Key Points
STIM1-mediated calcium entry is critical for neutrophil superoxide release via activation of calcium-sensitive PKCα and PKCβ.
STIM1 deficiency results in profound susceptibility to bacterial infection, but also protection in hepatic ischemia/reperfusion injury.
Abstract
The stromal-interacting molecule 1 (STIM1) is a potent sensor of intracellular calcium, which in turn regulates entry of external calcium through plasma membrane channels to affect immune cell activation. Although the contribution of STIM1 to calcium signaling in lymphocytes has been well studied, the role of this protein in neutrophil-mediated inflammation and host defense is unknown. We report that STIM1-deficient murine neutrophils show loss of store-operated calcium entry (SOCE) in response to both soluble ligands that activate G-proteins as well as Fcγ-receptor or integrin ligation that activates tyrosine kinase signaling. This results in modest defects in phagocytosis and degranulation responses but a profound block in superoxide production by the phagocyte oxidase. We trace the primary intracellular target of calcium to be protein kinase C isoforms α and β (PKCα and PKCβ), which in turn phosphorylate subunits of the oxidase leading to superoxide production. In vivo the loss of SOCE in stim1−/− chimeric mice results in marked susceptibility to bacterial infections but also protection from tissue injury in hepatic ischemia/reperfusion injury. These results demonstrate the critical role of STIM1-mediated SOCE and define major protein targets of calcium signaling in neutrophil activation during inflammatory disease.
Introduction
Neutrophils are the first line of host defense against pathogenic infections but are also responsible for causing tissue injury during inflammatory reactions, such as ischemia/reperfusion injury.1,2 Activation of neutrophils leads to release of reactive oxygen species (ROS) via the phagocyte oxidase as well as release of granule contents (proteases, peroxidases, and other inflammatory mediators), which together affect both pathogen killing and tissue injury. The majority of neutrophil-activating receptors induce extracellular calcium entry as an early signaling response. Influx of calcium has been shown to be required for a number of neutrophil effector functions, including phagocytosis, phagosome maturation, phagosomal ROS production, degranulation, and chemotaxis.3-5 Most of these studies have used depletion of extracellular calcium, calcium chelators, or broadly acting calcium channel blockers to demonstrate a functional role for calcium signaling in ex vivo neutrophil activation assays. Here we use a genetic approach to assess the physiologic outcome of altered calcium signaling in neutrophils in vivo.
Entry of calcium into neutrophils is mediated through both store-operated calcium entry (SOCE) pathways and non-SOCE mechanisms. SOCE is initiated by either tyrosine kinase or G-protein–coupled receptor (GPCR) signaling, which causes activation of phospholipase C (PLC) isoforms that in turn produce intracellular inositol 1,4,5, triphosphate (IP3).6 IP3 binds to receptors within the endoplasmic reticulum (ER), resulting in release of internal calcium stores into the cytoplasm and activating plasma membrane channels to allow extracellular entry of calcium to initiate effector functions. Stromal-interacting molecule 1 (STIM1) and STIM2 are the main calcium sensors within the ER that connect reduction of ER calcium stores to the plasma membrane channel proteins Orai1, -2, and -3.7,8 Studies in neutrophil-like cell lines suggest that STIM1/Orai1-regulated calcium entry plays a significant role in activation of neutrophil phagocytosis and ROS production.9,10 Neutrophils can also be activated by non-SOCE mechanisms that are independent of initial IP3-mediated depletion of ER calcium stores, primarily through transient receptor potential channel (TRPC) proteins.11 The TRPC family of proteins has also been linked to ROS production in neutrophil-like cell lines.12
The role of STIM-mediated SOCE has been studied mainly in lymphocytes. Genetic disruption of STIM1 and STIM2 leads to complete loss of SOCE in B and T cells, resulting in defects in proliferation, cytokine secretion, and altered immune responses in vivo.13,14 Indeed, lymphocyte dysfunction is thought to underlie the immunodeficiency seen in patients with mutations in STIM1 or ORAI1, although these individuals often present with bacterial infections that are more characteristic of defective innate immune cells.15 There have been no studies of STIM1-mediated SOCE in primary neutrophils either ex vivo or more importantly in vivo.
To study the role of extracellular calcium influx in neutrophil function, we have examined the response of neutrophils from stim1−/− chimeric mice following activation of either GPCR or tyrosine kinase signaling pathways. We found that STIM1 is involved in chemoattractant, Fcγ-receptor (FcγR), and integrin-induced calcium influx, which is essential for activating the neutrophil respiratory burst. We provide evidence that the mechanism in which STIM1-mediated calcium entry regulates neutrophil superoxide release is via activation of calcium-sensitive protein kinase C isoforms α and β (PKCα and PKCβ), which in turn phosphorylate subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Lack of this calcium signaling pathway results in profound sensitivity to bacterial challenge in vivo but also induces protection from neutrophil-mediated tissue damage during ischemia/reperfusion injury. These data suggest that therapeutic manipulation of SOCE in neutrophils may provide a powerful tool to treat inflammatory disease.
Methods
Mice
stim1−/− and littermate stim1+/+ (wild-type [WT]) fetal liver cells were obtained 16 days postconception from heterozygous breeders (obtained from Dr Tomohiro Kurosaki, RIKEN) backcrossed to C57BL/6 for 10 generations.16 Bone marrow chimeras were generated by intravenous injection of fetal liver cells (106 per recipient) into lethally irradiated congenial recipients (CD45.1+) (Taconic Farms). The complete repopulation of the neutrophil compartment was confirmed by flow cytometry for CD45.1 vs CD45.2 alleles. Chimeras were used 6 to 8 weeks after the bone marrow transplantation. CD18-deficient mice were purchased from Jackson Laboratory. PKCα/β double mutant mice and p47phox-deficient animals were kindly provided by Dr Max Krummel and Dr Anthony DeFranco (University of California at San Francisco [UCSF]). All animals were kept in a specific pathogen free facility at UCSF and used according to protocols approved by the UCSF Institutional Animal Care and Use Committee.
Calcium flux in suspension or following adhesion
Isolated neutrophils (107 cells per milliliter) in Hanks balanced salt solution (HBSS) with 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HBSS/H) with 0.5% fetal calf serum were loaded with 3 μM Indo-1 AM (Molecular Probes) in the dark at room temperature for 45 minutes. Cells were washed twice, resuspended at 2 × 107 cells per milliliter, and kept at 4°C in the dark until used. Prior to analysis, cells were aliquoted into fluorescence-activated cell sorter tubes containing HBSS/H with 1 mM Ca2+ and 0.5 mM Mg2+, warmed to 37°C for 2 minutes, then run on the flow cytometer for 30 seconds to establish a baseline reading. Stimuli were added, and the samples were analyzed continuously for 3 to 6 minutes. For experiments done in the presence of EGTA, the cells were preincubated for 1 minute before reading.
