ T cells, B cells, and interleukin-4. Transfer of serum antibodies from DTA-1–treated mice, which contain high levels of DTA-1–specific immunoglobulin G1 (IgG1), can induce anaphylaxis in naive mice upon administration of an additional dose of DTA-1, suggesting that anaphylaxis results from anti-DTA-1 antibodies. Depletion of basophils and blockade of platelet-activating factor, the key components of the IgG1 pathway of anaphylaxis, rescues the mice from DTA-1–induced anaphylaxis. These results demonstrate a previously undescribed lethal side effect of repetitive doses of an agonist immunomodulatory antibody as well as insight into the mechanism of toxicity, which may offer a means of preventing adverse effects in future clinical trials using anti-GITR or other agonist antibodies as immunotherapies.
Introduction
Immune modulation using monoclonal antibodies has a significant impact on the overall survival of patients with cancer, based on the results of clinical trials using antibodies to block CTLA-4 and PD-1.1-6 In an approach that differs from using antibodies to mitigate immune checkpoint, agonist monoclonal antibodies can be used to directly stimulate T-cell function. Antibodies that engage members of the tumor necrosis factor receptor (TNFR) superfamily have shown promising tumor protection in preclinical models.3,7-13 Glucocorticoid-induced TNFR-related (GITR) is a costimulatory receptor in the TNFR superfamily with high homology to the other TNFR superfamily members OX40, 4-1BB, and CD27.14 GITR and OX40 are expressed primarily on activated CD4+ and CD8+ effector T cells as well as on CD4+Foxp3+ regulatory T cells (Tregs).15,16 Engagement of GITR and OX40 through agonist monoclonal antibodies results in increased T-cell activation, cytokine secretion, proliferation, and survival.17-23 We and others have shown that the GITR agonist antibody DTA-1 and the OX40 agonist antibody OX86 are very effective antitumor therapies in murine tumor models by increasing antitumor CD4+ and CD8+ T-cell effector function as well as destabilizing and causing apoptosis of Tregs in the tumor microenvironment.7,24-32 Additionally, B cells are required for DTA-1–mediated protection from certain tumor models, indicating a humoral component to the antitumor effects of DTA-1.33
Although antibodies targeting costimulatory pathways have shown unquestionable potential in preclinical models, clinical trials using a CD28 superagonist antibody and preclinical experiments using a 4-1BB agonist antibody have had severe adverse immune-mediated side effects.34,35 This indicates that agonist monoclonal antibodies must be treated with great caution, and potential side effects should be investigated comprehensively. In this study, we show that engagement of the TNFR superfamily members GITR and OX40 with repetitive intraperitoneal doses of the agonist antibodies DTA-1 and OX86, respectively, causes anaphylaxis in mice. Anaphylaxis induced by repetitive doses of DTA-1 is caused by serum antibodies and is dependent on CD4+ T cells, B cells, basophils, platelet-activating factor (PAF), and GITR. We suggest a mechanism in which anaphylaxis results from generation of anaphylactic anti-DTA-1 antibodies. Anaphylaxis caused by DTA-1 can be reduced or prevented by an antibody that neutralizes interleukin-4 (IL-4), a PAF antagonist, or a basophil-depleting antibody. These results suggest that anaphylactic antidrug antibody generation may be of particular concern when using agonist antibodies targeting GITR and OX40.
Methods
Mice and tumor cell lines
All mouse procedures were performed in accordance with Institutional Animal Care and Use Committee protocol guidelines at Memorial Sloan-Kettering Cancer Center (MSKCC) under an approved protocol. Veterinary care was given to any animals requiring medical attention. C57BL/6J and Kitw/Kitw-v mice were obtained from the Jackson Laboratory. Major histocompatibility complex (MHC) class I–deficient (strain B2MN12) and MHC class II–deficient (strain ABBN12) were obtained from Taconic. GITR−/− and littermate controls (Sv129 × C57BL/6 background)36 were a gift from Dr P. P. Pandolfi (MSKCC, New York, NY) and were backcrossed >10 generations onto C57BL/6J background by using a speed congenic system.37 Mice with the µMT mutation were purchased from the Jackson Laboratory and backcrossed >10 generations onto C57BL/6J background and bred at MSKCC. The B16-F10 mouse melanoma line was originally obtained from I. Fidler (MD Anderson Cancer Center, Houston, TX). In therapeutic tumor protection experiments, mice were challenged with 0.75 to 1.0 × 105 B16-F10 cells injected intradermally into the flank (50 microliters per injection) and monitored every 2 to 3 days for 80 days.
