Key Points
This study shows the effective anticancer activity of a T-cell receptor mimic antibody targeting WT1 in resistant human Ph+ ALL.
In combination with tyrosine kinase inhibitors, ESKM can result in cure of Ph+ ALL in murine models.
Abstract
Acute and chronic leukemias, including CD34+ CML cells, demonstrate increased expression of the Wilms tumor gene 1 product (WT1), making WT1 an attractive therapeutic target. However, WT1 is a currently undruggable, intracellular protein. ESKM is a human IgG1 T-cell receptor mimic monoclonal antibody directed to a 9–amino acid sequence of WT1 in the context of cell surface HLA-A*02. ESKM was therapeutically effective, alone and in combination with tyrosine kinase inhibitors (TKIs), against Philadelphia chromosome–positive acute leukemia in murine models, including a leukemia with the most common, pan-TKI, gatekeeper resistance mutation, T315I. ESKM was superior to the first-generation TKI, imatinib. Combination therapy with ESKM and TKIs was superior to either drug alone, capable of curing mice. ESKM showed no toxicity to human HLA-A*02:01+ stem cells under the conditions of this murine model. These features of ESKM make it a promising nontoxic therapeutic agent for sensitive and resistant Ph+ leukemias.
Introduction
Wilms tumor gene 1 protein (WT1) is a zinc finger transcription factor involved in the embryonic development of multiple organ systems including the kidney, with restricted expression in adult tissues.1-4 The function of WT1 is complicated and unclear; it regulates a variety of proteins as a transcription factor, some as an activator and some as a repressor.5 Multiple cancers demonstrate significantly increased expression of WT1, including myelodysplastic syndromes, acute myeloblastic leukemias, acute lymphoblastic leukemias (ALL), chronic myelogenous leukemia (CML),6-10 and many solid tumors11 including mesotheliomas,12 ovarian cancer,13 and various gastrointestinal14,15 and central nervous system (CNS) tumors.16 Overexpression of WT1 has also been demonstrated in the CD34+ stem cells of patients with CML,6 making it an attractive therapeutic target for eradication of this disease.
As an intracellular transcription factor, WT1 currently cannot be inhibited by small-molecule drugs or directly targeted by antibody therapies. However, after processing within the cell, peptides from WT1 protein are presented on the cell surface in the context of human leukocyte antigen (HLA) class I molecules. Previous work creating WT1 vaccines to stimulate human cytotoxic T cells has identified key immunogenic peptide sequences of WT1 presented by HLA17 ; several new epitopes were recently discovered.18 The widespread expression of this gene product in tumors, its involvement in oncogenesis, and its suppression in normal cells after birth19 make this a useful tumor marker and an ideal antigen target for cancer therapy.20
We have developed an antibody, ESKM, directed against the 9–amino acid peptide RMFPNAPYL (RMF), expressed in the context of HLA-A*02:01.21-23 ESKM is a T-cell receptor mimic (TCRm) monoclonal human IgG1 in which the Fc portion has been modified by alternative glycosylation that results in stronger binding to effector cell–activating Fcγ receptors and increased antibody-dependent cell cytotoxicity (ADCC).23 Although T cell–based therapies have been attempted against WT1-expressing cancers, monoclonal TCRm antibodies have several advantages over vaccines, TCR constructs, and adaptive T cells: ESKM can be produced and administered easily; and it has greater potency, more predictable and simpler pharmacokinetics, and high efficacy. Although several TCRm antibodies have been developed for other antigens,24 none has entered human trials.
The current standard of care for chronic-phase CML is treatment with tyrosine kinase inhibitors (TKIs), but this therapy is not curative, is extremely expensive, and may be required throughout life.25 Variable compliance with long-term therapy, with approximately one-third of patients stopping TKIs altogether, and the development of mutations that provide resistance to TKIs, frequently results in treatment failure, which sometimes leads to accelerated phase or blast crisis.26,27 Long-term use of TKIs used to suppress leukemia leads to a myriad of side effects, including pleural edema, effusions, pulmonary hypertension, sepsis, gastrointestinal problems, and lethal cardiovascular events.28,29 Furthermore, outside of stem cell transplantation (SCT), there is no effective therapy of CML in blast crisis or Philadelphia chromosome positive (Ph+) ALL30 ; treatment with TKIs results in brief responses only. The presence of WT1 expression in CML and its progenitors allows us to test for the first time a curative strategy for this disease, by use of ESKM alone or in conjunction with TKIs. In this study, we found ESKM alone was a more effective therapy than TKIs in murine models of human Ph+ ALL. Importantly, the efficacy of the antibody was not affected by the presence of the BCR-ABL “gatekeeper” resistance mutation T315I that inactivates all the first- and second-generation Food and Drug Administration–approved TKIs in clinical use. In combination with TKIs, ESKM was capable of curing mice of Ph+ ALL.
Materials and methods
ADCC
ADCC was evaluated by chromium release assay, incubating target cells in 50 µCi Cr51 for 1 hour before 3 washes, with the determined optimal ADCC time of 6 hours. Three effector to target (E:T) ratios were used (either 10:1, 30:1, and 100:1 or 25:1, 50:1, and 100:1). Effector peripheral blood mononuclear cells (PBMCs) were derived from healthy donors by Ficoll density centrifugation, after obtaining informed consent on a Memorial Sloan-Kettering institutional review board–approved protocol. This study was conducted in accordance with the Declaration of Helsinki.