For calcium flux during adhesion, microfluor 2 black plates (Fisher) were coated with 0.2% milk to block adhesion, fibrinogen (150 μg/mL), or fibronectin-like binding fragment (Sigma-Aldrich; hereafter termed pRGD) (20 μg/mL) at room temperature for 30 minutes, and then washed twice with phosphate-buffered saline (PBS). Neutrophils were loaded with fluo-4 as described above for Indo-1 AM. Ca2+ (1 mM) and Mg2+ (0.5 mM) were added prior to plating. Fluorescence was measured by using a Spectramax M5 reader.
Neutrophil adhesion and migration
Neutrophil adhesion was measured in tissue culture plates precoated with 0.2% milk, fibrinogen, or pRGD as described above. Cells were loaded with calcein AM (Molecular Probes) in the presence of F-127 detergent at 37°C for 30 minutes. Cells were washed twice, diluted to 2 × 106 cells per milliliter, and kept at 4°C in the dark. Then, 2 × 105 cells were plated with macrophage-inflammatory protein 2 (MIP-2; 20 ng/mL), formyl-methionyl-leucyl phenylalanine (fMLF; 10 μM), tumor necrosis factor-α (TNF-α; 10 ng/mL), or phorbol 12-myristate 13-acetate (PMA; 1 ng/mL) and incubated at 37°C for 15 minutes. Fluorescence was read with a Spectramax M5 reader to confirm equal loading. Plates were washed 5 times with HBSS, and the remaining fluorescence was considered to be from adherent cells.
Neutrophil migration in vitro was performed in 24-well transwells (5 μm; Costar) as described.17 Intraperitoneal injection of 3% aged thioglycollate was used to assess neutrophil migration in vivo as described.17 Neutrophil migration into the footpad was performed by injection of 20 μL MIP-2 (50 ng/mL) into hind footpads between the second and third toe. The feet were dissected 4 hours after injection, fixed in 10% formalin for 2 days, and stained with hematoxylin and eosin (H&E).
Neutrophil respiratory burst in suspension or in adhesion
Neutrophil respiratory burst was measured by a luminol-based chemiluminescent assay. Isolated neutrophils were suspended in HBSS/H with or without Ca2+ (1 mM), in the presence of luminol (100 μM; Sigma-Aldrich) and horseradish peroxidase (8 U/mL; Sigma-Aldrich). Cells (2 × 105) were placed in 96-well plates precoated with milk, fibrinogen, or pRGD as described above. Agonists fMLF, TNF-α, immunoglobulin G (IgG)-opsonized zymosan (5:1) or IgG-opsonized sheep red blood cells (SRBCs; 10:1) were added as indicated. Chemiluminescence was measured in a Spectramax M5 plate reader at 37°C.
Zymosan particles were opsonized with rabbit–anti-zymosan IgG per manufacturer’s instructions (Life Technologies). SRBCs (MP Biologicals) were opsonized with anti-SRBC IgG (Rockland Immunochemicals) as previously described.18
IP3 production
IP3 production was measured by using an IP3 HitHunter Fluorescence Polarization Assay Kit (GE Healthcare). Isolated neutrophils were plated on pRGD-coated 6-well plates and incubated at 37°C for 15 minutes; IP3 quantification was then performed following the manufacturer’s protocol.
Staphylococcus aureus pneumonia
S aureus (Newman strain) was donated by Dr Arun Prakash (UCSF). Bacteria were grown to mid-log phase in Luria-Bertani (LB) broth, washed twice with PBS, and resuspended at 6 × 109 colony-forming units (CFUs) per milliliter. Then, 100 μL was injected into the trachea by direct laryngoscopy. At 24 hours, the mice were euthanized and the lungs were isolated. The left lobe was homogenized in PBS with 0.1% triton-X100 to release any live bacteria contained within cells. Serial dilutions were plated on LB agar to obtain bacterial counts. The right lobes were minced and digested in HBSS/2% fetal bovine serum with collagenase D for 45 minutes at 37°C with continuous shaking. The reaction was halted by the addition of EDTA (20 mM). The tissue was dispersed into a single-cell suspension by passage through a 70-μm cell strainer and used for total cell count and analysis via flow cytometry.
Listeria monocytogenes infection
L monocytogenes (clinical isolate) was provided by the UCSF Clinical Microbiology Laboratories. Bacteria were grown to mid-log phase in LB broth, washed with PBS, and resuspended at 5 × 106 CFU/mL. Mice were anesthetized with 2.5% avertin and then infected by intravenous injection of 100 μL into the retroorbital sinus. Livers or spleens were isolated 2 days postinfection. Total tissue homogenates were prepared in LB medium, and samples were plated on LB agar for colony-forming assays. Liver samples were fixed in 10% formalin, sectioned, and stained with trichrome to visualize leukocyte abscesses.
Hepatic ischemia/reperfusion injury
Mice were anesthetized with 50 µg/g pentobarbital given intraperitoneally. A long midline abdominal incision was made to expose the hepatic artery and portal vein with retractors; under the microscope, the hepatic artery and portal vein were ligated with 6-0 suture to occlude the blood supply to the liver. During ischemic injury, the mice were kept on a 37°C heating pad and covered by HBSS immersed gauze sponges. The ligatures were removed after 30 minutes, and the abdomen was closed with 6-0 suture. After 3 hours of reperfusion, blood was obtained from the retroorbital sinus for detection of alanine aminotransferase and/or aspartate aminotransferase via veterinary clinical chemical analyzer and interleukin-6 (IL-6) via serum enzyme-linked immunosorbent assay (eBioscience). The liver was fixed in 10% formalin, sectioned, and stained with trichrome or H&E). This study was conducted in accordance with the Declaration of Helsinki. For additional methods (reagents, flow cytometry, neutrophil isolation, subcellular fractionation, and immunoprecipitation), see the supplemental Methods (available on the Blood Web site).
Results
STIM1 is required for chemoattractant and FcγR-induced extracellular calcium influx
Stimulation of murine neutrophils with various chemokines and chemoattractants results in rapid extracellular calcium flux.17 To examine GPCR-induced calcium flux, we focused on two agonists, the chemokine MIP-2 (CXCL2) and the chemoattractant fMLF. In murine cells, fMLF induced a rapid, dose-dependent calcium influx followed by sustained elevation in intracellular calcium. MIP-2 generated a similar response; however, the prolonged calcium entry was less pronounced, consistent with fMLF being a more potent neutrophil agonist17 (Figure 1A). This biphasic response is typical of SOCE in which the first peak corresponds to calcium release from intracellular stores whereas the sustained calcium influx originates from extracellular calcium sources. Neutrophils isolated from stim1−/− chimeric mice showed a complete block in the second phase of calcium entry induced by fMLF and MIP-2 (Figure 1B-C, left panels). In the presence of EGTA to chelate extracellular calcium, the sustained calcium influx was abolished in WT cells; however, the first calcium peak was unaffected in both WT and stim1−/− cells (Figure 1B-C), suggesting that STIM1 is required for coupling intracellular store release with extracellular calcium entry.