Monoclonal antibodies and drug treatments
DTA-1 (anti-GITR), OX86 (anti-OX40), FGK45.5 (anti-CD40), GK1.5 (anti-CD4), 11B11 (anti-IL-4), and TA99 (anti-Tyrp-1) were produced and purified by the Monoclonal Antibody Core Facility at MSKCC. LOB12.3 (anti-4-1BB), 1A8 (anti-Ly6G), LTF-2 (rat immunoglobulin G2b [IgG2b] isotype control), and HRPN (rat IgG1 isotype control) were purchased from Bio X Cell. Murinized DTA-1 (mDTA-1) was obtained from Merck Research Laboratories. Ba103 (anti-CD200R3) was purchased from Hycult Biotech. Unless otherwise stated, all antibodies were administered by intraperitoneal injection in 200 µL of sterile phosphate-buffered saline (PBS). For tumor protection experiments, 0.2 mg of TA99 was injected on days 5 and 9 after tumor challenge, and 1 mg of DTA-1 was injected on days 5, 9, and 16 after tumor challenge. For experiments investigating anaphylaxis, 1 mg of DTA-1, OX86, FGK45.5, LOB12.3, LTF-2, or OVA was injected on days 0, 4, and 11. For IL-4 neutralization experiments, 500 µg 11B11 was injected on day 0 of DTA-1 treatment. For granulocyte depletion experiments, 500 µg 1A8 or 25 µg Ba103 (intravenous injection into the tail vein) was administered on day 10 of DTA-1 treatment. For PAF inhibition experiments, 100 µg CV6209 (Santa Cruz Biotechnology) was administered by intraperitoneal injection 30 minutes prior to the final dose of DTA-1. For histamine inhibition experiments, 125 µg triprolidine (Sigma-Aldrich) was administered by intraperitoneal injection 30 minutes prior to the final dose of DTA-1.
Temperature measurement
The temperature of each animal was measured at baseline and every 10 to 30 minutes following induction of anaphylaxis by using a rectal probe (Physitemp) or infrared thermometer (Fisher Scientific).
Cell transfer and passive transfer of immune sera
For cell transfer experiments, C57BL/6J mice were sublethally irradiated (600 rad) 4 hours before transfer. Spleens and lymph nodes from DTA-1–treated C57BL/6J mice were purified, and 50 × 106 cells was administered by intravenous tail vein injection in 200 µL of sterile PBS. For passive transfer of immune sera, blood was collected from anesthetized mice by cardiac puncture as a terminal procedure. Then, 200 µL of pooled sera was administered by intravenous injection into the tail vein of recipient mice.
Fractionation of sera
All reagents were purchased from Thermo Scientific unless otherwise stated. A disposable 5-mL polypropylene column was packed with 1 mL of Protein A/G agarose. Sera were diluted 1:1 in (A/G) IgG Binding Buffer, added to the column, washed, and eluted in IgG Elution Buffer according to the manufacturer’s instructions. Antibody and flowthrough fractions were dialyzed by using Slide-A-Lyzer MINI Dialysis Devices (3.5 kDa molecular weight cut off) and concentrated with Amicon Ultra Centrifugal Filters (50K) (Millipore).
ELISA
Mice were treated with DTA-1 or LTF-2 on days −11 and −7 relative to sera collection. Sera were analyzed by enzyme-linked immunosorbent assay by using plates coated with either DTA-1 or LTF-2 in PBS and biotinylated rat anti-mouse IgG1 (BD Biosciences) as a secondary antibody. The SAv-HRP enzyme reagent and the OptEIA detection reagent were purchased from BD Biosciences. Plates were read with a SpectraMax 340PC (Molecular Devices) at 650 nm.
Statistical analysis
Statistical differences between experimental groups were determined by using the unpaired 2-tailed Student t test and GraphPad Prism software.
Results
Repetitive intraperitoneal doses of DTA-1 cause anaphylaxis in a GITR-dependent manner
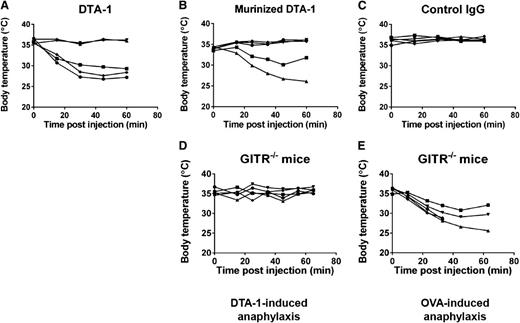
We and others have previously shown that DTA-1 is a very potent immunotherapy when used as a single agent.24-27,38,39 A single dose of DTA-1 protects up to 60% of mice bearing established tumors.25 To improve on the antitumor effects of DTA-1, we administered multiple doses of DTA-1 in combination with a tumor antigen–specific antibody (TA99) to mice bearing established tumors. Although the combination therapy of TA99 and DTA-1 provided considerable protection from tumor challenge, we observed that about 20% of the mice succumbed to a treatment-related toxicity (supplemental Figure 1). Upon monitoring mice immediately following DTA-1 injections, we observed that most of the mice became ill-appearing and lethargic by 20 minutes after the final dose of DTA-1. Additionally, we observed erythema of the footpads and piloerection in all affected animals, as well as very low blood volume when the affected mice were euthanized. These effects were observed only after the final dose and were not observed after the first or second doses of DTA-1. In addition, the adverse effects of DTA-1 were independent of tumor challenge and TA99 treatment. The symptoms and time course were suggestive of anaphylaxis,40-42 which we confirmed by examining body temperature after each dose of DTA-1. Mice experienced a rapid and severe drop in body temperature following the third intraperitoneal dose of DTA-1 (Figure 1A and supplemental Figure 2A). mDTA-1 caused the same anaphylactic symptoms as DTA-1, demonstrating that the foreign nature of DTA-1 is not the cause of anaphylaxis (Figure 1B and supplemental Figure 2B). mDTA-1 caused more severe anaphylaxis symptoms when administered at lower (75 µg) doses by intravenous injection into the tail vein (supplemental Figure 3), but neither DTA-1 nor mDTA-1 caused anaphylaxis when administered subcutaneously. Three doses of isotype control antibody (rat IgG2b) did not cause symptoms of anaphylaxis (Figure 1C).