BV173R Synthesis
The BV173R cell line was engineered to harbor the T315I BCR-ABL using a Clonetech pMSCV Puro vector with the T315I BCR-ABL gene, a kind gift from Charles Sawyers.31 BV173-luc-gfp cells were transduced with the retroviral vector, selected, and expanded in media with puromycin. The presence of the resistant T315I mutation was confirmed by polymerase chain reaction (PCR) for the plasmid, as well as ABL sequencing of cDNA and genomic DNA in the resultant BV173R cell line.
Flow cytometry and real-time PCR
Evaluation of ESKM binding to target cells and cell surface HLA-A*02:01 expression was done by direct flow cytometry. ESK was labeled with APC using the Innova Biosciences Lightning-Link Allophycocyanin (APC) kit. BB7 (anti-HLA-A*02:01) was purchased from various vendors. Minimal residual disease of cell lines in mouse bone marrow (BM) was evaluated using custom-made primers to the Firefly Luciferase gene with SYBR Green using glyceraldehyde 3-phosphate dehydrogenase as a control.
Trials of ESKM with TKIs in vivo in mice
Trials of ESKM were done using xenograft models, in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice with IL2 γ receptor knockout (NSG) mice32 engrafted with BV173 Ph+ leukemias. The BV173 and BV173R cell lines used for all in vivo studies stably expressed the firefly luciferase gene, and disseminated engraftment of leukemia was confirmed 6 days after tail vein injection by bioluminescent imaging (BLI) before treatments. Mouse research was approved by the MSKCC institutional review board under protocol 96-11-044.
Preliminary drug dosing studies in NSG mice demonstrated the maximum tolerated dose (MTD) of imatinib to be 50 mg/kg intraperitoneally (IP) daily, with higher dosing resulting in diarrhea and severe toxicity. Dasatinib was also initially tested, with the MTD of 40 mg/kg (0.8 mg/mouse) resulting in no appreciable short-term toxicity. However, longer-term trials of dasatinib resulted in mouse sudden deaths, and the dose was therefore lowered for subsequent experiments to 20 mg/kg and then to 10 mg/kg to eliminate drug-related mortality. For mice receiving ponatinib, the initial published dose of 5 mg/kg showed inadequate leukemic affect without any evident health side effects, and therefore ponatinib doses were increased to 10 mg/kg. At this dose, toxicity was comparable with 50 mg/kg of imatinib, with intermittent diarrhea and poor growth.
All TKIs, including imatinib, dasatinib, and ponatinib, as well as plerixafor, were purchased from Selleckchem or Fisher Scientific. The plerixafor stock solution was made in phosphate-buffered saline at 200 µg/mL and stored at room temperature. Mice received 1 mg/kg (20 µg/mouse) daily by IP injection, as was used in previous studies.33 No toxicity was noted during therapy. Before its use in vivo, stock solutions of TKIs were prepared in dimethyl sulfoxide (DMSO), with the scheduled dose of TKI in 50 µL DMSO per mouse. All TKIs and ESKM treatments were administered IP at these schedules: ESKM 100 µg twice weekly23 and TKIs daily. Male NSG mice, aged 6 to 8 weeks, were purchased from Jackson Labs.32 Three million leukemic cells (BV173 or BV173R) were injected per mouse by tail vein on day 0. Luciferin/luciferase BLI was then performed on day 6, with therapy commencing immediately after confirmation of equivalent levels of disseminated leukemia by imaging. IVIS 200 and IVIS Spectrum machines were used for BLI.
CD34+ cell transplantation
CD34+ cells were obtained by leukapheresis from 2 healthy donors as part of a harvest for sibling SCT on an MSKCC institutional review board–approved protocol. The leukapheresis product was purified by the CliniMACS system by the Memorial Hospital clinical stem cell laboratory. Twenty NSG mice, aged 6 to 8 weeks, were irradiated with 320 cGy on day −1 and received 3 × 106 CD34+ cells per mouse on day 0 by dorsal penile vein injection. Ten mice received cells from patient A and 10 from patient B. These 2 cohorts were then divided, with 5 mice from each group treated with ESKM 100 µg IP twice weekly starting on day +6 for 4 weeks, and the other 5 mice receiving the same dosing schedule of IgG isotype control. No specific supportive therapy or antibiotics were provided.
Results
ESKM therapy of Ph+ ALL in NSG mice
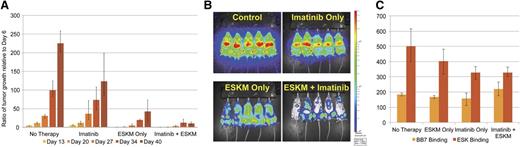
Imatinib is a first-generation TKI that has been in the longest use in humans for the treatment of CML. BV173 is a Ph+ HLA-A*02:01+ leukemia cell line.34 BLI was used to document disseminated engraftment of luciferase-marked BV173 Ph+ ALL in NSG mice 6 days after tail vein injection. Mice were then split into 4 groups of equal mean tumor burden and treated with: no therapy, imatinib only, ESKM only, or a combination of imatinib and ESKM. After 5 weeks of treatment, ESKM therapy was significantly superior to imatinib therapy (78% vs 52% tumor growth reduction, P < .01) (Figure 1A). The combination of ESKM and imatinib was significantly better than either imatinib or ESKM alone (P < .001), reducing tumor growth by 94% and possibly stabilizing progression of disease at that low level (Figure 1B). BM from all 4 groups were evaluated for HLA expression and ESKM binding immediately after completion of therapy (Figure 1C), with no group showing elimination of BV173 cells.