Stim1−/−neutrophils show defective SOCE in response to soluble agonists and thapsigargin. (A) WT neutrophils were labeled with Indo-1 AM and then stimulated with the indicated doses of fMLF (left panel) or MIP- 2 (right panel), and calcium flux was determined by flow cytometry. (B) WT and stim1−/− neutrophils were treated with 1 μM fMLP, 10 ng/mL MIP-2 (C), or 120 μg/mL bovine serum albumin/antibovine serum albumin immune complexes (IC) (D) in the absence (left panels) or presence (right panels) of 3 mM EGTA to chelate extracellular Ca2+. (E) WT and stim1−/− neutrophils were stimulated with thapsigargin (TG) (10 nM) in the presence (right panel) or absence (left panel) of 3 mM EGTA. Arrows indicate the addition of stimuli. Data are representative of at least 3 independent experiments.
Stim1−/−neutrophils show defective SOCE in response to soluble agonists and thapsigargin. (A) WT neutrophils were labeled with Indo-1 AM and then stimulated with the indicated doses of fMLF (left panel) or MIP- 2 (right panel), and calcium flux was determined by flow cytometry. (B) WT and stim1−/− neutrophils were treated with 1 μM fMLP, 10 ng/mL MIP-2 (C), or 120 μg/mL bovine serum albumin/antibovine serum albumin immune complexes (IC) (D) in the absence (left panels) or presence (right panels) of 3 mM EGTA to chelate extracellular Ca2+. (E) WT and stim1−/− neutrophils were stimulated with thapsigargin (TG) (10 nM) in the presence (right panel) or absence (left panel) of 3 mM EGTA. Arrows indicate the addition of stimuli. Data are representative of at least 3 independent experiments.
To test whether STIM1 is required for calcium entry–triggered tyrosine kinase–based signaling, we examined FcγR responses. STIM1 has been implicated in FcγR-induced calcium flux in neutrophil-like HL-60 cells by small interfering RNA (siRNA) knockdown.9 WT primary neutrophils stimulated with immune complexes also demonstrated a biphasic calcium influx, with the second phase abolished by EGTA (Figure 1D, left and right panels). In the absence of STIM1, the initial calcium transient is preserved and unaffected by extracellular calcium chelation (Figure 1D). Finally, we tested receptor-independent release of ER calcium stores with the sarco/endoplasmic reticulum Ca2+-ATPase inhibitor thapsigargin and found similar results (Figure 1E). Notably, the baselines and calcium release in the presence of EGTA were equivalent in WT and stim1−/− neutrophils for all stimuli, suggesting that internal calcium stores are unaffected by STIM1 deficiency. Together, these data indicate STIM1 is required for SOCE in murine neutrophils following receptor-dependent and independent stimuli.
STIM1 is required for integrin-induced extracellular calcium influx
In neutrophils, integrins are essential for many inflammatory responses, including adhesion, migration, and adhesion-dependent degranulation and respiratory burst. Tyrosine kinase–based integrin ligation induces calcium entry in lymphocytes and platelets19,20 but has been less well studied in neutrophils.21,22 By using video microscopy, we were able to observe sustained calcium flux in murine neutrophils adhering to surfaces coated with pRGD (not shown). To develop a more quantitative assessment of integrin-induced calcium entry, we established a plate-based assay. Fluo-4–loaded cells were placed in pRGD-coated wells; then calcium flux during adhesion and spreading were followed in a fluorescent plate reader. As shown in Figure 2A, neutrophils displayed two phases of calcium flux, with the majority of calcium entry occurring in the second phase. Plating cells on the nonbinding milk protein–coated surface did not induce calcium entry. Neutrophils from CD18-deficient mice also showed no calcium flux following plating on pRGD surfaces, establishing the integrin dependence of the assay. Calcium flux following adhesion to pRGD-coated surfaces was blocked when cells were incubated in calcium free-media or following treatment with the pan-PLC inhibitor U73122 (data not shown).
Stim1−/−neutrophils show defective SOCE in response to integrin-mediated adhesion. (A) WT or CD18–/– neutrophils were labeled with fluo-4 and then plated on pRGD-coated or 0.2% milk–coated plates, and the fluorescence was read by a Spectramax M5 reader every 6 seconds for 15 minutes. (B) WT and stim1−/− neutrophils were plated on pRGD-coated plates, and calcium flux was recorded. Cells in (A) and (B) were added at time 0. (C) Neutrophils from WT and stim1−/− littermate chimeras were plated on pRGD-coated plates for 15 minutes; adherent cells were lysed and the lysates were then immunoprecipitated with anti-PLCγ2 antibody followed by blotting with 4G10 antibody to detect p-PLCγ or total PLCγ antibody to normalize for protein levels. (D) Neutrophils from WT and stim1−/− littermate chimeras were plated on pRGD surfaces for 15 minutes and lysed; IP3 levels were then determined by HitHunter fluorescence polarization assay. ***P < .001. Data shown are representative of 3 to 4 independent experiments.
Stim1−/−neutrophils show defective SOCE in response to integrin-mediated adhesion. (A) WT or CD18–/– neutrophils were labeled with fluo-4 and then plated on pRGD-coated or 0.2% milk–coated plates, and the fluorescence was read by a Spectramax M5 reader every 6 seconds for 15 minutes. (B) WT and stim1−/− neutrophils were plated on pRGD-coated plates, and calcium flux was recorded. Cells in (A) and (B) were added at time 0. (C) Neutrophils from WT and stim1−/− littermate chimeras were plated on pRGD-coated plates for 15 minutes; adherent cells were lysed and the lysates were then immunoprecipitated with anti-PLCγ2 antibody followed by blotting with 4G10 antibody to detect p-PLCγ or total PLCγ antibody to normalize for protein levels. (D) Neutrophils from WT and stim1−/− littermate chimeras were plated on pRGD surfaces for 15 minutes and lysed; IP3 levels were then determined by HitHunter fluorescence polarization assay. ***P < .001. Data shown are representative of 3 to 4 independent experiments.
Stim1−/− neutrophils showed a complete block in the prolonged second phase of calcium entry following adhesion to pRGD-coated surfaces (Figure 2B); however, the initial peak of calcium flux was unaffected. These data are consistent with STIM1 being required for prolonged calcium entry from extracellular sources following activation of tyrosine kinase signaling by integrin ligation.
To validate that STIM1 deficiency did not affect the proximal steps of the calcium signaling pathway, we examined activation of PLCγ2 and IP3 generation in cells adherent to pRGD-coated surfaces. WT and stim1−/− cells showed equivalent tyrosine phosphorylation of PLCγ2 (Figure 2C) and intracellular IP3 production (Figure 2D) within 15 minutes following adhesion. These results are consistent with the intact first phase of intracellular cellular flux observed in stim1−/− cells, likely from emptying of ER stores. We conclude that STIM1 deficiency results in a loss of SOCE following stimulation by either GPCR or tyrosine kinase–based neutrophil agonists.