DTA-1 causes anaphylaxis in a GITR-dependent manner. C57BL/6J mice were treated with 1 mg of (A) DTA-1, (B) mDTA-1, or (C) isotype control (rat IgG2b, clone LTF-2) on days 0, 4, and 11. GITR−/− mice were treated with 1 mg (D) DTA-1 or (E) OVA. Rectal temperatures of individual mice were monitored for 1 hour after each dose, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group).
DTA-1 causes anaphylaxis in a GITR-dependent manner. C57BL/6J mice were treated with 1 mg of (A) DTA-1, (B) mDTA-1, or (C) isotype control (rat IgG2b, clone LTF-2) on days 0, 4, and 11. GITR−/− mice were treated with 1 mg (D) DTA-1 or (E) OVA. Rectal temperatures of individual mice were monitored for 1 hour after each dose, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group).
We found that GITR−/− mice were protected from DTA-1–induced anaphylaxis (Figure 1D). GITR−/− mice showed symptoms of severe anaphylaxis in an anaphylaxis model caused by ovalbumin, indicating that protection of GITR−/− mice from DTA-1–induced anaphylaxis is not a result that can be generalized to all anaphylaxis models (Figure 1E). These results show that GITR expression is critical for DTA-1–induced anaphylaxis.
Anaphylaxis is caused by repetitive dosing of antibodies targeting GITR and OX40
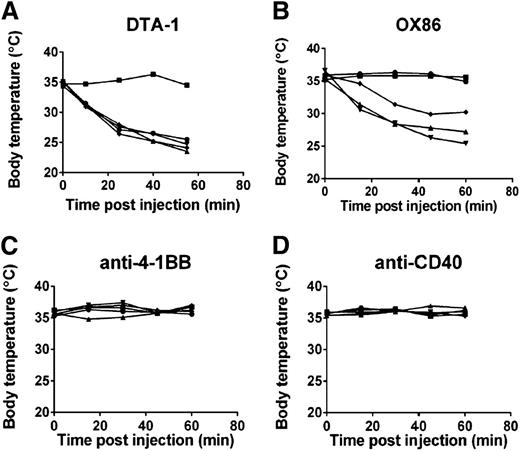
To determine whether anaphylaxis caused by DTA-1 was a general side effect of repetitive dosing of antibodies targeting the TNFR superfamily of costimulatory receptors, we treated mice with agonist antibodies to OX40 (clone OX86), 4-1BB, and CD40. We observed that mice treated with repetitive intraperitoneal doses of OX86, but not anti-4-1BB or anti-CD40, underwent anaphylaxis (Figure 2 and supplemental Figure 4). Anaphylaxis caused by OX86 was comparable in incidence and severity to anaphylaxis caused by DTA-1, suggesting that the mechanism behind anaphylaxis caused by both antibodies is similar. We used DTA-1 to study the mechanism of anaphylaxis in the subsequent experiments.
Repeated intraperitoneal doses of the TNFR superfamily agonist antibodies DTA-1 and OX86 cause anaphylaxis. C57BL/6J mice were treated with 1 mg of (A) DTA-1, (B) OX86, (C) anti-4-1BB (clone LOB.12), or (D) anti-CD40 (clone FGK45.5) on days 0, 4, and 11. Rectal temperatures of individual mice were monitored for 1 hour following the final injection, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group).
Repeated intraperitoneal doses of the TNFR superfamily agonist antibodies DTA-1 and OX86 cause anaphylaxis. C57BL/6J mice were treated with 1 mg of (A) DTA-1, (B) OX86, (C) anti-4-1BB (clone LOB.12), or (D) anti-CD40 (clone FGK45.5) on days 0, 4, and 11. Rectal temperatures of individual mice were monitored for 1 hour following the final injection, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group).