BV173 engrafted xenograft NSG mouse model treated with imatinib at MTD (50 mg/kg, 1 mg/mouse IP daily) and ESKM at optimal efficacious dose (100 µg IP twice weekly). Mice received 5 weeks of ESKM, imatinib, combination therapy, or no therapy (control). Error bars show the fifth and 95th percentiles. (A) Leukemic growth as measured by luciferase bioluminescent imaging for each of the 4 groups of mice. (B) End of therapy (5 weeks of therapy) image for each of the 4 groups of mice. (C) Three of 5 mice were randomly chosen from each of the 4 groups for BM harvest, and cells were evaluated by flow cytometry. Median staining by flow of the live hCD19+ cells (confirmed BV173) were evaluated for BB7 (HLA-A*02:01) and ESK binding.
BV173 engrafted xenograft NSG mouse model treated with imatinib at MTD (50 mg/kg, 1 mg/mouse IP daily) and ESKM at optimal efficacious dose (100 µg IP twice weekly). Mice received 5 weeks of ESKM, imatinib, combination therapy, or no therapy (control). Error bars show the fifth and 95th percentiles. (A) Leukemic growth as measured by luciferase bioluminescent imaging for each of the 4 groups of mice. (B) End of therapy (5 weeks of therapy) image for each of the 4 groups of mice. (C) Three of 5 mice were randomly chosen from each of the 4 groups for BM harvest, and cells were evaluated by flow cytometry. Median staining by flow of the live hCD19+ cells (confirmed BV173) were evaluated for BB7 (HLA-A*02:01) and ESK binding.
ESKM therapy with the second-generation TKI dasatinib
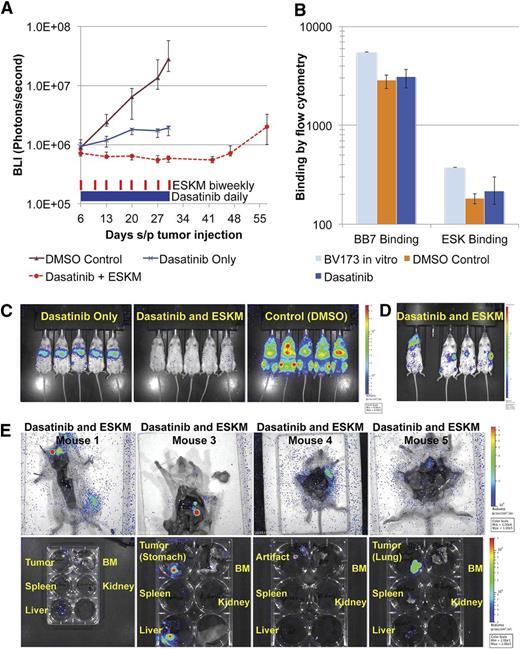
Dasatinib is a second-generation TKI with far greater potency than imatinib in humans and in animal models.35 Using the same mouse model as described before, dasatinib administered at 40 mg/kg IP daily in concurrent combination with ESKM resulted in the clinical and molecular cure of 3 of 4 mice (Figure 2A-B), which was confirmed by BM quantitative PCR 3 weeks after the end of therapy (supplemental Table 1 on the Blood Web site, MRD < 0.001%). No cures were seen in mice treated with either drug alone. The one mouse treated with both ESKM and dasatinib that relapsed first developed recurrence of disease in the CNS on day 27 (Figure 2C). Because of their large size, antibodies do not enter the CNS.36 At this dasatinib dose, all mice receiving the TKI became ill about 1 week into therapy, with 1 mouse fatality on day 8 of treatment. All dasatinib-treated mice sustained temporary BLI remissions, but those not treated with ESKM relapsed 2 to 3 weeks after cessation of therapy.
BV173 engrafted xenograft NSG mouse model treated with dasatinib at doses above the MTD. Error bars show the fifth and 95th percentiles. (A) Leukemic growth measured by BLI in mice treated with high-dose dasatinib therapy (40 mg/kg × 8 days, then lowered to 20 mg/kg ×3 days for a total 11 days of treatment and then stopped altogether secondary to high toxicity), ESKM for 4 weeks only, combination therapy, and control (no therapy). (B) BLI on day 55, 3 weeks after the end of therapy. Mouse #1 in dasatinib with ESKM combination died of dasatinib toxicity on day 13, and mouse #4 appeared to have relapsed in CNS. (C) The high-dose dasatinib plus ESKM group BLI images, showing progression of relapse in mouse #4. (D) Leukemic growth measured by BLI in mice treated with dasatinib therapy at 20 mg/kg IP daily given ×18 days and then stopped owing to high toxicity. ESKM was given to 6 mice (days 18 to 42). All mice relapsed, although ESKM mice relapsed significantly more slowly, and lifespan was longer ×1 week. The last point on each line pertains to when the mice were sacrificed because of illness. (E) BLI at the end of ESKM therapy (day 42). Two cages of mice received ESKM after discontinuation of dasatinib (dasatinib → ESKM 1 and 2) and compared with 1 cage (5 mice) that received no additional therapy. ESKM-salvaged mice have substantially less tumor burden then dasatinib-only mice, although all mice eventually relapsed.