STIM1 deficiency does not affect neutrophil adhesion or migration
Calcium flux has been reported to play an important role in neutrophil adhesion and migration.23 To examine the contribution of STIM1-mediated SOCE in these neutrophil functions, we examined adhesion and migration both ex vivo and in vivo. Depletion of extracellular calcium reduced adhesion of WT neutrophils to milk- or fibrinogen-coated surfaces in the presence of the strong neutrophil agonist fMLF or the weaker agonist TNF-α, while adhesion to the high-affinity pRGD ligand occurred independently of extracellular calcium (Figure 3A). Surprisingly, stim1−/− neutrophils displayed no defect in adhesion to these surfaces (Figure 3B), indicating that extracellular calcium flux does not affect integrin inside-out signaling. The normal adhesion of STIM1-deficient neutrophils correlated with normal surface expression of the major adhesion molecules LFA-1 (CD11a) and Mac-1 (CD11b) (supplemental Figure 1).
Stim1−/−neutrophils have normal adhesion and migration. (A) WT littermate chimera neutrophils (2 × 105 cells per well) were loaded with calcein AM plated on 0.2% milk– or different integrin ligand–coated plates in the presence of indicated stimuli with or without calcium for 15 minutes. Following 5 gentle washes, the fluorescence of the remaining adherent cells was determined. ***P < .001. (B) WT and stim1−/− chimera neutrophils (2 × 105) were plated on 0.2% milk– or different integrin ligand–coated plates, and adhesion was determined as above. (C) WT and stim1−/− neutrophils (106) were studied for chemotactic responses to MIP-2 (left) or fMLF (right) using 24-well transwell plates. The concentration of the stimuli in the lower wells is indicated. Migration was carried out for 45 minutes, and cells entering the lower chamber were counted by hemocytometer. (A-C) Data shown are representative of 3 to 4 independent experiments. (D) Left: WT and stim1−/− chimeras were injected intraperitoneally with 3% thioglycollate, and the number of neutrophils in peritoneal lavage fluid at 4 hours after injection was determined by flow cytometry (double staining for CD45.2/Gr-1). Right: peripheral blood was obtained from WT and stim1−/− chimeras at 4 hours following thioglycollate injection, and neutrophil counts were determined by using a HemaVet analyzer. Data shown are pooled from 2 independent experiments with at least 4 mice per experiment. (E) The footpads of either WT or stim1−/− chimeric mice were injected with 0.1 mL of 50 ng/mL MIP-2 and dissected 4 hours after injection and were formalin fixed and paraffin embedded. Tissue sections were stained with H&E. Photos are at ×40 magnification and are representative of 3 mice per group. ns, not significant.
Stim1−/−neutrophils have normal adhesion and migration. (A) WT littermate chimera neutrophils (2 × 105 cells per well) were loaded with calcein AM plated on 0.2% milk– or different integrin ligand–coated plates in the presence of indicated stimuli with or without calcium for 15 minutes. Following 5 gentle washes, the fluorescence of the remaining adherent cells was determined. ***P < .001. (B) WT and stim1−/− chimera neutrophils (2 × 105) were plated on 0.2% milk– or different integrin ligand–coated plates, and adhesion was determined as above. (C) WT and stim1−/− neutrophils (106) were studied for chemotactic responses to MIP-2 (left) or fMLF (right) using 24-well transwell plates. The concentration of the stimuli in the lower wells is indicated. Migration was carried out for 45 minutes, and cells entering the lower chamber were counted by hemocytometer. (A-C) Data shown are representative of 3 to 4 independent experiments. (D) Left: WT and stim1−/− chimeras were injected intraperitoneally with 3% thioglycollate, and the number of neutrophils in peritoneal lavage fluid at 4 hours after injection was determined by flow cytometry (double staining for CD45.2/Gr-1). Right: peripheral blood was obtained from WT and stim1−/− chimeras at 4 hours following thioglycollate injection, and neutrophil counts were determined by using a HemaVet analyzer. Data shown are pooled from 2 independent experiments with at least 4 mice per experiment. (E) The footpads of either WT or stim1−/− chimeric mice were injected with 0.1 mL of 50 ng/mL MIP-2 and dissected 4 hours after injection and were formalin fixed and paraffin embedded. Tissue sections were stained with H&E. Photos are at ×40 magnification and are representative of 3 mice per group. ns, not significant.
WT vs stim1−/− neutrophils showed no difference in transwell chemotaxis assays in response to MIP-2 or fMLF (Figure 3C). In vivo, we observed no significant difference in neutrophil recruitment between WT and stim1−/− chimeric mice in either thioglycollate sterile peritonitis or following injection of MIP-2 into the foot pad (Figure 3D-E). Moreover, the STIM1-deficient mice showed normal mobilization of neutrophils from the bone marrow into the peripheral blood during thioglycollate peritonitis, indicating that this step of the inflammatory cascade was unaffected. Thus, we conclude that, surprisingly, SOCE is not required for neutrophil adhesion or migration.
STIM1-deficient neutrophils show reduced FcγR phagocytosis and degranulation
Braun et al24 have previously reported that macrophages derived from stim1−/− chimeric mice show defective FcγR phagocytosis. As has been observed in previous reports, we observed that phagocytosis was reduced by approximately 50% in stim1−/− neutrophils, perhaps because of impaired recruitment of ER membranes to the developing phagosome24,25 (supplemental Figure 2A-C). In contrast to STIM1-deficient mast cells that manifest a profound defect in FcεR-mediated degranulation responses,16 stim1−/− neutrophils showed a modest reduction in both fMLF and integrin-mediated degranulation measured by lactoferrin release (supplemental Figure 2D). Importantly, treatment of stim1−/− cells with PMA reversed the modest degranulation defect. Thus, similar to other immune cell types, deficiency of STIM1 in neutrophils leads to impaired phagocytosis and degranulation.
STIM1-deficient neutrophils are defective in GPCR- and tyrosine kinase–mediated activation of respiratory burst
It is well established that human neutrophils require extracellular calcium to mount an effective respiratory burst through the NADPH oxidase following activation by GPCR ligands.26,27 We confirmed this for murine neutrophils, finding a dose-dependent reduction in ROS production at decreasing concentrations of extracellular calcium (supplemental Figure 3A-B).
WT cells showed rapid activation of the NADPH oxidase following stimulation by fMLF, which was completely dependent upon extracellular calcium (Figure 4A). Neutrophils from stim1−/− chimeric mice showed a complete block in the respiratory burst initiated by fMLF (Figure 4A). Similarly, adhesion-mediated superoxide generation was significantly reduced in both stim1−/− neutrophils and in WT cells depleted of extracellular calcium (Figure 4B-C). Activation of the NADPH oxidase was completely abolished by the intracellular calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, suggesting that intracellular calcium release supports the residual superoxide production in adherent stim1−/− neutrophils. As expected, respiratory burst was blocked by addition of the pan-PLC inhibitor U73122 (supplemental Figure 3C). We also measured integrin and fMLP superoxide production by using cytochrome c reduction and obtained comparable results (supplemental Figure 3D-E).