CD4+ T cells, B cells, and IL-4 are required for DTA-1–induced anaphylaxis
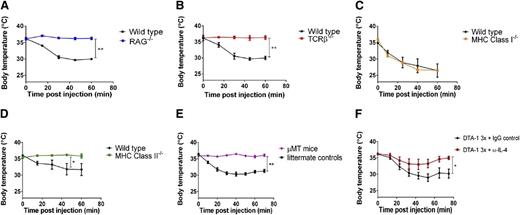
Because GITR is expressed most highly on T cells, we were particularly interested in whether T cells are involved in anaphylaxis caused by repetitive dosing of DTA-1. We observed that both RAG−/− and TCRβ−/− mice were protected from DTA-1–induced anaphylaxis (Figure 3A-B). We found that MHC class I–deficient mice were not protected from DTA-1–induced anaphylaxis, indicating that CD8+ T cells do not play a major role in anaphylaxis caused by DTA-1 (Figure 3C). Conversely, mice that were depleted of CD4+ T cells (supplemental Figure 5) as well as MHC class II–deficient mice (Figure 3D) were protected from DTA-1–induced anaphylaxis, suggesting a role for CD4+ T cells in anaphylaxis caused by DTA-1. Although GITR is expressed at much lower levels on B cells than on CD4+ T cells (supplemental Figure 6), we also examined whether B cells were required for DTA-1–induced anaphylaxis, because GITR agonist antibodies have recently been shown to have potent humoral adjuvant effects.33,43 We found that µMT mice, which lack mature B cells, were protected from anaphylaxis caused by DTA-1 (Figure 3E). Because CD4+ T cells typically do not secrete vasoactive mediators such as histamine that are important for the pathology of anaphylaxis, the role of CD4+ T cells in DTA-1–induced anaphylaxis is most likely to provide helper signals to B cells to facilitate an anaphylactic antibody response. One such signal may be IL-4, a critical cytokine for anaphylaxis as well as other Th2-type allergic responses.44,45 We observed that neutralizing IL-4 considerably reduced anaphylaxis severity (Figure 3F).
CD4+ T cells, B cells, and IL-4 are required for DTA-1–induced anaphylaxis. (A) RAG−/− mice, (B) TCRβ-deficient mice, (C) MHC class I–deficient mice, (D) MHC class II–deficient mice, and (E) µMT mice were injected with 1 mg DTA-1 on days 0, 4, and 11. Rectal temperatures of mice were monitored for 1 hour following the final injection. (F) C57BL/6J mice were treated with 1 mg DTA-1 on days 0, 4, and 11. Concurrently with the day 0 injection, mice also received 0.5 mg anti-IL-4 (clone 11B11) or isotype control (rat IgG1, clone HRPN). Rectal temperatures were monitored following the day –11 dose of DTA-1. Lines in the graphs represent the mean of each group of mice. The data are representative of 2 independent experiments (n = 5 mice per group). *P < .05; **P < .0001 by unpaired 2-tailed Student t test.
CD4+ T cells, B cells, and IL-4 are required for DTA-1–induced anaphylaxis. (A) RAG−/− mice, (B) TCRβ-deficient mice, (C) MHC class I–deficient mice, (D) MHC class II–deficient mice, and (E) µMT mice were injected with 1 mg DTA-1 on days 0, 4, and 11. Rectal temperatures of mice were monitored for 1 hour following the final injection. (F) C57BL/6J mice were treated with 1 mg DTA-1 on days 0, 4, and 11. Concurrently with the day 0 injection, mice also received 0.5 mg anti-IL-4 (clone 11B11) or isotype control (rat IgG1, clone HRPN). Rectal temperatures were monitored following the day –11 dose of DTA-1. Lines in the graphs represent the mean of each group of mice. The data are representative of 2 independent experiments (n = 5 mice per group). *P < .05; **P < .0001 by unpaired 2-tailed Student t test.
Anaphylactic activity can be transferred via serum antibodies
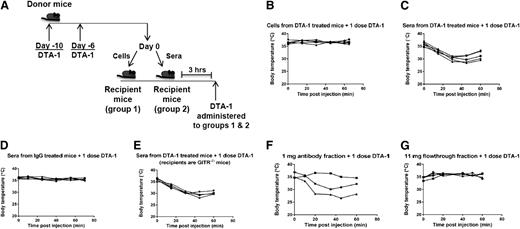
The findings that CD4+ T cells and B cells were required for DTA-1–induced anaphylaxis suggested that an antibody response is the cause of the observed symptoms. Indeed, the difference between anaphylaxis and other types of shock such as sepsis is the involvement of antibodies, which trigger release of inflammatory mediators from innate immune cells through Fc receptors.46 To further elucidate the mechanism underlying anaphylaxis induced by repetitive dosing of DTA-1, we attempted to transfer the anaphylactic activity from one cohort of mice to another through either cells or serum. Mice were treated with 2 doses of DTA-1 administered 4 days apart. Six days after the second dose of DTA-1, the mice were euthanized, and cells from lymphoid organs or pooled sera were transferred into naive animals following the schema in Figure 4A. No toxicity was observed immediately following transfer of cells or sera. Three hours after the transfer, all mice were treated with a single dose of DTA-1. The mice that had received the serum fraction displayed symptoms of anaphylaxis upon DTA-1 administration, whereas the mice that had received cells did not (Figure 4B-C). Additionally, control mice that had received sera from IgG-treated mice did not show signs of anaphylaxis upon an additional dose of DTA-1 (Figure 4D). Although the sera transferred from DTA-1–treated mice most likely contained DTA-1, we do not believe that the transferred DTA-1 was responsible for anaphylaxis, because naive mice that were treated with 2 doses of DTA-1 (3 hours apart) did not undergo anaphylaxis (supplemental Figure 7). Therefore, DTA-1 induces production of a factor present in the serum that can elicit anaphylaxis in mice upon 1 additional dose of DTA-1. Serum from DTA-1–treated wild-type mice could also induce anaphylaxis upon an additional dose of DTA-1 in GITR−/− mice (Figure 4E), indicating that although GITR is required for anaphylaxis caused by repeated doses of DTA-1 (Figure 1D), GITR triggering is not required for the actual symptoms of anaphylaxis observed upon the final dose.