BV173 engrafted xenograft NSG mouse model treated with dasatinib at doses above the MTD. Error bars show the fifth and 95th percentiles. (A) Leukemic growth measured by BLI in mice treated with high-dose dasatinib therapy (40 mg/kg × 8 days, then lowered to 20 mg/kg ×3 days for a total 11 days of treatment and then stopped altogether secondary to high toxicity), ESKM for 4 weeks only, combination therapy, and control (no therapy). (B) BLI on day 55, 3 weeks after the end of therapy. Mouse #1 in dasatinib with ESKM combination died of dasatinib toxicity on day 13, and mouse #4 appeared to have relapsed in CNS. (C) The high-dose dasatinib plus ESKM group BLI images, showing progression of relapse in mouse #4. (D) Leukemic growth measured by BLI in mice treated with dasatinib therapy at 20 mg/kg IP daily given ×18 days and then stopped owing to high toxicity. ESKM was given to 6 mice (days 18 to 42). All mice relapsed, although ESKM mice relapsed significantly more slowly, and lifespan was longer ×1 week. The last point on each line pertains to when the mice were sacrificed because of illness. (E) BLI at the end of ESKM therapy (day 42). Two cages of mice received ESKM after discontinuation of dasatinib (dasatinib → ESKM 1 and 2) and compared with 1 cage (5 mice) that received no additional therapy. ESKM-salvaged mice have substantially less tumor burden then dasatinib-only mice, although all mice eventually relapsed.
To determine whether combination therapy with TKIs and ESKM could be optimized by sequential therapies rather than concurrent treatment, BV173-engrafted mice were treated with dasatinib at a lower dose of 20 mg/kg alone for 18 of the planned 21 days. By this point, 4 mice receiving dasatinib died, and the remaining 11 mice were randomized to receive further treatment: 6 received ESKM, and 5 received no additional therapy. Four weeks of ESKM therapy did delay relapse (Figure 2D), but no mice were cured, and ESKM therapy did not result in complete remission as measured by BLI at the end of therapy (Figure 2E).
BV173 in NSG mice was next treated with the MTD of concurrent dasatinib and ESKM. Combination therapy reduced leukemia, whereas the mice treated with dasatinib alone doubled their leukemic burden (Figure 3A). Cells were analyzed by flow cytometry upon completion of therapy, showing no change in antigen expression (Figure 3B). No leukemia was seen by BLI at the end of therapy in mice that received both dasatinib and ESKM (Figure 3C). The mice treated with combination therapy were followed with BLI scans until day 58. Mouse 2 died on day 31, likely from dasatinib toxicity, and, interestingly, the other 4 mice all showed focal tumor relapse but not recurrent leukemia (Figure 3D). This striking pattern of ALL relapse as discrete tumors (Figure 3E) is unusual and was only seen with the combination therapy.
BV173 engrafted in NSG mice, treated with dasatinib 10 mg/kg daily ± ESKM 100 µg twice weekly for 25 days, beginning on day 6. The error bars show the fifth and 95th percentiles. (A) Logarithmic tumor growth curve as measured by BLI. (B) Control and dasatinib-alone mice were sacrificed on day 30 for evaluation of ESKM binding and HLA-A*02:01 expression by flow cytometry. BV173 cells were harvested from the livers of all NSG mice immediately after cessation of therapy. Live hCD19+ cells (confirmed BV173) were evaluated for BB7 (HLA-A*02:01) and ESK staining and compared with BV173 in culture. (C) BLI imaging from day 27, showing persistence of leukemia at the end of therapy in dasatinib-treated mice and eradication of leukemic signal in mice receiving combination therapy. (D) The BLI image of the dasatinib + ESKM group on day 55, 24 days after cessation of therapy, showing focal lymphoid relapse. (E) Dasatinib + ESKM–treated mice dissected and reimaged individually to localize tumor. The organs were removed and imaged separately for precise tumor localization. Mouse 1: Primary tumor in the lymph node, with small liver metastasis detected. Mouse 3: Primary tumor in the stomach with focal liver metastasis and small renal metastasis. Mouse 4: Tumor seen in the left upper quadrant, but unable to be isolated by organ excision. Mouse 4: Diffuse right lung tumor, unable to visualize right upper quadrant tumor seen in prior imaging.