Stim1−/−neutrophils are defective in superoxide production. Superoxide production by WT and stim1−/− neutrophils was measured by luminol-based chemiluminescence in the presence (left panels) or absence (right panels) of extracellular calcium. (A) WT and stim1−/− neutrophils (2 × 105 cells per well) were plated in 96-well plates precoated with 0.5% milk (to block adhesion) in the presence of fMLF (10 μM), and luminescence was monitored in a Spectramax plate reader at 37°C over the indicated time period. Integrin-dependent ROS production was measured in plates coated with 15 μg/mL pRGD (B) or 150 μg/mL fibrinogen in the presence of 10 ng/mL TNF-α (C). The intracellular calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid AM (10 μM) was added as indicated. FcγR-generated superoxide was measured by incubating WT and stim1−/− neutrophils with IgG-opsonized zymosan (D) or SRBCs with TNF-α priming (10 ng/mL) (E). Luminescence was monitored over the indicated time period. Data are mean (± standard deviation; n = 3 wells each) and are representative of at least 3 independent experiments. Statistical analysis at peak response was P < .001 between WT and stim1−/− cells in the presence of calcium.
Stim1−/−neutrophils are defective in superoxide production. Superoxide production by WT and stim1−/− neutrophils was measured by luminol-based chemiluminescence in the presence (left panels) or absence (right panels) of extracellular calcium. (A) WT and stim1−/− neutrophils (2 × 105 cells per well) were plated in 96-well plates precoated with 0.5% milk (to block adhesion) in the presence of fMLF (10 μM), and luminescence was monitored in a Spectramax plate reader at 37°C over the indicated time period. Integrin-dependent ROS production was measured in plates coated with 15 μg/mL pRGD (B) or 150 μg/mL fibrinogen in the presence of 10 ng/mL TNF-α (C). The intracellular calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid AM (10 μM) was added as indicated. FcγR-generated superoxide was measured by incubating WT and stim1−/− neutrophils with IgG-opsonized zymosan (D) or SRBCs with TNF-α priming (10 ng/mL) (E). Luminescence was monitored over the indicated time period. Data are mean (± standard deviation; n = 3 wells each) and are representative of at least 3 independent experiments. Statistical analysis at peak response was P < .001 between WT and stim1−/− cells in the presence of calcium.
FcγR-mediated ROS production yielded similar results. Stim1−/− neutrophils demonstrated a substantial deficit in superoxide production, matching the response of WT cells in the absence of extracellular calcium when stimulated with IgG-opsonized zymosan or SRBCs (Figure 4D-E). As seen for degranulation, normal function of NADPH oxidase could be restored to STIM1-deficient neutrophils by treatment with PMA (supplemental Figure 3F), indicating that there were no intrinsic defects in the oxidase complex in stim1−/− cells. Thus, we conclude that STIM1-mediated SOCE is required for activation of the NADPH oxidase by agonists that activate either GPCR or tyrosine kinase–signaling pathways.
Defective PKCα/β activation and phosphorylation of NADPH oxidase subunits in stim1−/− neutrophils
To define a biochemical mechanism for how SOCE is linked to activation of the NADPH oxidase, we focused on potential intracellular targets of calcium. Several molecules have been proposed as calcium-binding proteins involved in neutrophil activation, including the structural S100A8/A9 proteins, the cytosolic phospholipases (cPLA2), and the PKC enzymes.9,28,29 Given our observations that the impaired NAPDH oxidase activation (and degranulation) of stim1−/− neutrophils could be reversed by direct activation of PKCs through treatment with PMA, we focused on the PKC isoforms as targets of SOCE.
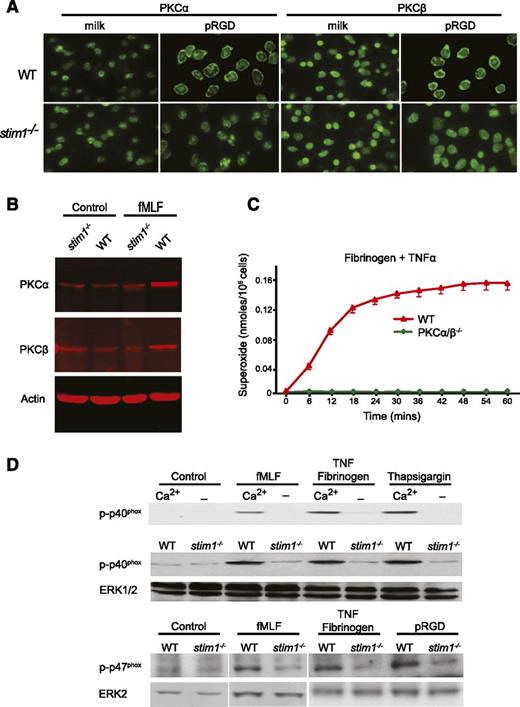
Murine neutrophils express abundant amounts of PKCα, -β, -δ, and -ζ30 ; however, only the classical PKCα/β isoforms contain both calcium and diacylglycerol binding domains and are thought to be regulated by both mechanisms.31 Insofar as activation of PKCs is accompanied by translocation to the plasma membrane, we examined PKCα and -β membrane translocation in stim1−/− neutrophils.32 As shown in Figure 5A, WT neutrophils adherent to pRGD-coated surfaces showed clear membrane relocalization of PKCα and -β compared with cells plated on a nonadherent surface. By contrast, adherent stim1−/− neutrophils did not show relocalization of PKCα or -β, despite attaching and spreading on the pRGD surface. Similarly, both PKCα and -β were increased in membrane fractions of WT but not stim1−/− cells following treatment with fMLF (Figure 5B). We conclude that activation of PKCα and -β are impaired in stim1−/− neutrophils.
Stim1−/−neutrophils have defective activation of PKCα/β and fail to phosphorylate the p40phoxsubunit of the NADPH oxidase. (A) WT and stim1−/− neutrophils were plated onto milk- or pRGD-coated coverslips for 10 minutes, fixed, and then stained with anti-PKCα or anti-PKCβ antibodies. (B) Total cellular membranes were isolated from WT or stim1−/− neutrophils, either resting (control) or stimulated for 3 minutes with fMLF. Equal amounts of protein were electrophoresed and then immunoblotted with anti-PKCα, anti-PKCβ, or antiactin to verify equal loading. Immunoblots were imaged with a Licor system. (C) WT and PKCα/β−/− neutrophils were plated on fibrinogen in the presence or absence of TNF-α. Reduction of cytochrome C was determined in a Spectramax plate reader at 37°C. Statistical analysis at peak response was P < .001 between WT and PKCα/β−/− cells. (D) Upper panel: WT neutrophils in HBSS with or without Ca2+ were plated on 0.2% milk–coated plates with fMLF or on fibrinogen-coated plates with TNF-α or thapsigargin for 15 minutes. Total protein was immunoblotted with the phospho-specific anti-p40phox monoclonal antibody. Lower panels: WT or stim1−/− neutrophils were stimulated as indicated, and phosphorylation of the p40phox and p47phox proteins was determined. The same blot was reprobed with anti-ERK antibodies to verify equal loading. Immunoblots were imaged by electrochemiluminescence detection. Lanes shown for p47phox are from the same experiment but run on different gels. Data shown are representative of 2 to 4 independent experiments.