Serum antibodies from DTA-1–treated animals transfer anaphylactic activity to naive mice. (A-E) C57BL/6J mice were treated with 1 mg DTA-1 or isotype control (rat IgG2b, clone LTF-2) on days −10 and −6. On day 0, mice were euthanized, and sera, spleens, and lymph nodes were removed. Either (B) 50 × 106 cells from pooled spleens and lymph nodes or (C-E) 200 µL pooled sera were transferred by intravenous tail vein injection into naive (C,D) C57BL/6J or (E) GITR−/− mice. Three hours later, a single dose of DTA-1 was administered by intraperitoneal injection, and rectal temperatures were monitored for 1 hour, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group). (F-G) Donor mice were treated as in (A). After sera were harvested, antibodies were fractionated from the pooled sera by using a protein A/G column. Either (F) 1 mg of the antibody fraction or (G) 11 mg of the antibody-depleted fraction (termed “flowthrough” fraction) was transferred by intravenous tail vein injection into naive mice. Three hours later, 1 mg DTA-1 was administered by intraperitoneal injection, and rectal temperatures of individual mice were monitored, represented by individual lines in the graphs. Data are representative of 2 independent experiments (n = 3 to 5 mice per group).
Serum antibodies from DTA-1–treated animals transfer anaphylactic activity to naive mice. (A-E) C57BL/6J mice were treated with 1 mg DTA-1 or isotype control (rat IgG2b, clone LTF-2) on days −10 and −6. On day 0, mice were euthanized, and sera, spleens, and lymph nodes were removed. Either (B) 50 × 106 cells from pooled spleens and lymph nodes or (C-E) 200 µL pooled sera were transferred by intravenous tail vein injection into naive (C,D) C57BL/6J or (E) GITR−/− mice. Three hours later, a single dose of DTA-1 was administered by intraperitoneal injection, and rectal temperatures were monitored for 1 hour, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group). (F-G) Donor mice were treated as in (A). After sera were harvested, antibodies were fractionated from the pooled sera by using a protein A/G column. Either (F) 1 mg of the antibody fraction or (G) 11 mg of the antibody-depleted fraction (termed “flowthrough” fraction) was transferred by intravenous tail vein injection into naive mice. Three hours later, 1 mg DTA-1 was administered by intraperitoneal injection, and rectal temperatures of individual mice were monitored, represented by individual lines in the graphs. Data are representative of 2 independent experiments (n = 3 to 5 mice per group).
To determine which component in the serum of DTA-1–treated mice is responsible for rendering naive mice susceptible to DTA-1–induced anaphylaxis, we fractionated sera from DTA-1–treated mice over a protein A/G column into a fraction containing only serum antibodies and a fraction from which antibodies had been depleted (termed “flowthrough” fraction). We observed that transfer of the antibody fraction, but not the flowthrough fraction, from DTA-1–treated mice into naive mice resulted in anaphylaxis when mice were treated with a single dose of DTA-1 (3 hours later) (Figure 4F-G). As mentioned above, although DTA-1 is likely to be present in the antibody fraction we purified, we do not believe that the DTA-1 transferred in the antibody fraction is responsible for transferring the susceptibility to anaphylaxis. Rather, taking into account the humoral adjuvant properties of DTA-1, we believe that the mice are making an anaphylactic antibody response to DTA-1 itself, and that the final intraperitoneal bolus injection of DTA-1 forms immune complexes with anti–DTA-1 antibodies and triggers release of inflammatory mediators that cause vasodilation, leading to the observed drop in body temperature and other anaphylactic symptoms in the affected mice.
Repetitive dosing of DTA-1 induces anaphylaxis through the IgG1-basophil-PAF pathway
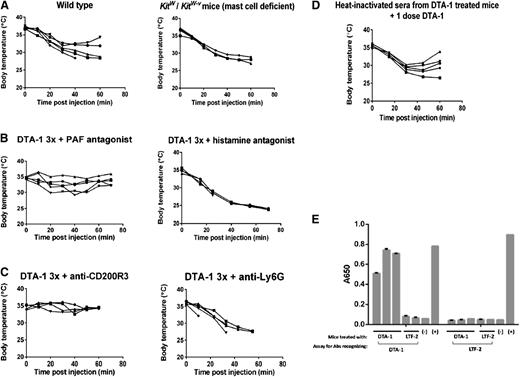
It has become apparent that 2 pathways of anaphylaxis exist in mice: the best characterized is caused by antigen crosslinking of IgE on mast cells followed by release of histamine,47-50 while the other pathway is mediated by IgG1 and basophil or neutrophil release of PAF.51-56 We observed that mast cell–deficient Kitw/Kitw-v mice were not protected from anaphylaxis caused by DTA-1 (Figure 5A). This led us to investigate the IgG1- and PAF-mediated pathway of anaphylaxis. Treatment with the PAF inhibitor CV6209 30 minutes before the final dose of DTA-1 resulted in decreased anaphylaxis severity, whereas the histamine inhibitor triprolidine had no effect on anaphylaxis caused by DTA-1 (Figure 5B), suggesting that PAF-secreting cells mediate anaphylaxis in our model. Depletion of basophils, but not neutrophils, 24 hours before the final dose of DTA-1 resulted in protection from anaphylaxis (Figure 5C), supporting the finding of Tsujimura et al56 that basophils, while present in very small numbers in peripheral blood, can be critical effector cells of anaphylaxis.