BV173 engrafted in NSG mice, treated with dasatinib 10 mg/kg daily ± ESKM 100 µg twice weekly for 25 days, beginning on day 6. The error bars show the fifth and 95th percentiles. (A) Logarithmic tumor growth curve as measured by BLI. (B) Control and dasatinib-alone mice were sacrificed on day 30 for evaluation of ESKM binding and HLA-A*02:01 expression by flow cytometry. BV173 cells were harvested from the livers of all NSG mice immediately after cessation of therapy. Live hCD19+ cells (confirmed BV173) were evaluated for BB7 (HLA-A*02:01) and ESK staining and compared with BV173 in culture. (C) BLI imaging from day 27, showing persistence of leukemia at the end of therapy in dasatinib-treated mice and eradication of leukemic signal in mice receiving combination therapy. (D) The BLI image of the dasatinib + ESKM group on day 55, 24 days after cessation of therapy, showing focal lymphoid relapse. (E) Dasatinib + ESKM–treated mice dissected and reimaged individually to localize tumor. The organs were removed and imaged separately for precise tumor localization. Mouse 1: Primary tumor in the lymph node, with small liver metastasis detected. Mouse 3: Primary tumor in the stomach with focal liver metastasis and small renal metastasis. Mouse 4: Tumor seen in the left upper quadrant, but unable to be isolated by organ excision. Mouse 4: Diffuse right lung tumor, unable to visualize right upper quadrant tumor seen in prior imaging.
Therapy of T315I-resistant Ph+ leukemia therapy
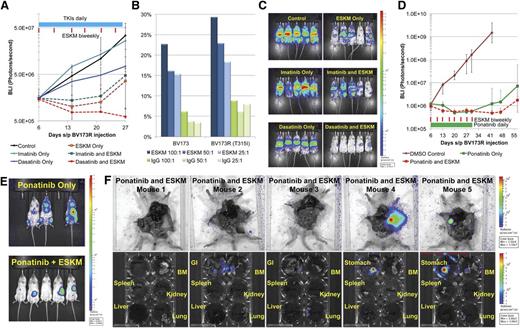
BV173R (TKI pan-resistant T315I mutant) leukemia xenografted NSG mice were initially treated with ESKM, imatinib, dasatinib, and concurrent combinations of imatinib plus ESKM and dasatinib plus ESKM. Only 3 weeks of therapy were given, because the leukemia proved resistant to the first- and second-generation TKIs. In contrast, ESKM retained full and equivalent efficacy against the resistant BV173R leukemia compared with TKI-sensitive leukemia BV173 (Figure 4A), as quantified by luciferase imaging during treatment. These findings are consistent with in vitro studies indicating that in ADCC assays, ESKM is as active against BV173R as it is against BV173 (Figure 4B). Dasatinib provided only modest slowing of leukemic growth, with leukemic burden rising fivefold over the same time period. The combination of ESKM and dasatinib showed substantial reduction in leukemia burden, though no mice were cured. As in the prior experiments with BV173 treated with the combination ESKM plus dasatinib, relapse was in the form of lymphoid tumors rather than diffuse leukemia (Figure 4C). The lack of ESKM efficacy on BV173 growing as primary subcutaneous lymphomas was documented, suggesting that these cells escape by lack of penetration of the few NSG effector cells that circulate (supplemental Figure 1). How this form of escape will be manifested in humans is uncertain because the NSG mice lack NK cells.
BV173R (with T315I mutation) treated with dasatinib 10 mg/kg and imatinib 50 mg/kg IP daily for 21 days with and without biweekly ESKM therapy (6 doses of ESKM). The error bars show the fifth and 95th percentiles. (A) Exponential growth curves, from the start of therapy (day 6). The blue bar shows the duration of TKI therapy and the red lines are doses of ESKM. (B) The susceptibility of cell line BV173, resistant cell line BV173R (with BCR-ABL T315I mutation) to ESKM directed ADCC with human PBMCs, and different E:T ratios. (C) The BLI imaging at the end of ESKM/TKI therapy (day 27). ESKM is superior to imatinib and dasatinib for treatment of resistant Ph+ ALL. (D) The BLI of mice engrafted with BV173R treated with ponatinib 10 mg/kg alone and in combination with ESKM × 25 days (days 6 to 30). The green bar shows the duration of ponatinib therapy and the red lines are doses of ESKM. (E) The BLI image on day 57 of mice treated in panel (D), immediately before organ analysis for tumor location and MRD evaluation. (F) Ponatinib + ESKM mice from panel (D) were dissected and the organs imaged for tumor localization. Mouse 1: No detectable disease on any imaging, possible cure. Mouse 2: Gastrointestinal and BM leukemia detected. Mouse 3: Gastrointestinal, BM, and lung leukemia detected, with an uncertain primary source. Mouse 4: Lymphoid tumor in the stomach, no other tumor detected. Mouse 5: Primary lymphoid tumor in the stomach, BM leukemia detected.
BV173R (with T315I mutation) treated with dasatinib 10 mg/kg and imatinib 50 mg/kg IP daily for 21 days with and without biweekly ESKM therapy (6 doses of ESKM). The error bars show the fifth and 95th percentiles. (A) Exponential growth curves, from the start of therapy (day 6). The blue bar shows the duration of TKI therapy and the red lines are doses of ESKM. (B) The susceptibility of cell line BV173, resistant cell line BV173R (with BCR-ABL T315I mutation) to ESKM directed ADCC with human PBMCs, and different E:T ratios. (C) The BLI imaging at the end of ESKM/TKI therapy (day 27). ESKM is superior to imatinib and dasatinib for treatment of resistant Ph+ ALL. (D) The BLI of mice engrafted with BV173R treated with ponatinib 10 mg/kg alone and in combination with ESKM × 25 days (days 6 to 30). The green bar shows the duration of ponatinib therapy and the red lines are doses of ESKM. (E) The BLI image on day 57 of mice treated in panel (D), immediately before organ analysis for tumor location and MRD evaluation. (F) Ponatinib + ESKM mice from panel (D) were dissected and the organs imaged for tumor localization. Mouse 1: No detectable disease on any imaging, possible cure. Mouse 2: Gastrointestinal and BM leukemia detected. Mouse 3: Gastrointestinal, BM, and lung leukemia detected, with an uncertain primary source. Mouse 4: Lymphoid tumor in the stomach, no other tumor detected. Mouse 5: Primary lymphoid tumor in the stomach, BM leukemia detected.
NSG mice engrafted with this T315I Ph+-resistant leukemia were then treated with the third-generation drug, ponatinib. Leukemic regression was seen in all ponatinib-treated mice, with negative BLI showing no disease at the end of therapy on day 34 (supplemental Figure 2A). Mice treated with ponatinib monotherapy subsequently developed systemic leukemic relapse within 2 weeks after end of treatment, whereas those treated with the combination of ESKM and ponatinib developed either focal lymphoid relapse (3/5) or had no evidence of disease (2/5) 4 weeks after completion of therapy (supplemental Figure 2B). These results were confirmed by repeating the experiment with a complete 4-week course of ponatinib 10 mg/kg alone and in combination with ESKM (Figure 4D). Mice were followed for 4 weeks after the end of therapy (Figure 4E), at which time their tumors were evaluated by resection and BLI (Figure 4F and supplemental Figure 3A-B). Of note, ponatinib at 10 mg/kg was fairly toxic (eg, causing diarrhea, poor weight gain), and although all treated mice survived 4 weeks of therapy, 4 of the 20 mice treated in these experiments died of late side effects.
Effects in vitro of ESKM and TKIs against Ph+ ALL
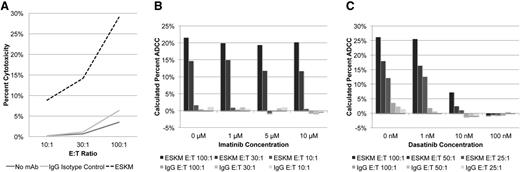
The striking activity of ESKM in combination with the 2 TKIs led us to ask whether there was a mechanistic interaction of the TKIs on the ability of ESKM to kill target cells through its known cytotoxic mode of action, ADCC. Chromium release (Cr51) assay was used to evaluate ESKM-directed human PBMC–driven ADCC in vitro against BV173. The addition of ESKM resulted in 21% to 29% ADCC across multiple experiments, using the E:T of 100:1 (Figure 5A). Control IgG1 isotype was not cytotoxic. ADCC was also measured in the presence of imatinib and dasatinib. Imatinib had no effect on ADCC at concentrations as high as 10 µM (Figure 5B), at 10 times the peak concentrations of this drug in humans. However, dasatinib showed a profound inhibition on ADCC in repeated experiments, with complete inhibition of PBMC-mediated ADCC at concentration of 100 nM (Figure 5C), the peak concentration of drug in humans.
Effects of TKIs on ADCC. (A) ADCC by Cr51 assay with variable E:T ratios using ESKM vs IgG1 isotype control. The effects of imatinib (B) and dasatinib (C) on ADCC. No significant effect on ADCC by imatinib was seen on human PBMC-mediated ADCC in concentrations as high as 10 µM. Dasatinib inhibited human PBMC-mediated ADCC starting at concentrations of 10 nM.
Effects of TKIs on ADCC. (A) ADCC by Cr51 assay with variable E:T ratios using ESKM vs IgG1 isotype control. The effects of imatinib (B) and dasatinib (C) on ADCC. No significant effect on ADCC by imatinib was seen on human PBMC-mediated ADCC in concentrations as high as 10 µM. Dasatinib inhibited human PBMC-mediated ADCC starting at concentrations of 10 nM.
We also examined whether TKIs might affect expression of the WT1/HLA epitope, ESKM binding and, hence, efficacy. This issue was interrogated both in vitro and in vivo. BV173 cells were grown in culture with imatinib (1 µM), dasatinib (100 nM), and ponatinib (100 nM), in the approximate peak concentrations achieved in vivo by these drugs. Consistent with previously reported studies,37,38 exposure of Ph+ cells to high doses of TKIs resulted in significant downregulation of HLA expression and a mild decrease of WT1 (supplemental Figure 4). However, this downregulation of HLA was seen in vitro by culturing cells in the prolonged presence of a constant peak dose of TKIs, conditions that are not physiological.
To evaluate whether TK1 treatment could also decrease HLA or RMF cell surface expression in vivo and thereby contribute to ESKM resistance, BV173 leukemia was extracted immediately after termination of therapy from the imatinib-treated mice BM (Figure 1B) and from the livers of mice treated with dasatinib 10 mg/kg (Figure 3C). Five weeks of therapy in vivo with imatinib alone, ESKM alone, or a combination of both drugs did not alter HLA surface expression on CD19+ cells as measured by flow cytometry (supplemental Figure 5A). A small decrease in ESK median binding was seen in cells treated with imatinib (Figure 1C), though it could not be discerned on the flow histogram (supplemental Figure 5B). There was no difference in HLA or ESK surface binding between BV173 cells obtained from the livers of mice treated with 25 days of dasatinib compared with vehicle control (DMSO), though both had equally decreased HLA expression compared with freshly cultured BV173 (Figure 3B).
Leukemia escape mechanisms
BV173 leukemia treated with monotherapy for as much as 5 weeks with ESKM monotherapy was noted to overgrow in the BM. To evaluate whether BM stromal cells were protective against TCRm therapy, NSG mice engrafted with BV173 were treated for 4 weeks with a combination of ESKM and the CXCR4 inhibitor plerixafor to inhibit binding of the BV173 cells to the stroma. Treatment with plerixafor was associated with reproducible but not statistically significant reduction in BV173 growth. A concomitant reduction of tumor growth with plerixafor and ESKM treatment was also observed, but the difference in growth inhibition was not significant (supplemental Figure 6A-B).
We also evaluated whether alterations in HLA expression or presentation of WT1 peptide as measured by ESK binding could alter sensitivity to ESKM-mediated ADCC in the presence or absence of TKIs. To do this, we evaluated ADCC against fresh leukemic blasts from a patient with blast crisis CML. This leukemia was selected for its having low expression of HLA-A*02:01 (supplemental Figure 7A: 7 fold shift vs 30-40–fold shift for BV173) and low ESK binding (supplemental Figure 7B). ADCC with ESKM was background level, compared with 21% to 29% for BV173 (supplemental Figure 7C). As expected, in vivo, ESKM alone had no efficacy against this CML with low HLA expression and ESK binding. However, BV173 did not show significant losses of antigen expression that would account for relapse (Figure 3B and supplemental Figure 5B).
Activity of ESKM against normal hematopoietic cells
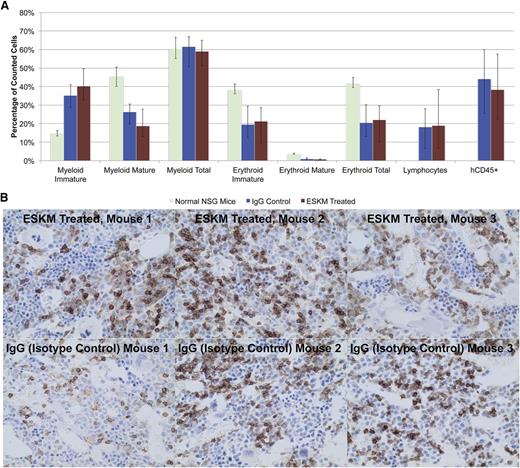
Although the potent therapeutic effects in mice appear to be promising, cytotoxicity against normal hematopoietic cells is a potential risk. Previously we showed that ESKM does not affect hematopoietic stem cell numbers or frequencies, LSK cells, or mature white blood cell subpopulations in HLA-A*02:01 transgenic mice that express WT1.39 ESKM was investigated for cross-reactivity against healthy CD34+ human hematopoietic stem cells by evaluating ESKM vs IgG isotype control therapy of mice transplanted with human HLA-A*02:01 CD34+ cells. Two mice died of early irradiation toxicity, and on days +45 to 46, 8 mice died of SCT across all 4 groups, all attributable to late radiation effects. The remaining mice (3 ESKM-treated and 6 IgG-treated) were submitted to pathology for BM and organ evaluation. These mice were found to have anemia with good reticulocytosis, suggesting severe anemia as the cause of death on days +45 to 46. There was no histologic difference between the BM of mice treated with ESKM compared with those that received isotype control (Figure 6A). Importantly, human CD45+ staining showed 20% to 60% engraftment in all mice (Figure 6B). No toxicity was observed secondary to ESKM therapy in the BM or in any of the mouse organs by pathology compared with IgG controls.
BM pathology from NSG mice treated with 4 weeks of ESKM or isotype IgG control after human HLA-A*02:01 CD34+ transplant, compared with normal NSG mice. (A) No differences were seen between ESKM- and IgG-treated mice, and both groups have increased immature myeloid and erythroid lineages compared with NSG mice. The transplanted groups also have lymphocytes, which NSG mice lack. Engraftment of human cells was the same in ESKM- and IgG-treated mice, ranging from 20% to 60%. The error bars show the fifth and 95th percentiles. (B) Immunohistochemical staining of NSG mouse BM transplanted with human CD34+ cells for hCD45. There was no significant difference between the mice treated with ESKM (top panels) and those treated with isotype control (bottom panels). Normal NSG mice samples were did not stain positive for hCD45 (not shown).
BM pathology from NSG mice treated with 4 weeks of ESKM or isotype IgG control after human HLA-A*02:01 CD34+ transplant, compared with normal NSG mice. (A) No differences were seen between ESKM- and IgG-treated mice, and both groups have increased immature myeloid and erythroid lineages compared with NSG mice. The transplanted groups also have lymphocytes, which NSG mice lack. Engraftment of human cells was the same in ESKM- and IgG-treated mice, ranging from 20% to 60%. The error bars show the fifth and 95th percentiles. (B) Immunohistochemical staining of NSG mouse BM transplanted with human CD34+ cells for hCD45. There was no significant difference between the mice treated with ESKM (top panels) and those treated with isotype control (bottom panels). Normal NSG mice samples were did not stain positive for hCD45 (not shown).
Discussion
Compared with TKIs, ESKM is more potent, has a longer half-life, and is not susceptible to tumor escape by gatekeeper mutations. ESKM is also a therapeutically effective antibody in mouse models of other human leukemias and solid tumors.23 However, as monotherapy, the antibody was unable to achieve cures in these models. In combination, TKIs and ESKM did not exhibit enhanced toxicity, had at least an additive therapeutic effect, and resulted in molecular cures of Ph+ ALL in mice. BM relapse of leukemia post treatment may reflect inadequate effector cells rather than loss of antigen, or local stromal cell protection, because RMF/HLA-A*02:01 was still expressed on leukemia cells at the end of therapy, and reducing leukemic cell adherence to stromal cells with plerixafor did not significantly increase antileukemic activity. This hypothesis is further supported by the consistent and efficient leukemic cell clearance in the liver, where there are abundant Kupffer cells for ADCC (Figure 1B) and not in the BM, which lack many effectors in these NSG mice.
The data support a more generally accepted hypothesis that mAb therapies would likely benefit from a second agent to decrease cancer burden and keep cancer growth rates low, thereby fostering more effective ADCC in vivo at increased E:T ratios. Thus, patients with CML in remission on TKIs may be ideal candidates for ESKM treatment, since they have minimal residual leukemic burden and slow cancer growth. The main mechanism of escape of ALL for ESKM monotherapy seemed to be inadequate effector function. Surprisingly, the combination therapy with dasatinib or ponatinib resulted in lymphoid tumor relapse, which is exceedingly rare in CML in humans. We speculate that a lack of effector cells infiltrating the leukemic tumor or the reduced ESKM penetration into the lymphoid tumors may account for this observation.
An important advantage to TCRm antibody therapy was its efficacy against pan-resistant Ph+ leukemias. First- and second-generation TKIs are not effective against leukemias that carry the BCR-ABL T315I resistance mutation. Such mutations occur in 12% to 15% of screened patients with CML and are a source of relapse and failure of treatment.40,41 Although a third-generation TKI, ponatinib, is effective in the presence of this mutation, its use is currently restricted because of severe vascular toxicity. Therefore there is an urgent need for alternative therapies in patients with these mutations. ESKM works through an entirely different mechanism than the TKIs, and our experiments demonstrated equal efficacy of the TCRm-mediated ADCC against both TKI-sensitive BV173 and its resistant mutant BV173R in vitro and in vivo. Combining ESKM with another TKI could result in faster remissions and possible cure, which may allow a shorter course of these expensive, potentially toxic and noncurative drugs.
Although these data strongly support the effectiveness of combining TKIs with ESKM, there are limitations. The patients must be HLA-A*02+ and have leukemias expressing WT1. Leukemias with reduced HLA-A*02 expression may be resistant to ESKM. In addition, low WT1 expression, or inadequate concentrations of effector cells such as in a leukemia-packed BM, may also limit cytotoxic activity of this TCRm antibody. Importantly, despite its potent activity against Ph+ ALL, ESKM had no discernable effect on normal human BM progenitor cells within these model systems. Because of its specificity for the leukemia cells, ESKM should cause minimal toxicity. In addition, this antibody could be evaluated in human clinical trials during the peri–stem cell transplant time frame.
Presented in oral abstract form at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 10, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Charles Sawyers for the T315I plasmid, Tanya Korontsvit and Victoriya Zakhaleva for their assistance, Emily Casey for labeling ESK with APC for flow cytometry, and Nick Veomett for helpful discussions.
This work was supported by the National Institutes of Health, National Cancer Institute (grants T32CA62948-18, R01CA55349, and P01CA23766) and National Institute of General Medical Sciences (grant MSTP-GM0773), the Leukemia and Lymphoma Society, the Lymphoma Foundation, Tudor and Glades funds, and the MSKCC Technology Development Fund and the Experimental Therapeutics Center.
Authorship
Contribution: L.D., E.J.B., T.D., C.L., and D.A.S. designed the study; R.J.O. and D.A.S. coordinated the study; L.D. conducted all experiments and analysis with assistance from D.P., E.J.B., and A.S.; D.P. synthesized the BV173R cell line; S.Y. and C.L. supplied and synthesized ESKM; and L.D. drafted the manuscript, which was subsequently revised by all co-authors.
Conflict-of-interest disclosure: L.D., T.D., and D.A.S. are inventors of intellectual property that is owned by MSKCC and for which a license has been obtained. None own equity or are paid by the licensee or receive grants. S.Y. and C.L. are employed by and have an equity stake in Eureka, which co-owns the intellectual property described above. The remaining authors declare no competing financial interests.
Correspondence: David A. Scheinberg, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: d-scheinberg@ski.mskcc.org.
References
Author notes
D.P. and E.J.B. contributed equally to this study.