Stim1−/−neutrophils have defective activation of PKCα/β and fail to phosphorylate the p40phoxsubunit of the NADPH oxidase. (A) WT and stim1−/− neutrophils were plated onto milk- or pRGD-coated coverslips for 10 minutes, fixed, and then stained with anti-PKCα or anti-PKCβ antibodies. (B) Total cellular membranes were isolated from WT or stim1−/− neutrophils, either resting (control) or stimulated for 3 minutes with fMLF. Equal amounts of protein were electrophoresed and then immunoblotted with anti-PKCα, anti-PKCβ, or antiactin to verify equal loading. Immunoblots were imaged with a Licor system. (C) WT and PKCα/β−/− neutrophils were plated on fibrinogen in the presence or absence of TNF-α. Reduction of cytochrome C was determined in a Spectramax plate reader at 37°C. Statistical analysis at peak response was P < .001 between WT and PKCα/β−/− cells. (D) Upper panel: WT neutrophils in HBSS with or without Ca2+ were plated on 0.2% milk–coated plates with fMLF or on fibrinogen-coated plates with TNF-α or thapsigargin for 15 minutes. Total protein was immunoblotted with the phospho-specific anti-p40phox monoclonal antibody. Lower panels: WT or stim1−/− neutrophils were stimulated as indicated, and phosphorylation of the p40phox and p47phox proteins was determined. The same blot was reprobed with anti-ERK antibodies to verify equal loading. Immunoblots were imaged by electrochemiluminescence detection. Lanes shown for p47phox are from the same experiment but run on different gels. Data shown are representative of 2 to 4 independent experiments.
Although the PKC isoforms have been implicated in phosphorylation and membrane translocation of the NADPH oxidase subunits, there remains controversy about their relative importance in ROS production.33,34 To directly test the contribution of PKCα and -β to activation of the phagocyte oxidase, we examined ROS production in neutrophils from PKCα/β−/− double mutant mice. Bone marrow neutrophils from PKCα/β−/− mice were present in normal numbers and showed normal morphology and surface marker staining (not shown); however, they were completely defective in ROS production following adhesion to fibrinogen in the presence of TNF-α (Figure 5C). Single mutant PKCα or PKCβ showed an intermediate defect in ROS production (not shown). Moreover, phosphorylation of p40phox was significantly impaired in PKCα/β−/− neutrophils (not shown). These genetic experiments validate previous inhibitor studies suggesting that PKCα/β activity is required for activation of the NADPH oxidase in neutrophils.
Impaired calcium-mediated PKC activation should be manifest in reduced phosphorylation of the subunits of the NADPH oxidase. WT neutrophils stimulated with fMLF or following adhesion to integrin ligands showed robust phosphorylation of the p40phox and p47phox subunits of the NADPH oxidase (Figure 5D). Depletion of extracellular calcium completely blocked p40phox phosphorylation, correlating with a block in oxidase activity. In response to these same stimuli, stim1−/− neutrophils showed a significant reduction in p40phox and p47phox phosphorylation (Figure 5D, lower panels). Together, these findings suggest that impaired SOCE in STIM1-deficient neutrophils leads to poor activation of PKCα and -β, resulting in reduced phosphorylation of the NADPH oxidase subunits and therefore diminished ROS production.
Stim1−/− chimeric mice are more susceptible to S aureus and L. monocytogenes infections
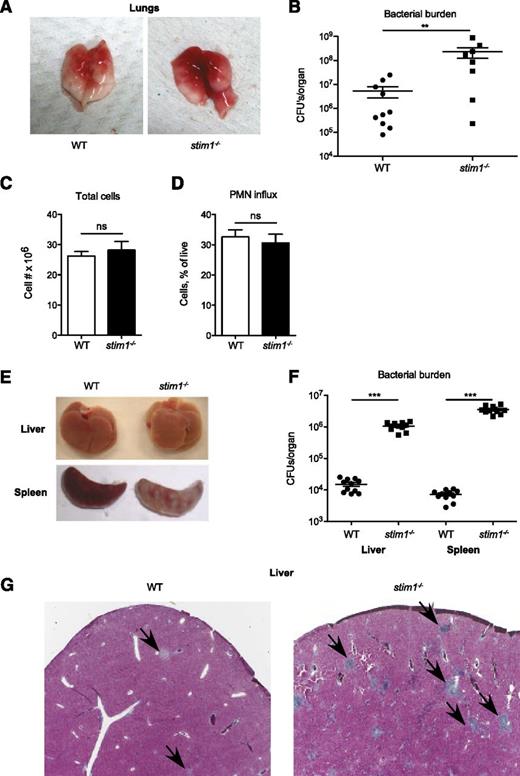
Impaired activation of the neutrophil oxidase combined with modest defects in phagocytosis and degranulation predict that stim1−/− chimeric mice should be susceptible to infection. To test this, we challenged stim1−/− chimeric mice in Staphylococcus pneumonia or Listeria bacteremia models; these mice were highly susceptible to both of these pathogens. The lungs of stim1−/− chimeric mice were markedly more hemorrhagic and manifested 1 to 2 orders of magnitude higher bacterial colony counts 24 hours after Staphylococcus infection (Figure 6A-B). However, the number of total leukocytes and neutrophils present in the pulmonary tissues was equivalent in both strains of mice, validating that STIM1 deficiency did not diminish neutrophil recruitment (Figure 6C-D). Stim1−/− chimeras were similarly susceptible to intravenous Listeria infection, with grossly visible abscesses and markedly higher bacterial counts in liver and spleen tissue after 2 days of infection (Figure 6E-F). Histologically, the livers of infected stim1−/− chimeras were filled with large numbers of neutrophil-containing abscesses (Figure 6G). These data demonstrate that stim1−/− chimeric mice are unable to mount an effective host defense to Staphylococcus or Listeria infections, despite the ability to recruit large numbers of neutrophils to the infected tissues.