Anaphylaxis caused by DTA-1 is mediated by PAF, basophils, and IgG1 antibodies. (A) C57BL/6J or Kitw/KitW-v mice were treated with 1 mg DTA-1 on days 0, 4, and 11. Rectal temperatures of individual mice were monitored for 1 hour following the final injection, represented by individual lines in the graphs. Data are representative of 2 independent experiments (n = 5 mice per group). (B) C57BL/6J mice were treated with 1 mg DTA-1 on days 0, 4, and 11. Thirty minutes prior to the final DTA-1 injection, mice were injected intraperitoneally with either 125 µg of the histamine inhibitor triprolidine or 125 µg of the PAF inhibitor CV6209. Rectal temperatures of individual mice were monitored for 1 hour following the final dose of DTA-1, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group). (C) C57BL/6J mice were treated with 1 mg DTA-1 on days 0, 4, and 11. One day before the final dose of DTA-1, mice were either injected intravenously with 25 µg of anti-CD200R3 (clone Ba103) or intraperitoneally with 0.5 mg of anti-Ly6G (clone 1A8). Rectal temperatures of individual mice were monitored for 1 hour following the final dose of DTA-1, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group). (D) Sera from DTA-1–treated mice were collected as in Figure 4A. The pooled sera were heat inactivated at 56°C for 3 hours. Heat-inactivated sera was then transferred by intravenous injection into the tail vein of recipient mice. Three hours later, a single dose of DTA-1 was administered, and rectal temperatures were monitored, represented by individual lines in the graphs. Data are representative of 2 independent experiments (n = 5 mice per group). (E) C57BL/6J mice were treated with DTA-1 or isotype control (rat IgG2b, clone LTF-2) on days 0 and 4. On day 11, sera were collected from individual mice and assayed by enzyme-linked immunosorbent assay for IgG1 antibodies recognizing DTA-1 or LTF-2. Data are representative of 3 independent experiments.
Anaphylaxis caused by DTA-1 is mediated by PAF, basophils, and IgG1 antibodies. (A) C57BL/6J or Kitw/KitW-v mice were treated with 1 mg DTA-1 on days 0, 4, and 11. Rectal temperatures of individual mice were monitored for 1 hour following the final injection, represented by individual lines in the graphs. Data are representative of 2 independent experiments (n = 5 mice per group). (B) C57BL/6J mice were treated with 1 mg DTA-1 on days 0, 4, and 11. Thirty minutes prior to the final DTA-1 injection, mice were injected intraperitoneally with either 125 µg of the histamine inhibitor triprolidine or 125 µg of the PAF inhibitor CV6209. Rectal temperatures of individual mice were monitored for 1 hour following the final dose of DTA-1, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group). (C) C57BL/6J mice were treated with 1 mg DTA-1 on days 0, 4, and 11. One day before the final dose of DTA-1, mice were either injected intravenously with 25 µg of anti-CD200R3 (clone Ba103) or intraperitoneally with 0.5 mg of anti-Ly6G (clone 1A8). Rectal temperatures of individual mice were monitored for 1 hour following the final dose of DTA-1, represented by individual lines in the graphs. Data are representative of 3 independent experiments (n = 5 mice per group). (D) Sera from DTA-1–treated mice were collected as in Figure 4A. The pooled sera were heat inactivated at 56°C for 3 hours. Heat-inactivated sera was then transferred by intravenous injection into the tail vein of recipient mice. Three hours later, a single dose of DTA-1 was administered, and rectal temperatures were monitored, represented by individual lines in the graphs. Data are representative of 2 independent experiments (n = 5 mice per group). (E) C57BL/6J mice were treated with DTA-1 or isotype control (rat IgG2b, clone LTF-2) on days 0 and 4. On day 11, sera were collected from individual mice and assayed by enzyme-linked immunosorbent assay for IgG1 antibodies recognizing DTA-1 or LTF-2. Data are representative of 3 independent experiments.
To further assess the involvement of IgG1 antibodies in DTA-1–induced anaphylaxis, we heated serum from DTA-1–treated mice at 56°C to inactivate IgE antibodies. In further agreement with the involvement of the IgG1 anaphylaxis pathway in DTA-1–induced anaphylaxis, we found that the heat-inactivated serum retained anaphylactic activity (Figure 5D). Additionally, we found high levels of DTA-1–specific IgG1 antibodies in serum from individual mice treated with DTA-1(Figure 5E), supporting the hypothesis stated above that DTA-1–induced anaphylaxis results from an anti-DTA-1 anaphylactic antibody response.
Inhibiting anaphylaxis does not reduce the efficacy of DTA-1 as a tumor therapy
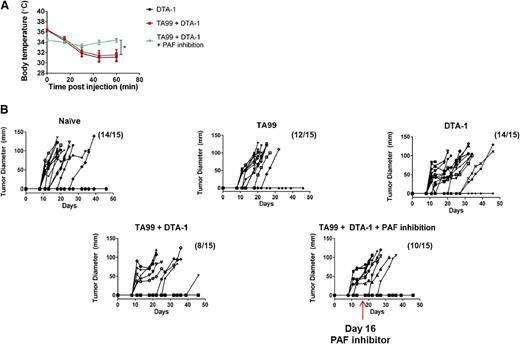
We next investigated whether anaphylaxis caused by repetitive dosing of DTA-1 could be prevented while preserving the antitumor activity of DTA-1. Mice were challenged with B16 melanoma and treated with the combination therapy of DTA-1 and the tumor antigen–specific antibody TA99 (supplemental Figure 1). As in Figure 5, PAF was inhibited 30 minutes prior to the final dose of DTA-1. Blocking PAF protected tumor-bearing mice from anaphylaxis, as observed in non–tumor-bearing mice (Figures 5B and 6A). Additionally, protection from anaphylaxis did not adversely affect the tumor protection resulting from TA99 plus DTA-1 therapy (Figure 6B). These results suggest that DTA-1–induced anaphylaxis can be realistically mitigated in therapeutic settings.
Inhibition of PAF protects mice from anaphylaxis while maintaining tumor protection provided by DTA-1 as part of a combination therapy. C57BL/6J mice were challenged intradermally with 1 × 105 B16 melanoma cells. Groups of mice were either left untreated (naive) or were treated with 200 µg TA99 on days 5 and 9 after tumor challenge, 1 mg DTA-1 on days 5, 9, and 16 after tumor challenge, or a combination of both TA99 and DTA-1 at the aforementioned doses and schedules. One group treated with TA99 plus DTA-1 received 125 µg CV6209 by intraperitoneal injection in PBS 30 minutes before the day 16 dose of DTA-1. (A) Rectal temperatures were monitored following the day 16 dose of DTA-1. (B) Tumor growth was measured every 2 to 3 days, represented by individual lines in the graphs. Mice were euthanized when tumor diameter reached 1 cm. Data are representative of 3 independent experiments (n = 15 mice per group). *P < .05, unpaired 2-tailed Student t test.
Inhibition of PAF protects mice from anaphylaxis while maintaining tumor protection provided by DTA-1 as part of a combination therapy. C57BL/6J mice were challenged intradermally with 1 × 105 B16 melanoma cells. Groups of mice were either left untreated (naive) or were treated with 200 µg TA99 on days 5 and 9 after tumor challenge, 1 mg DTA-1 on days 5, 9, and 16 after tumor challenge, or a combination of both TA99 and DTA-1 at the aforementioned doses and schedules. One group treated with TA99 plus DTA-1 received 125 µg CV6209 by intraperitoneal injection in PBS 30 minutes before the day 16 dose of DTA-1. (A) Rectal temperatures were monitored following the day 16 dose of DTA-1. (B) Tumor growth was measured every 2 to 3 days, represented by individual lines in the graphs. Mice were euthanized when tumor diameter reached 1 cm. Data are representative of 3 independent experiments (n = 15 mice per group). *P < .05, unpaired 2-tailed Student t test.
Discussion
Preclinical models have demonstrated that stimulation of the GITR and OX40 pathways using DTA-1 or OX86 can result in striking immune modulation, leading to tumor protection and induction of autoimmunity. In this report, we show that repetitive doses of the GITR agonist antibody DTA-1 cause anaphylaxis in mice, which is mediated by IgG1, basophils, and PAF. We observed similar effects using the OX40 agonist antibody OX86, suggesting that antibodies engaging members of the TNFR superfamily may be particularly prone to anaphylaxis upon repeated dosing. Interestingly, GITR was critically required for anaphylaxis caused by repetitive dosing of DTA-1, but not for anaphylaxis caused by a single dose of DTA-1 after transfer of serum antibodies from previously dosed mice. This strongly suggests that costimulation of T cells through GITR drives anaphylactic sensitization. The critical mechanistic role for GITR in DTA-1–mediated anaphylaxis is strongly supported by the observation that GITR−/− mice were protected from DTA-1–induced, but not ovalbumin-induced, anaphylaxis. DTA-1–induced anaphylaxis was mediated by serum antibodies generated after DTA-1 injection, and because no additional foreign antigens were injected into the mice, the data suggest that anti-DTA-1 antibodies are responsible for the rapid symptoms observed after the final dose of DTA-1. Therefore, it appears that engaging GITR using DTA-1 enhances the immunogenicity of DTA-1 itself, leading to anaphylaxis upon repetitive dosing.