Stim1−/−chimeras have major impairment in host defense to S aureus pneumonia and L monocytogenes septicemia. WT or stim1−/− chimeras were inoculated with S aureus (Newman strain) via direct tracheal intubation. At 24 hours, the mice were euthanized and the lungs were isolated. Representative gross specimens are shown in (A). (B) The left lobe of the lung was homogenized, and serial dilutions were plated for assessment of bacterial colony-forming assays (shown as CFU/organ). Data shown are pooled from 2 independent experiments. n = 10 mice per group. **P < .03. The right lobes were digested with collagenase and cells were collected for total cell count (C) and analysis via flow cytometry (D). Neutrophils were identified by double-staining for CD11b and Ly6G. (E) WT or stim1−/− chimeras were infected by intravenous injection of L monocytogenes into the retroorbital sinus. Liver or spleens were isolated 2 days postinfection. Representative organs are shown. (F) Total tissue homogenates were prepared in LB medium, and samples were plated on LB plates for Listeria colony-forming assays (shown as CFU/mL). Results are pooled from 2 separate experiments. n = 11 mice per group. ***P < .001. (G) Liver samples from WT or stim1−/− chimeric mice obtained 2 days following Listeria infection were stained with trichrome to visualize leukocyte abscesses (shown by arrows). Microscopy sections are at ×10 magnification and are representative of 3 different animals in 2 separate experiments.
Stim1−/−chimeras have major impairment in host defense to S aureus pneumonia and L monocytogenes septicemia. WT or stim1−/− chimeras were inoculated with S aureus (Newman strain) via direct tracheal intubation. At 24 hours, the mice were euthanized and the lungs were isolated. Representative gross specimens are shown in (A). (B) The left lobe of the lung was homogenized, and serial dilutions were plated for assessment of bacterial colony-forming assays (shown as CFU/organ). Data shown are pooled from 2 independent experiments. n = 10 mice per group. **P < .03. The right lobes were digested with collagenase and cells were collected for total cell count (C) and analysis via flow cytometry (D). Neutrophils were identified by double-staining for CD11b and Ly6G. (E) WT or stim1−/− chimeras were infected by intravenous injection of L monocytogenes into the retroorbital sinus. Liver or spleens were isolated 2 days postinfection. Representative organs are shown. (F) Total tissue homogenates were prepared in LB medium, and samples were plated on LB plates for Listeria colony-forming assays (shown as CFU/mL). Results are pooled from 2 separate experiments. n = 11 mice per group. ***P < .001. (G) Liver samples from WT or stim1−/− chimeric mice obtained 2 days following Listeria infection were stained with trichrome to visualize leukocyte abscesses (shown by arrows). Microscopy sections are at ×10 magnification and are representative of 3 different animals in 2 separate experiments.
Stim1−/− chimeric mice are resistant to hepatic ischemia/reperfusion injury
Uncontrolled neutrophil activation is a major cause of tissue damage during ischemia/reperfusion injury.35 Generation of ROS mediates much of this injury insofar as gp91phox-deficient mice are protected from hepatic ischemia/reperfusion injury.36 Therefore, we used a model of hepatic ischemia/reperfusion injury to study the impact of STIM1 deficiency. Histologic examination of livers from WT mice showed large areas of hepatic necrosis visualized by trichrome staining as nonstaining tissue (Figure 7A). H&E stains revealed extensive hepatic necrosis with loss of hepatocyte cytoplasmic contents. By contrast, livers from stim1−/− mice showed no evidence of hepatic necrosis or alteration in hepatocyte morphology. Livers from both genotypes had obvious neutrophil infiltration seen on H&E stains (arrowheads, Figure 7A) and by immunostaining with anti–Gr-1 (supplemental Figure 4). The lack of hepatic injury in stim1−/− chimeric mice was confirmed by measurement of liver enzymes and the inflammatory cytokine IL-6 in the peripheral blood; WT mice showed significant elevations of aspartate aminotransferase/alanine aminotransferase and IL-6 whereas stim1−/− chimeras did not (Figure 7B-C). Similarly, p47phox−/− mice, which lack NADPH oxidase activity, also failed to show hepatic injury or elevated IL-6 following ischemia/reperfusion, confirming that ROS production mediates tissue injury in this model. We conclude that loss of SOCE in STIM1-deficient neutrophils leads to loss of NADPH oxidase activation and hence protection from tissue injury in hepatic ischemia/reperfusion.
Stim1−/−chimeras have less liver damage following ischemia/reperfusion injury. (A) WT or stim1−/− chimeric mice were subjected to liver ischemia/reperfusion injury by ligation of the portal vein and hepatic artery for 30 minutes followed by 3 hours of reperfusion. Mice were then euthanized, and liver samples were obtained for histology. Liver sections were stained with trichrome (upper 4 panels) or H&E (lower 2 panels). Areas of poor trichome staining (arrowheads) indicate hepatic necrosis in the WT mice, which were absent in livers from stim1−/− chimeras. Arrowheads indicate inflammatory cell infiltrate. Magnification is ×10 (upper panels), ×20 (middle panels) and ×40 (lower panels). Images shown are representative of 10 mice per group subjected to ischemia/reperfusion injury in separate experiments. (B) WT, stim1−/− chimeras, or p47phox-deficient mice were subjected to liver ischemia/reperfusion injury, and blood samples were obtained 3 hours after reperfusion. Liver enzymes aspartate aminotransferase and alanine aminotransferase were determined by a veterinary clinical chemistry analyzer. (C) The serum samples from above were analyzed for IL-6 by enzyme-linked immunosorbent assay. Data are averaged from 3 mice per group and are representative of 2 independent experiments. ***P < .001.
Stim1−/−chimeras have less liver damage following ischemia/reperfusion injury. (A) WT or stim1−/− chimeric mice were subjected to liver ischemia/reperfusion injury by ligation of the portal vein and hepatic artery for 30 minutes followed by 3 hours of reperfusion. Mice were then euthanized, and liver samples were obtained for histology. Liver sections were stained with trichrome (upper 4 panels) or H&E (lower 2 panels). Areas of poor trichome staining (arrowheads) indicate hepatic necrosis in the WT mice, which were absent in livers from stim1−/− chimeras. Arrowheads indicate inflammatory cell infiltrate. Magnification is ×10 (upper panels), ×20 (middle panels) and ×40 (lower panels). Images shown are representative of 10 mice per group subjected to ischemia/reperfusion injury in separate experiments. (B) WT, stim1−/− chimeras, or p47phox-deficient mice were subjected to liver ischemia/reperfusion injury, and blood samples were obtained 3 hours after reperfusion. Liver enzymes aspartate aminotransferase and alanine aminotransferase were determined by a veterinary clinical chemistry analyzer. (C) The serum samples from above were analyzed for IL-6 by enzyme-linked immunosorbent assay. Data are averaged from 3 mice per group and are representative of 2 independent experiments. ***P < .001.
Discussion
In this study, we demonstrate that the STIM1 calcium sensor is required for SOCE in murine neutrophils. Loss of SOCE in stim1−/− cells results in modest effects on phagocytosis and degranulation but a more substantial defect in activation of the NADPH oxidase in response to a number of stimuli. The lack of SOCE is correlated with impaired activation of PKCα and -β leading to reduced phosphorylation of oxidase subunits, which accounts for the impaired production of ROS by stim1−/− cells. As a result, stim1−/− chimeric mice show severely impaired host response to Staphylococcus and Listeria infections but also reduced tissue injury in hepatic ischemia/reperfusion injury, both of which are mediated in large part by neutrophil activation.
This work relied on generation of stim1−/− chimeric mice for studies of host defense and ischemia/reperfusion injury in vivo, insofar as stim1−/− mice die perinatally.37 Thus, it is a possibility that some of the in vivo phenotypes may be a result of the loss of SOCE in other immune cells. Neutrophils and the NADPH oxidase are clearly necessary for clearance of S aureus since this pathogen is prevalent in patients with neutropenia or defects in NADPH oxidase components.38-40 The susceptibility displayed by stim1−/− chimeras in this model is almost certainly due to impaired neutrophil activation. The role of neutrophils in host defense to Listeria infection has recently been revisited by using the highly specific neutrophil-depleting monoclonal antibody Ly6G.41 Depletion of neutrophils with this monoclonal antibody results in poor control of Listeria replication in the liver. Loss of NADPH oxidase activity (in gp91phox-deficient mice) also results in impaired control of Listeria in the liver at early periods of infection, suggesting a dominant role for neutrophils at this stage.42,43 However, targeting neutrophils with anti-Ly6G has a much less deleterious effect on host defense to Listeria than treatment with anti–Gr-1 (Ly6G and Ly6C), which depletes neutrophils, inflammatory monocytes, and subsets of dendritic cells and CD8+ T cells.44 Thus, loss of SOCE in other cell types may contribute to the susceptibility of stim1−/− chimeras to Listeria infection.45 By contrast, the short time frame of the ischemia/reperfusion injury model favors the hypothesis that loss of neutrophil NADPH oxidase activity is responsible for protection in this model; at these very early time points, monocytes and macrophages would be less likely to be involved. Stim1−/− chimeric mice are also resistant to ischemic brain \ injury following transient occlusion of the middle cerebral artery, a phenotype that was suggested to be the result of reduced thrombus formation from loss of SOCE in platelets.46 Hence, it is also possible that some aspect of the reduced tissue injury in the hepatic ischemia/reperfusion model may be due to impaired platelet function in the stim1−/− chimeras. Generation of neutrophil lineage-specific mutants of stim1 will be required to formally address the role of neutrophil functional defects in the in vivo immune defects we observed in stim1−/− chimeras.
Several reports have implicated PKC family members in the activation of NADPH oxidase components. In transfected cell lines, the novel isoform PKCδ participates in fMLF or adhesion-dependent ROS production via phosphorylation of p40phox and p47phox subunits.47-49 These results are corroborated by inhibitor studies and in PKCδ−/− murine neutrophils, which demonstrate a partial defect in respiratory burst in response to these stimuli.30,47,48 Less is known about the classical calcium-sensitive PKC isoforms PKCα and PKCβ. siRNA knockdown in neutrophil-like HL-60 cells suggests that PKCα also has a role in ROS production generated by fMLF and PMA.50 We found that PKCα/β−/− neutrophils display a striking defect in ROS production and phox protein phosphorylation. Likely, multiple PKC family members cooperate to phosphorylate NADPH oxidase components. Further study is required to dissect the relative contributions of PKC family members downstream of distinct receptors.
It was somewhat surprising to find that loss of SOCE in primary neutrophils did not affect adhesion or chemotaxis in light of recent findings that migrational guidance of neutrophils under shear flow is mechano-transduced by integrin-induced calcium flux requiring Orai1.5 This study demonstrated that under high shear stress, high-affinity states of integrin LFA-1 induce calcium flux, which is required to orient the polymerization of cellular actin along a uropod-pseudopod axis to establish cell polarity and migratory direction. However, lower-affinity LFA-1 interactions do not induce intracellular calcium flux, F-actin polymerization, cell polarization, or directional migration under shear flow. It is possible that adhesive interactions other than high-affinity integrin ligation are sufficient to guide neutrophil recruitment in vivo, at least in the infection or hepatic ischemia/reperfusion models, independently of calcium flux.
The majority of studies, especially in lymphocytes, have focused on the role of STIM1 coupling to the Orai family members of channel proteins to mediate SOCE. However, there is significant evidence to suggest that STIM molecules are involved in activating other calcium channel proteins in nonlymphoid cells. In endothelial cells, STIM1 activates SOCE through the TRPC1 and -4 calcium channel proteins.51 Similar interactions between STIM1 and TRPC family members have been observed in cell transfection experiments or siRNA knockout methods in cell lines.52-54 Mast cell–expressed TRPC1 has been shown to contribute to SOCE, which is required for degranulation responses.55 TRPC1 and -6 have been implicated in SOCE entry and activation of the NADPH oxidase in myeloid HL-60 cells.12 Therefore, it remains unclear in primary neutrophils whether all the STIM1-mediated activation of SOCE occurs through Orai1 or whether other calcium channels are involved. Comparison of neutrophils deficient in these channel proteins with stim1−/−-deficient neutrophils will be required to resolve which are the dominant STIM1 gated calcium channels in neutrophils.
These results demonstrate that loss of STIM1-mediated SOCE in neutrophils has a profound effect on activation of the NADPH oxidase, likely through impaired activation of PKCα and -β. We propose that much of the immunodeficiency in patients with mutations in STIM1 or ORAI1 may result from defects in neutrophil function. Several of these patients presented with acute bacterial sepsis, which may reflect neutrophil dysfunction.15 Although lymphocytes from these patients have been well studied, neutrophils have not. Hence, there is little appreciation for the potential contribution of neutrophils to the overall immune defects seen in STIM1- or ORAI1-deficient individuals.
These results also imply that therapeutic blockade of SOCE in neutrophils would provide a novel approach to treat inflammatory disease. Of course, such a blockade would have to be balanced with potential effects on immune defense, as with any immunosuppressive agent. Nevertheless, the profound effect on neutrophil function caused by loss of SOCE justifies further work in this area.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Clare Abram for critical reading of the manuscript.
Supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI65495 and AI68150 to C.A.L.), by Grant-in-Aids for Japan Science and Technology Agency and Core Research for Evolutional Science and Technology (T.K.), and by the Ministry of Education, Culture, Sports, Science and Technology, Japan (Y.B. and T.K.). R.A.C. is a Fellow of the Pediatric Scientist Development Program, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12-HD000850).
Authorship
Contribution: H.Z., R.A.C., F.L., and Y.H. conducted the experimental work; Y.B. and T.K. provided valuable reagents; P.T. and C.A.L. provided laboratory oversight; and H.Z., R.A.C., F.L., Y.H., Y.B., P.T., T.K., and C.A.L. participated in manuscript composition and editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.Z. is BioLegend, San Diego, CA.
Correspondence: Clifford A. Lowell, Department of Laboratory Medicine, University of California at San Francisco, 513 Parnassus Ave, MSB-1058, San Francisco, CA 94143-0451; e-mail: clifford.lowell@ucsf.edu.
References
Author notes
H.Z. and R.A.C. contributed equally to this study.