The observation that the OX40 agonist antibody OX86 also caused anaphylaxis whereas the 4-1BB agonist antibody did not cause any anaphylactic symptoms supports further examination of the similarities between OX40 and GITR in signaling and expression. Interestingly, Tregs express very high levels of GITR and OX40, but not 4-1BB; this expression pattern may explain the more potent antibody responses caused by agonizing GITR and OX40, especially in light of recent findings that Tregs are involved in control of the germinal center response and that depletion of Tregs can enhance anaphylaxis.57-63
An important question is whether anaphylaxis caused by repetitive intraperitoneal dosing of DTA-1 and OX86 in mice is translatable to humans treated with humanized versions of these antibodies. Our preclinical observations will heighten awareness that anaphylaxis may be of particular concern when using antibodies targeting GITR and OX40. Indeed, anaphylaxis caused by antidrug antibodies has been observed in patients retreated with OKT3 (mouse anti-CD3) and basiliximab (chimeric anti-CD25).64-66 Careful immune monitoring of sera from these patients may have detected high levels of antidrug IgE antibodies and could have precluded these patients from receiving another dose of antibody. Although the foreign and chimeric natures of OKT3 and basiliximab, respectively, were considered the primary reasons for anaphylaxis upon retreatment, it is interesting that both OKT3 and basiliximab target and modulate the function of T lymphocytes. Together with our data showing anaphylaxis upon retreatment with DTA-1 and OX86 in mice, this suggests that antibodies targeting T cells may be particularly susceptible to anaphylactic sensitization. Our results indicate that caution should be exercised when administering multiple doses of anti-GITR or anti-OX40 to patients, especially if high levels of antidrug IgE or IgG1 antibodies are observed after the first dose. Of additional concern when translating anti-GITR to the clinic is our observation that anaphylaxis was also observed in mice treated with mDTA-1. Also of note is that mDTA-1 and OX86 are different isotypes from rat DTA-1; therefore, the observations that mDTA-1 and OX86 cause anaphylaxis invalidates concerns that anaphylaxis caused by DTA-1 is an isotype-specific artifact. The finding that repetitive dosing of mDTA-1 causes anaphylaxis when dosed by intravenous bolus, but not when dosed subcutaneously, indicates that intravenous doses in the clinic should be delivered slowly. Regardless of the method of administration, however, administering a drug when high levels of antidrug antibodies are present will result in reduced bioavailability of the drug even if anaphylaxis does not occur, because the drug will be cleared from the bloodstream by antidrug antibodies. Given the high costs of monoclonal antibodies as therapies, administering an antibody to patients with high levels of antidrug antibodies should be avoided from an economic as well as a safety standpoint.
Additionally, several recent advances have been made in antibody engineering technology that enables the creation of less immunogenic antibodies. Algorithms have been developed to predict epitopes within a given protein sequence that will bind to MHC molecules, as well as to determine the structural consequences of mutations that will abrogate the immunogenic peptides.67 Epitopes specific for Treg cells (Tregitopes) have also been determined in conserved domains of Fc and F(ab) portions of antibodies in mice and humans.68 Activation of regulatory T cells through engagement with Tregitope-MHC complexes results in expansion and activation of regulatory T cells, along with enhancement of the immunosuppressive Treg functions such as IL-10 and transforming growth factor-β secretion.69 Deimmunization strategies and engineering to incorporate Treg epitopes may be of particular importance when designing antibodies targeting GITR and OX40 for clinical use.
In conclusion, we found that repetitive intraperitoneal bolus doses of DTA-1 cause anaphylaxis in a manner that requires GITR, CD4+ T cells, B cells, basophils, PAF, IL-4, and serum antibodies. These data are important because patients with cancer are currently being treated with anti-GITR and anti-OX40 in clinical trials, and anticipating immune-based side effects will result in safer trials and will possibly expedite FDA approval for these and other immunotherapies. Understanding anaphylaxis caused by antidrug antibodies is particularly significant because it is a phenomenon that has been observed in patients re-treated with other antibodies. It is tempting to speculate that antibodies targeting T cells, especially Tregs, are more susceptible to anaphylactic sensitization than antibodies targeting tumor or stromal cells. It will be important to dose slowly and include quantitative monitoring of antidrug IgE and IgG1 antibodies in clinical trials using GITR agonist antibodies. Moreover, the availability of anti-PAF drugs in the clinic for use in cardiac rehabilitation70 suggests that inclusion of these drugs in future clinical trials to mitigate adverse immune-mediated events is a possibility.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Xia Yang and Hong Zhong for technical help and Dr Daniel Hirschhorn-Cymerman for critically reading the manuscript.
This work was supported in part by research funding from Merck (A.M.B., D.G., and L.G.P.) and by grants from the National Cancer Institute (R01 CA56821, P01 CA33049, and P01 CA59350), Swim Across America, the Lita Annenberg Hazen Foundation, the T.J. Martell Foundation, Mr William H. Goodwin and Mrs Alice Goodwin and the Commonwealth Cancer Foundation for Research, the Joanna M. Nicolay Melanoma Foundation, and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center.
Authorship
Contribution: J.T.M., A.M.B., T.M., and J.D.W. designed the research; J.T.M., A.P.B., D.G., and L.G.P. performed research; A.M.B., D.G., and L.G.P. contributed a new reagent; J.T.M., A.M.B., D.G., L.G.P., T.M., and J.D.W. analyzed data; and J.T.M., T.M., and J.D.W. wrote the paper.
Conflict-of-interest disclosure: A.M.B., D.G., and L.G.P. are employees of Merck Research Laboratories. The remaining authors declare no competing financial interests.
Correspondence: Jedd D. Wolchok, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Room Z-1462, New York, NY 10065; e-mail: wolchokj@mskcc.org.
References
Author notes
T.M. and J.D.W. contributed equally to this paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal