Key Points
Analysis of 20 samples from CML-BC patients showed that MMP-9 was highly expressed in three, with two exhibiting high levels of HES1.
MMP-9 is upregulated by Hes1, and MMP-9 deficiency impaired the development of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT.
Abstract
High levels of HES1 expression are frequently found in BCR-ABL+ chronic myelogenous leukemia in blast crisis (CML-BC). In mouse bone marrow transplantation (BMT) models, co-expression of BCR-ABL and Hes1 induces CML-BC–like disease; however, the underlying mechanism remained elusive. Here, based on gene expression analysis, we show that MMP-9 is upregulated by Hes1 in common myeloid progenitors (CMPs). Analysis of promoter activity demonstrated that Hes1 upregulated MMP-9 by activating NF-κB. Analysis of 20 samples from CML-BC patients showed that MMP-9 was highly expressed in three, with two exhibiting high levels of HES1 expression. Interestingly, MMP-9 deficiency impaired the cobblestone area–forming ability of CMPs expressing BCR-ABL and Hes1 that were in conjunction with a stromal cell layer. In addition, CMPs expressing BCR-ABL and Hes1 secreted MMP-9, promoting the release of soluble Kit-ligand (sKitL) from stromal cells, thereby enhancing proliferation of the leukemic cells. In accordance, mice transplanted with CMPs expressing BCR-ABL and Hes1 exhibited high levels of sKitL as well as MMP-9 in the serum. Importantly, MMP-9 deficiency impaired the development of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT models. The present results suggest that Hes1 promotes the development of CML-BC, partly through MMP-9 upregulation in leukemic cells.
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm originating in an abnormal pluripotent hematopoietic stem cell (HSC) and characterized by the formation of the BCR-ABL fusion gene caused by a specific chromosomal translocation t(9;22). BCR-ABL, a constitutively active tyrosine kinase, stimulates proliferation and survival of CML cells. In CML, an initial indolent chronic phase (CP) is followed by the accelerated phase (AP) and blast crisis (BC), resulting in expansion of immature leukemic cells. The differentiation block by additional genetic events in progenitor stages of CML cells is responsible for the transition from CML in CP (CML-CP) to CML in BC (CML-BC).1 CML-CP has become relatively easy to control with the development of BCR-ABL inhibitors such as imatinib. However, once the disease progresses to BC, it often becomes resistant to therapies. We and others recently reported that high levels of HES1 expression were frequently found in patients with CML-BC, but not with CML-CP.2,3 In addition, we demonstrated that the combination of Hes1 and BCR-ABL induced fatal CML-BC–like disease in mouse bone marrow transplantation (BMT) models. These results suggested that Hes1 is a key molecule in transition to BC in CML.2
The balance between basic helix-loop-helix (bHLH) family transcriptional activators and repressors regulates the proper timing of cellular differentiation and tissue morphogenesis.4 Hairy enhancer of split 1 (Hes1) is a bHLH transcriptional repressor that often maintains cells in an immature state in various tissues. Hes1 is a downstream target of Notch signaling.5,6 During embryogenesis, Hes1 blocks differentiation of neural stem cells by antagonizing Mash1.7 In the hematopoietic system, retroviral expression of Hes1 blocks granulocyte colony-stimulating factor (G-CSF)–induced granulocytic differentiation of 32Dcl3 cells,8 immortalizes common myeloid progenitors (CMPs) and granulocyte-macrophage progenitors (GMPs) in the presence of interleukin-3 (IL-3),2 and preserves the long-term reconstituting ability of HSCs.9 Alternatively, Notch signaling is reported to promote mast-cell development at the expense of granulocyte differentiation through upregulation of both Hes1 and GATA-3 in CMPs and GMPs.10 In any event, Hes1-mediated differentiation block in hematopoietic cells is explained in part by downregulation of C/EBPα,2 an essential transcriptional factor for granulocytic differentiation.
Matrix metalloproteinase-9 (MMP-9) (also called 92 kDa gelatinase) degrades specific components of the extracellular matrix, including denatured collagens (gelatin) type IV, V, and XI, as well as elastin.11 MMP-9 also promotes the release of extracellular matrix-bound or cell-surface–bound cytokines, such as vascular endothelial growth factor, which promotes angiogenesis12 or early bone marrow (BM) development via osteoclast recruitment.13 Interestingly, BM ablation induces expression of stromal cell–derived factor-1 (SDF-1), which upregulates MMP-9, leading to shedding of soluble Kit-ligand (sKitL).14 In this setting, sKitL enables BM repopulating cells to translocate to a permissive vascular niche favoring differentiation and reconstitution of the stem/progenitor cell pool.14
Here we show that MMP-9 is strongly upregulated by Hes1 through activation of nuclear factor-κB (NF-κB). Importantly, MMP-9 deficiency impaired the development of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT models. In addition, MMP-9 deficiency attenuated in vitro migration and proliferation of CMPs expressing BCR-ABL and Hes1 in the presence of stromal cells. Given that plasma levels of sKitL as well as MMP-9 were elevated in mice transplanted with CMPs expressing BCR-ABL and Hes1, these results highlight the importance of the Hes1-MMP-9-sKitL cascade via the interaction between leukemic and stromal cells in the development of CML-BC.
Materials and methods
Mice
C57BL/6 (Ly5.1) mice (Sankyo Labo Service Corporation) and C57BL/6 (Ly5.2) mice (Charles River Laboratories, Japan) were used. MMP-9−/− 129Sv mice11 were used after at least 8 backcrosses to C57BL/6 (Ly5.2) mice. All animal studies were approved by the Animal Care Committee of the Institute of Medical Science, The University of Tokyo.
Transfection and retrovirus production
Rat Hes1 cDNA (a gift from R. Kageyama, Kyoto University, Kyoto, Japan) was inserted upstream of the internal ribosome entry site–human nerve growth factor receptor (IRES-NGFR) cassette of pMYs-INGFR to generate pMYs-Hes1-INGFR.15 BCR-ABL (p210) cDNA16 was inserted upstream of the IRES-enhanced green fluorescent protein (IRES-GFP) cassette of pMYs-IG to generate pMYs-BCR-ABL-IG. Retrovirus infection was done as described previously.15,17 Briefly, retroviruses were generated by transient transfection of Plat-E17 packaging cells with FuGENE 6 (Roche Diagnostics).
Infection to progenitors
Lin−c-Kit+Sca-1−FcγRloCD34+ CMPs18 were sorted with a FACSAria cell sorter (Becton Dickinson). Infection to progenitors was performed using RetroNectin (Takara Bio) as previously described.2 Iscove’s modified Dulbecco’s medium (IMDM) (Sigma-Aldrich) containing 20% fetal bovine serum (FBS), 50 ng/mL mouse stem cell factor (SCF), 20 ng/mL mouse thrombopoeitin (TPO), and 20 ng/mL mouse IL-3, 20 ng/mL mouse IL-6 (all from R&D Systems) was used for CMPs. The efficiency of infection was approximately 20%, with retrovirus harboring pMYs-Hes1-INGFR, and 10% with retrovirus harboring pMYs-BCR-ABL-IG.
Colony-forming assay
Retrovirus-infected cells were sorted 48 hours after infection with a FACSAria cell sorter (Becton Dickinson) and used for colony-forming assay using Methocult 3231 (StemCell Technologies), supplemented with 50 ng/mL mouse SCF, 20 ng/mL mouse TPO, and 20 ng/mL mouse IL-3 and 20 ng/mL mouse IL-6, as previously described.2
Mouse BM transplantation
CMPs prepared from MMP-9+/+ or MMP-9−/− mice were infected with retrovirus containing Hes1 or BCR-ABL, and 1.8 to 3.5 × 104 of Hes1/NGFR and BCR-ABL/GFP double-positive cells were sorted and intravenously injected into C57BL/6-Ly5.1–recipient mice after sublethal γ-irradiation. There was no difference in the efficiency of retrovirus infection between CMPs from wild-type (WT) or MMP-9–deficient mice. The efficiency of infection was Hes1: 16.5% to 25.0% and BCR-ABL: 7.0% to 12.8% in WT CMPs; and Hes1: 17.1% to 25.5% and BCR-ABL: 6.5% to 13.5% in MMP-9–deficient CMPs. Probabilities of overall survival of the transplanted mice were estimated using the Kaplan-Meier method. Statistical differences were determined by the Wilcoxon test.
Flow cytometric analysis
Cells were stained with the following monoclonal PE-conjugated antibodies: Ly-5.1, Gr-1, CD11b, B220, CD19, CD3, CD4, CD8, c-Kit, Sca-1, CD34, and Ter119. Flow cytometric analysis of the stained cells was performed with FACSCalibur (Becton Dickinson) equipped with CellQuest software (BD Biosciences) and FlowJo software (Tree Star), as previously described.2,19
Patients
CML patients were diagnosed at Hiroshima University Hospital and its affiliated hospitals. All studies were approved by the Institutional Review Board at Hiroshima University and the ethics committee of the University of Tokyo (approval no. 20-10-0620). Patients’ informed consents were obtained in accordance with the Declaration of Helsinki.
Real-time reverse-transcription polymerase chain reaction
Real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was performed as described previously.2 Reaction conditions are shown in the supplemental Methods available on the Blood Web site.
Cobblestonelike area-forming assays
Sorted cells were added to each well of a 6-well plate containing C3H10T1/2 cells (RIKEN Biosource) irradiated at a dose of 50 Gy. After co-culture for 6 days (culture volume, 2 mL; IMDM supplemented with 10% FBS and without cytokines), the numbers of cobblestone area-forming cells (CAFCs) per well were counted, as previously described.20
Immunoassay
The levels of MMP-9 and sKitL in mouse plasma or culture supernatants were measured with an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
Evaluation of NF-κB activity
NF-κB activity was measured in nuclear protein extracts using the TransAM NF-κB Family Transcription Factor Assay Kit (Active Motif), an ELISA-based method designed to specifically detect and quantify NF-κB components: p65, p50, p52, and Rel-B activation. The assay was performed according to the manufacturer’s protocol.
Statistical analysis
Statistical significance was calculated using the Student t test for independent variables. P values < .05 were considered statistically significant.
Results
MMP-9 expression was upregulated by Hes1
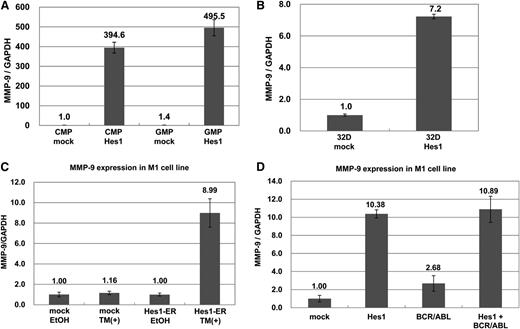
To uncover the transcriptional targets of Hes1, we performed microarray expression analysis (GEO accession #GSE56921) of Hes1- and mock-transduced CMPs. The results showed that several genes, including transglutaminase2 (Tgm2), CXCL3, CCL2, and MMP-9, were upregulated at least tenfold between Hes1-transduced CMPs vs mock-transduced CMPs (supplemental Table 1). Among them, we focused on MMP-9 because it is known that MMP-9 is critical to enhancing hematopoiesis through its ability to release the sKitL. Real-time RT-PCR analysis confirmed that transduction with Hes1 in GMPs as well as CMPs strongly upregulated MMP-9 (Figure 1A). Likewise, transduction with Hes1 upregulated MMP-9 in murine myeloid cell line 32Dcl3 (Figure 1B). To further examine the effect of Hes1 on MMP-9 expression, we used an estrogen receptor (ER)-based inducible system,21 in which murine myeloid cell line M1 was transduced with Hes1 fused to ER (Hes1-ER). The addition of 4-hydroxy-tamoxifen (4-OHT) strongly upregulated MMP-9 mRNA expression in Hes1-ER–transduced M1 cells but not in mock-transduced M1 cells (Figure 1C). Because MMP-9 was reported to be secreted by BCR-ABL+ cells,22 we also tested whether BCR-ABL upregulated MMP-9. Real-time RT-PCR analysis of M1 cells expressing BCR-ABL and/or Hes1 demonstrated that MMP-9 upregulation was induced by BCR-ABL alone, but at a lower level than by Hes1 alone (Figure 1D and supplemental Figure 1A). We found no synergistic upregulation of MMP-9 by BCR-ABL and Hes1 (Figure 1D). Thus, Hes1 strongly upregulated MMP-9 in CMPs, GMPs, and myeloid cell lines.
MMP-9 expression was upregulated by Hes1. (A) Real-time RT-PCR for MMP-9 in Hes1- or mock-transduced CMPs and GMPs. (B) Real-time RT-PCR for MMP-9 in Hes1- or mock-transduced 32Dcl3 cells. (C) Real-time RT-PCR for MMP-9 in Hes1-ER– or mock-transduced M1 cells cultured in the presence or absence of 4-hydroxy-tamoxifen (4-OHT) for 60 hours. (D) Real-time RT-PCR for MMP-9 in M1 cells transduced with Hes1, BCR-ABL, BCR-ABL and Hes1, or mock. (A-D) Expression levels were normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. The relative expression level of mock-transduced CMPs (A), mock-transduced 32Dcl3 cells (B), mock-transduced M1 cells cultured in the absence of 4-OHT (C), or mock-transduced M1 cells (D) was defined as 1. All data points correspond to the mean and the standard deviation (SD) of 3 independent experiments.
MMP-9 expression was upregulated by Hes1. (A) Real-time RT-PCR for MMP-9 in Hes1- or mock-transduced CMPs and GMPs. (B) Real-time RT-PCR for MMP-9 in Hes1- or mock-transduced 32Dcl3 cells. (C) Real-time RT-PCR for MMP-9 in Hes1-ER– or mock-transduced M1 cells cultured in the presence or absence of 4-hydroxy-tamoxifen (4-OHT) for 60 hours. (D) Real-time RT-PCR for MMP-9 in M1 cells transduced with Hes1, BCR-ABL, BCR-ABL and Hes1, or mock. (A-D) Expression levels were normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. The relative expression level of mock-transduced CMPs (A), mock-transduced 32Dcl3 cells (B), mock-transduced M1 cells cultured in the absence of 4-OHT (C), or mock-transduced M1 cells (D) was defined as 1. All data points correspond to the mean and the standard deviation (SD) of 3 independent experiments.
Hes1 upregulates MMP-9 by activating the NF-κB signaling pathway
To determine whether MMP-9 was transcriptionally upregulated by Hes1, 293T cells were transiently transfected with a luciferase vector driven by the MMP-9 promoter (Figure 2A, left panel) with Hes1 expression plasmid or empty vector. Luciferase activity was determined 48 hours after the transfection. Hes1-transfected cells exhibited twofold higher MMP-9 promoter activity compared with mock-transfected cells (Figure 2A, right panel). MMP-9 gene expression is known to be controlled by multiple transcription factors, including NF-κB, SP-1, and AP-1.23 To clarify which transcription factor was involved in Hes1-mediated activation of the MMP-9 promoter, 293T cells were transiently transfected with a luciferase vector driven by MMP-9 promoter constructs lacking the NF-κB binding site (Figure 2A, middle panel) or having the mutation in both the SP-1 and AP-1 sites (Figure 2A, lower panel) with Hes1 expression plasmid or empty vector. Deleting the NF-κB binding site from the full-length MMP-9 promoter remarkably lowered Hes1-mediated activation of MMP-9 promoter (Figure 2A, middle panel). Conversely, mutations of the SP-1 and AP-1 binding sites within the full-length MMP-9 promoter did not affect Hes1-mediated activation of MMP-9 promoter (Figure 2A, lower panel). These results suggested that NF-κB signaling was required for MMP-9 upregulation by Hes1. Next, to examine whether NF-κB was activated by Hes1, 293T cells were transfected with Hes1 expression plasmid or empty vector, and the super-repressor form of IκBα (SR-IκBα) expression plasmid or empty vector, together with NF-κB-luciferase reporter plasmid. Hes1 expression increased the luciferase activity of NF-κB, which was dampened by co-expression of SR-IκBα (Figure 2B, left panel). We confirmed that tumor necrosis factor-alpha (TNFα)-induced increase of NF-κB luciferase activity was suppressed by co-expression of SR-IκBα in 293T cells (Figure 2B, right panel). Thus, Hes1 activated the NF-κB signaling pathway. In addition, Hes1-mediated increase of MMP-9 promoter activity was inhibited by co-expression of SR-IκBα in 293T cells (Figure 2C). We also examined which components of NF-κB were activated by Hes1 using the TransAM NF-κB Family Transcription Factor Assay Kit, demonstrating that p65 and p50 were activated by ER-based Hes1 induction in M1 cells (Figure 2D). Collectively, these results indicated that Hes1 transcriptionally upregulated MMP-9 through NF-κB activation.
Hes1 upregulated MMP-9 through activation of NF-κB signaling pathway. (A) The full-length MMP-9 (−1284/+21) (upper panel), a truncation mutant (−584/+21) (middle panel), or the full-length MMP-9 with mutations of SP-1– and AP-1–binding sites (lower panel) inserted into pGL3 basic expression vector are shown as pGL3-MMP-9-Luc, pGL3-truncated MMP-9-Luc, or pGL3-mutated MMP-9-Luc, respectively. Binding sites for the 3 transcription factors (NF-κB, SP-1, and AP-1) are shown (left panel). 293T cells were transiently transfected with either pMYs-Hes1-IG or pMYs-IG together with pGL3-MMP-9-Luc (upper panel), pGL3-truncated MMP-9-Luc (middle panel), or pGL3-mutated MMP-9-Luc (lower panel). (B) 293T cells were transiently transfected with the indicated expression plasmids (pMYs-Hes1-IG, pMYs-IG, pMX-SR-IκBα-IRES-Zeocin, pMX-IRES-Zecoin) together with pGL3-NF-κB-Luc (left panel). 293T cells were transiently transfected with either pMX-SR-IκBα-IRES-Zeocin or pMX-IRES-Zecoin together with pGL3-NF-κB-Luc. After 36 hours, transfected cells were cultured in the presence or absence of 20 ng/mL TNFα for 12 hours (right panel). (C) 293T cells were transiently transfected with pMX-SR-IκBα-IRES-Zeocin and either pMYs-Hes1-IG or pMYs-IG together with pGL3-MMP-9-Luc. (A-C) Results represent the average values for relative luciferase activity. All transfection groups were normalized with a Renilla luciferase vector as an internal control. (D) ELISA assay for NF-κB subunits in Hes1-ER– or mock-transduced M1 cells cultured in the presence or absence of 4-hydroxy-tamoxifen (4-OHT) for 48 hours. (A-D) All data points correspond to the mean and SD. Data are representative of 3 independent experiments. Statistically significant differences are shown: *P < .05, **P < .01.
Hes1 upregulated MMP-9 through activation of NF-κB signaling pathway. (A) The full-length MMP-9 (−1284/+21) (upper panel), a truncation mutant (−584/+21) (middle panel), or the full-length MMP-9 with mutations of SP-1– and AP-1–binding sites (lower panel) inserted into pGL3 basic expression vector are shown as pGL3-MMP-9-Luc, pGL3-truncated MMP-9-Luc, or pGL3-mutated MMP-9-Luc, respectively. Binding sites for the 3 transcription factors (NF-κB, SP-1, and AP-1) are shown (left panel). 293T cells were transiently transfected with either pMYs-Hes1-IG or pMYs-IG together with pGL3-MMP-9-Luc (upper panel), pGL3-truncated MMP-9-Luc (middle panel), or pGL3-mutated MMP-9-Luc (lower panel). (B) 293T cells were transiently transfected with the indicated expression plasmids (pMYs-Hes1-IG, pMYs-IG, pMX-SR-IκBα-IRES-Zeocin, pMX-IRES-Zecoin) together with pGL3-NF-κB-Luc (left panel). 293T cells were transiently transfected with either pMX-SR-IκBα-IRES-Zeocin or pMX-IRES-Zecoin together with pGL3-NF-κB-Luc. After 36 hours, transfected cells were cultured in the presence or absence of 20 ng/mL TNFα for 12 hours (right panel). (C) 293T cells were transiently transfected with pMX-SR-IκBα-IRES-Zeocin and either pMYs-Hes1-IG or pMYs-IG together with pGL3-MMP-9-Luc. (A-C) Results represent the average values for relative luciferase activity. All transfection groups were normalized with a Renilla luciferase vector as an internal control. (D) ELISA assay for NF-κB subunits in Hes1-ER– or mock-transduced M1 cells cultured in the presence or absence of 4-hydroxy-tamoxifen (4-OHT) for 48 hours. (A-D) All data points correspond to the mean and SD. Data are representative of 3 independent experiments. Statistically significant differences are shown: *P < .05, **P < .01.
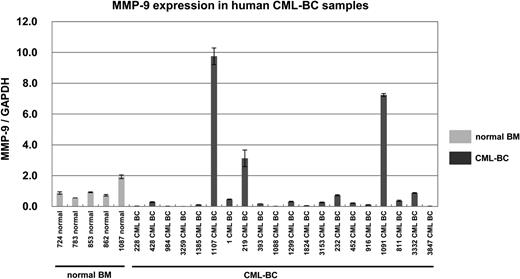
MMP-9 expression was elevated in some cases of human CML-BC samples
In a previous study, we found that HES1 mRNA levels were elevated in 8 of 20 human CML-BC samples.2 Because MMP-9 was shown to be upregulated by Hes1, we examined MMP-9 expression in the same samples used in the previous study.2 The levels of MMP-9 mRNA were measured by real-time RT-PCR in 20 samples (11 peripheral blood, 1 cerebrospinal fluid, and 8 BM) from CML-BC patients, as well as 5 BM samples from normal subjects. In 3 of 20 CML-BC samples, MMP-9 mRNA levels were found to be elevated more than threefold the average of normal BM samples (Figure 3). Clinical data and relative mRNA expression levels of HES1 and MMP-9 among samples from 20 patients with CML-BC are shown (supplemental Table 2). Notably, both HES1 and MMP-9 were highly expressed in 2 CML-BC samples. These results suggested that Hes1-mediated upregulation of MMP-9 played a certain role in the pathogenesis of CML-BC.
MMP-9 expression was elevated in 3 of 20 CML-BC patients. Real-time RT-PCR for MMP-9 in samples from healthy subjects (light shaded bar) or patients with CML-BC (dark shaded bar). Expression levels were normalized by GAPDH mRNA. RNA from normal BM cells served as a control (mean of 5 RNA levels of normal BM was defined as 1). MMP-9 mRNA levels exceeded 3.0 in 3 of 20 samples from CML-BC patients.
MMP-9 expression was elevated in 3 of 20 CML-BC patients. Real-time RT-PCR for MMP-9 in samples from healthy subjects (light shaded bar) or patients with CML-BC (dark shaded bar). Expression levels were normalized by GAPDH mRNA. RNA from normal BM cells served as a control (mean of 5 RNA levels of normal BM was defined as 1). MMP-9 mRNA levels exceeded 3.0 in 3 of 20 samples from CML-BC patients.
MMP-9 deficiency impaired cobblestonelike area-forming of CMPs, expressing BCR-ABL and Hes1, which were in conjunction with a stromal cell layer
We then asked whether MMP-9 played a role in the development of CML-BC. First, we confirmed that MMP-9 deficiency failed to influence the frequency of c-Kit+, Sca-1+, and lineage-negative HSC (KSL), CMP, or GMP in BM (supplemental Figure 2A), the frequency of spleen colony-forming unit (CFU-S) and colony-forming unit in culture (CFU-C) (supplemental Figure 2B-C), or competitive repopulation ability (supplemental Figure 2D). Thus, MMP-9 seemed to be dispensable in the function and development of normal hematopoietic stem/progenitor cells. We had previously demonstrated that the combination of Hes1 and BCR-ABL enabled CMPs to form colonies after repeated replating in the absence of cytokines.2 To examine the effect of MMP-9 on the in vitro colony formation and proliferation of CMPs expressing BCR-ABL and Hes1, WT or MMP-9-deficient CMPs were transduced with BCR-ABL and Hes1. MMP-9 deficiency did not alter the in vitro colony formation capacity of CMPs expressing BCR-ABL and Hes1 (Figure 4A). The colonies made from these 2 types of transduced CMPs showed similar morphology in the condition free from cytokines (Figure 4B). Irrespective of MMP-9 expression, BCR-ABL and Hes1 immortalized CMPs in the liquid culture without cytokines. In addition, MMP-9 deficiency did not influence the in vitro proliferation of CMPs expressing BCR-ABL and Hes1 (Figure 4C). The lack of MMP-9 activity in MMP-9–deficient CMPs, but not in WT CMPs, expressing BCR-ABL and Hes1 was verified by gelatinolytic zymography (Figure 4D). We next examined the effect of MMP-9 on in vivo migration of leukemic cells. To this end, the same numbers of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were injected through tail veins into nonirradiated recipient mice. Five days after the transplantation, the number of GFP+ and NGFR+ cells migrating to BM was estimated by flow cytometric analysis. We found that MMP-9 deficiency reduced the migration to BM of CMPs expressing BCR-ABL and Hes1 (Figure 4E). To next clarify the role of MMP-9 in self-renewal and migration of CMPs expressing BCR-ABL and Hes1 in the presence of a stromal cell layer, we used the CAFC assay. The same numbers of WT or MMP-9–deficient CMPs, expressing BCR-ABL and Hes1, were seeded in each well of a 6-well plate precoated with irradiated C3H10T1/2 cells. After co-culture for 6 days, the number of CAFCs per well was counted. The results showed that the MMP-9 deficiency decreased the number of CAFCs per inoculated CMPs expressing BCR-ABL and Hes1 (Figure 4F). These results indicated that MMP-9 promoted the migration and proliferation of leukemic cells expressing BCR-ABL and Hes1 that were in conjunction with BM stromal cells.
MMP-9 deficiency impaired cobblestone area-forming of CMPs expressing BCR-ABL and Hes1 that were in conjunction with a stromal cell layer. (A) Colony-forming assay of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1. Both types of the transduced cells were serially replatable more than 4 times. Bars indicate the number of colonies obtained per 1 × 103 cells after each round of plating in methylcellulose without cytokines. (B) Typical colonies derived from WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1. Images were obtained with an IX70 microscope and a DP70 camera (Olympus, Tokyo, Japan); an objective lens, UPlanFl (Olympus); original magnification ×100. (C) Sustained growth of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 in liquid culture without cytokines. The numbers of cells were determined every 4 days by trypan blue staining, and 1 × 105 cells per well were seeded into a 6-well plate. (D) Gelatin zymography for detection of MMP-9. Supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were collected after 24 hours of culture, and MMP-9 activity was measured by gelatinolytic zymography. (E) In vivo homing and retention of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1. Five days after 2.0 × 106 cells of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were intravenously injected into nonirradiated mice, GFP+ and NGFR+ cells migrated into BM were quantified using flow cytometry. (F) Graphic representation of CAFC numbers. Equal numbers of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were added to each well of a 6-well plate precoated with irradiated C3H10T1/2 cells. After co-culture for 6 days, CAFC numbers per well were counted. (A,C,E-F) All data points correspond to the mean and the SD of 3 independent experiments. Statistically significant differences are shown: *P < .05, **P < .01.
MMP-9 deficiency impaired cobblestone area-forming of CMPs expressing BCR-ABL and Hes1 that were in conjunction with a stromal cell layer. (A) Colony-forming assay of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1. Both types of the transduced cells were serially replatable more than 4 times. Bars indicate the number of colonies obtained per 1 × 103 cells after each round of plating in methylcellulose without cytokines. (B) Typical colonies derived from WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1. Images were obtained with an IX70 microscope and a DP70 camera (Olympus, Tokyo, Japan); an objective lens, UPlanFl (Olympus); original magnification ×100. (C) Sustained growth of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 in liquid culture without cytokines. The numbers of cells were determined every 4 days by trypan blue staining, and 1 × 105 cells per well were seeded into a 6-well plate. (D) Gelatin zymography for detection of MMP-9. Supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were collected after 24 hours of culture, and MMP-9 activity was measured by gelatinolytic zymography. (E) In vivo homing and retention of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1. Five days after 2.0 × 106 cells of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were intravenously injected into nonirradiated mice, GFP+ and NGFR+ cells migrated into BM were quantified using flow cytometry. (F) Graphic representation of CAFC numbers. Equal numbers of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were added to each well of a 6-well plate precoated with irradiated C3H10T1/2 cells. After co-culture for 6 days, CAFC numbers per well were counted. (A,C,E-F) All data points correspond to the mean and the SD of 3 independent experiments. Statistically significant differences are shown: *P < .05, **P < .01.
MMP-9 deficiency impaired the development of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT models
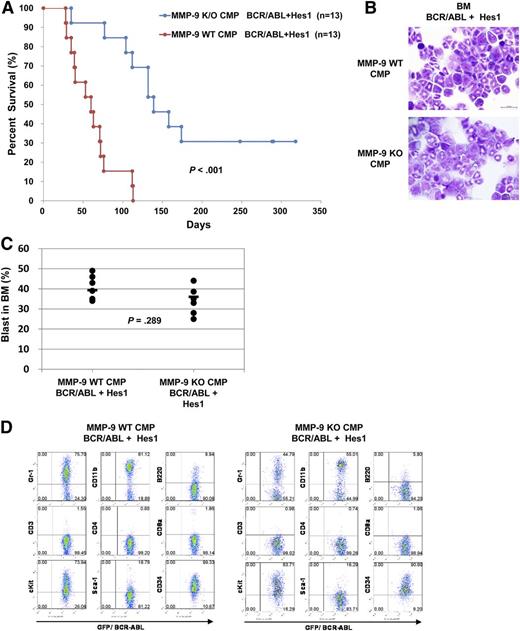
We then asked whether MMP-9 was involved in the development of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT models. WT or MMP-9–deficient CMPs infected with retroviruses harboring BCR-ABL and Hes1 were transplanted into sublethally irradiated mice. The numbers of cells injected ranged from 1.8 × 104 to 3.5 × 104. Mice receiving transplants of WT CMPs expressing BCR-ABL and Hes1 developed fatal CML-BC–like disease, with a significantly shorter latency than mice transplanted with MMP-9–deficient CMPs expressing BCR-ABL and Hes1 (Figure 5A). The tissue distribution of the disease was virtually the same among mice receiving WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1. The morbid mice invariably exhibited marked hepatosplenomegaly and an increased population of myeloid blast exceeding 20% of all nucleated BM cells, fulfilling criteria of AML or CML-BC (Figure 5B-C). The surface marker profiles of GFP+ leukemic BM cells from the morbid mice expressed CD11b, Gr-1, c-Kit, and CD34 at high levels, irrespective of whether they were derived from WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 (Figure 5D). Thus, MMP-9 deficiency delayed the progression of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT models, although it did not influence the phenotypes of the disease.
MMP-9 deficiency impaired the development of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT models. (A) Kaplan-Meier analysis for the survival of mice that received transplants of WT or MMP-9–deficient CMPs transduced with the combination of BCR-ABL and Hes1. The numbers of transplanted mice are shown. Statistically significant differences were observed in latency and penetrance (log-rank test, P = .000146). (B-D) The morbid mice transplanted with WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were used. (B) Cytospin preparations of BM cells were stained with Giemsa. A representative photo is shown. Images were obtained with a BX51 microscope and a DP12 camera (Olympus); original magnification ×100. (C) Blast ratios in the BM. The mean blast ratios in all nucleated BM cells were 39.3% ± 9.7% and 35.1% ± 8.9% in mice receiving WT or MMP-9–deficient CMPs transduced with the combination of Hes1 and BCR-ABL, respectively (2-sample Student t test with Welch correction, P = .289). (D) Flow cytometric analysis of BM cells. The dot plots show Gr-1, CD11b, B220, CD3, CD4, CD8a, c-Kit, Sca-1, and CD34 labeled with the corresponding phycoerythrin-conjugated mAb vs expression of GFP. GFP is a marker of BCR-ABL transduction.
MMP-9 deficiency impaired the development of CML-BC–like disease induced by BCR-ABL and Hes1 in mouse BMT models. (A) Kaplan-Meier analysis for the survival of mice that received transplants of WT or MMP-9–deficient CMPs transduced with the combination of BCR-ABL and Hes1. The numbers of transplanted mice are shown. Statistically significant differences were observed in latency and penetrance (log-rank test, P = .000146). (B-D) The morbid mice transplanted with WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were used. (B) Cytospin preparations of BM cells were stained with Giemsa. A representative photo is shown. Images were obtained with a BX51 microscope and a DP12 camera (Olympus); original magnification ×100. (C) Blast ratios in the BM. The mean blast ratios in all nucleated BM cells were 39.3% ± 9.7% and 35.1% ± 8.9% in mice receiving WT or MMP-9–deficient CMPs transduced with the combination of Hes1 and BCR-ABL, respectively (2-sample Student t test with Welch correction, P = .289). (D) Flow cytometric analysis of BM cells. The dot plots show Gr-1, CD11b, B220, CD3, CD4, CD8a, c-Kit, Sca-1, and CD34 labeled with the corresponding phycoerythrin-conjugated mAb vs expression of GFP. GFP is a marker of BCR-ABL transduction.
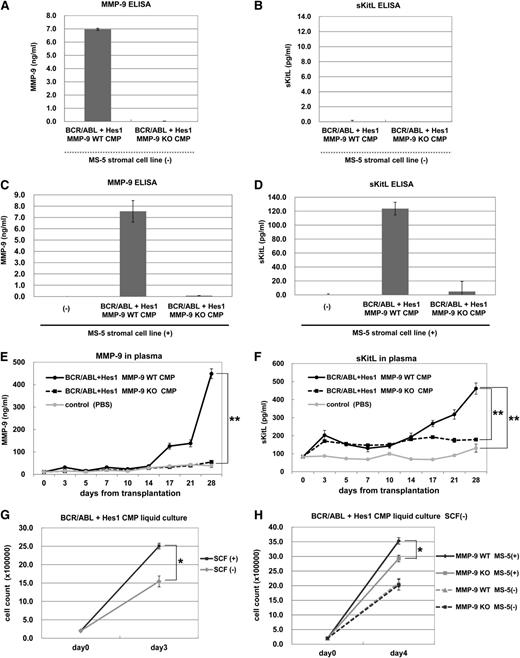
MMP-9 secreted by leukemic cells expressing BCR-ABL and Hes1 promoted the release of sKitL from stromal cells, leading to enhanced proliferation of leukemic cells
To test whether MMP-9 was secreted by leukemic cells expressing BCR-ABL and Hes1, MMP-9 levels in culture supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were measured by ELISA. The results showed that high levels of MMP-9 were secreted by WT, but not by MMP-9–deficient, CMPs expressing BCR-ABL and Hes1 (Figure 6A). We found no detectable levels of sKitL in culture supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 (Figure 6B). Because MMP-9 was known to promote the release of sKitL from stromal cells,14 we tested whether murine BM stromal cell line MS-5, expressing a membrane bound form of the Kit ligand (mKitL), released sKitL in the presence of WT CMPs expressing BCR-ABL and Hes1. We confirmed that MMP-9 was not secreted by MS-5 cells and that the co-culture of MS-5 cells and WT CMPs expressing BCR-ABL and Hes1 did not alter the levels of MMP-9 secreted by the latter cells (Figure 6C). Importantly, high levels of sKitL were detected in co-culture supernatants of WT CMPs, but not in those of MMP-9–deficient CMPs, with MS-5 cells (Figure 6D). It should be noted that sKitL was not released by MS-5 cells alone (Figure 6D). We also confirmed that transduction with SR-IκBα decreased protein levels of MMP-9 released by CMPs expressing BCR-ABL and Hes1 (supplemental Figure 3A), resulting in the decrease of sKitL secreted by MS-5 cells (supplemental Figure 3B). Moreover, downregulation of MMP-9 by SR-IκBα lowered the number of CAFCs in CMPs expressing BCR-ABL and Hes1 (supplemental Figure 3C). These results indicated that CMPs expressing BCR-ABL and Hes1 upregulated and secreted MMP-9 through NF-κB activation, which induced the release of sKitL from the stromal cells promoting the migration and proliferation of leukemic cells. We next asked whether plasma levels of MMP-9 and sKitL were elevated in mice with CML-BC–like disease induced by BCR-ABL and Hes1. WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were transplanted into the sublethally irradiated mice, and the plasma levels of MMP-9 and sKitL were measured after the transplantation. We found that the plasma levels of sKitL and MMP-9 gradually increased in mice transplanted with WT CMPs expressing BCR-ABL and Hes1 (Figure 6E-F). In contrast, the plasma levels of MMP-9 and sKitL in mice transplanted with MMP-9–deficient CMPs expressing BCR-ABL and Hes1 did not increase as well as those in phosphate buffered saline–injected mice as control (Figure 6E-F). To delineate the effect of sKitL on the proliferation of CMPs expressing BCR-ABL and Hes1, these leukemic cells were cultured in the presence or absence of SCF. The results showed that exogenous SCF stimulated the growth of CMPs expressing BCR-ABL and Hes1 (Figure 6G). Moreover, when WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 were cultured with or without MS-5 cells, co-culture with MS-5 cells more strongly induced the proliferation of WT leukemic cells compared with MMP-9–deficient leukemic cells. (Figure 6H). Taken together, these results indicated that leukemic cells expressing BCR-ABL and Hes1 secreted MMP-9, which stimulated the release of sKitL from stromal cells, leading to enhanced proliferation of leukemic cells.
MMP-9 secreted by leukemic cells expressing BCR-ABL and Hes1 promoted the release of sKitL from stromal cells. (A-B) Levels of MMP-9 (A) or sKitL (B) in culture supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 cultured for 24 hours were measured by ELISA. (C-D) Levels of MMP-9 (C) or sKitL (D) in culture supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 that had been co-cultured with MS-5 stromal cells for 24 hours were measured by ELISA. (E-F) Plasma levels of MMP-9 (E) or sKitL (F) at the indicated days in the recipient mice transplanted with WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 or in mice without transplantation were measured by ELISA. (G) Growth of WT CMPs expressing BCR-ABL and Hes1 in the presence or absence of 50 ng/mL SCF. (H) Growth of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 cultured with or without MS-5 cells without SCF. (G-H) The numbers of cells were determined by trypan blue staining. (G) 2 × 105 cells per well were seeded into a 6-well plate. (H) 1 × 105 cells per well were seeded into a 6-well plate. (A-H) All data points correspond to the mean and the SD of 3 independent experiments. (E-H) Statistically significant differences are shown: *P < .05, **P < .01.
MMP-9 secreted by leukemic cells expressing BCR-ABL and Hes1 promoted the release of sKitL from stromal cells. (A-B) Levels of MMP-9 (A) or sKitL (B) in culture supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 cultured for 24 hours were measured by ELISA. (C-D) Levels of MMP-9 (C) or sKitL (D) in culture supernatants of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 that had been co-cultured with MS-5 stromal cells for 24 hours were measured by ELISA. (E-F) Plasma levels of MMP-9 (E) or sKitL (F) at the indicated days in the recipient mice transplanted with WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 or in mice without transplantation were measured by ELISA. (G) Growth of WT CMPs expressing BCR-ABL and Hes1 in the presence or absence of 50 ng/mL SCF. (H) Growth of WT or MMP-9–deficient CMPs expressing BCR-ABL and Hes1 cultured with or without MS-5 cells without SCF. (G-H) The numbers of cells were determined by trypan blue staining. (G) 2 × 105 cells per well were seeded into a 6-well plate. (H) 1 × 105 cells per well were seeded into a 6-well plate. (A-H) All data points correspond to the mean and the SD of 3 independent experiments. (E-H) Statistically significant differences are shown: *P < .05, **P < .01.
Discussion
We previously reported that a substantial population of patients with CML-BC, but not CML-CP, displayed a high expression of HES1 in their leukemic cells. We also demonstrated that co-expression of BCR-ABL and Hes1 induced CML-BC–like disease in the mice BMT model and implicated Hes1-mediated differentiation block in hematopoietic cells.2 Because the differentiation block in progenitor stages is the mainstay for the BC transition in CML,1 downregulation of C/EBPα by Hes1 could be critical in BC transition in CML. In the present study, we sought to identify the downstream events of Hes1 other than C/EBPα downregulation, demonstrating that MMP-9 was upregulated by Hes1 via activation of NF-κB. In fact, MMP-9 was highly expressed in 3 of 20 samples from patients with CML-BC, among which two exhibited high levels of HES1 expression. Although MMP-9 was extremely enhanced by Hes1 in in vitro experiments, it is not clear why expression levels of HES1 and those of MMP-9 did not completely correlate in the human CML-BC samples. Because it is known that BC is caused by many different mechanisms and confers vast heterogeneity in CML patients, this heterogeneity could affect multiple regulators of MMP-9 expression, some of which might downregulate its expression. Concerning the importance of MMP-9 in the progression of CML-BC–like disease in mice, we showed that MMP-9 deficiency profoundly delayed the onset of CML-BC–like disease induced by BCR-ABL and Hes1 in the mouse BMT model. In the absence of MMP-9, some mice actually did not show any signs of the disease at the time of 248 to 318 days from transplantation. We speculate that the decreased migration and attenuated proliferation of MMP-9-KO CMPs were responsible for the engraftment and propagation of CML-BC cells, respectively (Figure 7). CMPs engineered to express BCR-ABL and Hes1 secreted MMP-9, which promoted the release of sKitL from co-culturing stromal cells in vitro. Mice transplanted with the CMPs expressing BCR-ABL and Hes1 exhibited high levels of sKitL as well as MMP-9 in the serum, thereby leading to enhanced proliferation of the leukemic cells (Figure 7). It is also possible that MMP-9 promotes not only the release of sKitL but also the release of other cell surface–bound cytokines that enhance the proliferation of leukemic cells.
Schematic model of the molecular mechanism by which high levels of Hes1 expression promote the development of CML-BC. Hes1 expression is frequently upregulated in BCR-ABL+ leukemic cells, in which Hes1 upregulates MMP-9 through NF-κB activation. MMP-9 is secreted by leukemic cells and promotes migration and proliferation of leukemic cells that are in conjunction with stromal cells. In addition, MMP-9 sheds sKitL from stromal cells, and increased sKitL enhances proliferation of leukemic cells.
Schematic model of the molecular mechanism by which high levels of Hes1 expression promote the development of CML-BC. Hes1 expression is frequently upregulated in BCR-ABL+ leukemic cells, in which Hes1 upregulates MMP-9 through NF-κB activation. MMP-9 is secreted by leukemic cells and promotes migration and proliferation of leukemic cells that are in conjunction with stromal cells. In addition, MMP-9 sheds sKitL from stromal cells, and increased sKitL enhances proliferation of leukemic cells.
The mechanism by which Hes1 is upregulated in CML-BC is not yet known. However, one study has suggested a possible mechanism of Hes1 upregulation in CML-BC: Ito et al reported that an RNA-binding protein Musashi2 (MSI2) expression is highly upregulated in CML-BC patients and that repression of NUMB by MSI2 promotes BC transition in CML, implicating MSI2 in BC transition.3 Consistent with our findings, they also showed that HES1 expression was elevated in a number of CML-BC patients with high expression of MSI2.3 Interestingly, Numb is known as a repressor of the Notch pathway; therefore, repression of NUMB by MSI2 could lead to HES1 upregulation in CML-BC patients. We also examined the expression levels of HES1-related genes such as JUNB,24 E2A,25 and GLI126 in human CML-BC samples by real-time RT-PCR in 20 samples from CML-BC patients (supplemental Table 3). Notably, 2 samples expressed high levels of both JUNB and HES1, whereas 3 samples expressed those of both GLI1, downstream of Sonic Hedgehog signaling, and HES1. These results suggested that upregulation of JUNB or activation of Sonic Hedgehog signaling, which is known to act upstream of Hes1, could lead to HES1 upregulation in CML-BC patients. Consistent with our findings, Zhao et al reported that Hedgehog signaling is essential for the maintenance of cancer stem cells in CML.27
Recently, it has been shown that the Notch/Hes1 pathway induces NF-κB activation through repression of a deubiquitinase CYLD, a negative regulator of the IκB kinase complex.28 In addition, it was previously reported that NF-κB stimulates expression of MMP-9.29 However, our report is the first to show the connection between overexpression of Hes1 and upregulation of MMP-9 in CML-BC cells. In our study, CYLD was not repressed by Hes1 (data not shown), implying that Hes1 could activate NF-κB through an alternative pathway other than repressing CYLD in CML cells.
Thus the current results indicate that the Hes1-NF-κB-MMP-9 axis is critical in the progression of CML-BC. MMPs are a family of endopeptidases excreted by a number of cell types, capable of cleaving several molecules of the extracellular matrix. Because of their capacity to degrade the extracellular matrix, MMPs (especially MMP-2 and MMP-9) are known to be critical to angiogenesis, tumor growth, and metastasis.30 In acute myeloid leukemia (AML), several studies have shown that expression of MMP-9 is critical to the progression of AML31,32 or to the migration of leukemic cells.33,34 Conversely, there are some reports suggesting that MMP-9 is not important in the progression of AML.35,36 This inconsistency may be related to the heterogeneity of the disease, and further studies will be needed to ascertain the significance of MMP-9 in AML.
There have been several reports indicating that MMP-9 is highly expressed in CML cells. First, a microarray analysis revealed that MMP-9 was highly upregulated in the leukemic cells of 27 CML patients when compared with normal cells.37 Another group reported that the expression of MMP-9 was generally high in mononuclear cells from CML patients.38 It was also reported that myeloblasts expanded from BM CD34+ cells derived from Philadelphia-positive CML patients secreted various angiogenesis-promoting factors, including MMP-9.22 Thus, overexpression of MMP-9 may occur frequently in CML. However, the mechanism by which MMP-9 is upregulated and the role of MMP-9 in the pathogenesis of CML have not been extensively investigated. Importantly, our study is the first paper reporting the significance of Hes1-NF-κB-MMP-9 axis in upregulation of MMP-9 in CML-BC. We also demonstrated that MMP-9 plays important roles in enhancing proliferation of CML-BC cells using an in vitro co-culture system of CML-BC cells and stromal cell lines, and that MMP-9 deficiency delayed the onset of CML-BC–like disease in the mouse BMT model.
We showed that transduction with Hes1 in CMPs, as well as in other cell lines such as 32Dcl3 or M1, strongly upregulated expression of MMP-9 (Figure 1A-C), and we observed that sKitL was upregulated by WT CMPs expressing BCR-ABL and Hes1 in vitro (Figure 6D) and in vivo (Figure 6F). However, considering that MMP-3 promotes hyperplastic epithelial growth in a nonproteolytic manner,39 it is possible that proliferation of CML-BC cells is also dependent on MMP-9 functions other than its enzymatic activity. Although we tried to test the importance of the enzymatic activity of MMP-9 in the CML-BC progression by transducing the WT MMP-9, an enzymatically inactive mutant of MMP-9, or an empty vector as a control to MMP-9–deficient CMPs transformed by BCR-ABL and Hes1, the transduced cells tended to partially lose their ability to induce the disease in vivo even when the empty vector was transduced. Thus, it was difficult to clarify this point at present. However, considering the increased concentrations of sKitL in the serum of the leukemic mice induced by BCR-ABL and Hes1 (Figure 6F), it is likely that the enzymatic activity of MMP-9 would contribute to the progression of CML-BC in the mice model. Further studies will be needed to address this point.
As indicated in our previous study, development of CML-BC by overexpression of Hes1 was perhaps mainly induced by differentiation block resulting from downregulation of C/EBPα by Hes1. In the present study, we demonstrated that Hes1 contributes to the progression of CML-BC, working as a positive regulator for migration and proliferation of CML-BC cells through extensive upregulation of MMP-9. Although further studies are needed, considering that MMP-9 enables BM repopulating cells to translocate to a vascular niche through shedding of sKitL,14 overexpression of MMP-9 by Hes1 may also enhance the translocation of CML-BC cells to vascular niches, leading to the effective proliferation and progression of the disease.
In summary, we have demonstrated that overexpression of MMP-9 induced by Hes1 upregulation plays some part in the progression of CML-BC. This novel finding may lead to a better therapeutic strategy for this difficult disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr R. Kageyama for the Hes1 cDNA, Dr Jianming Xu for the pGL3-MMP9-Luc vector, Dr A. Brent Carter for the luciferase vector of MMP-9 deletion construct and MMP-9 promoter with mutations in SP-1 and AP-1, and Dr Shoji Yamaoka for the pMX-SR-IκBα-IRES-Zeocin construct. They are grateful to Dr Dovie Wylie for her excellent editing of the English.
This study was supported by Grants-in-aid for Scientific Research on Innovative Areas, MEXT, Japan; Grants-in-aid for Scientific Research (A), JSPS, Japan; and Grants-in-aid for Young Scientists (B), JSPS, Japan.
Authorship
Contribution: F.N. performed all of the experiments and wrote most of the manuscript; J.K. actively participated in designing the experiments and manuscript writing; T.U. and C.N. actively assisted with the experiments and designing the experiments; K.T., D.I., T.M., Y.Kagiyama, Y.E., K.C.K., L.C.-Y., Y.Komeno, K.I., and T.O. assisted with the experiments; G.N. and H.A. assisted with microarray analysis; Y.H. and H.H. provided human samples; M.O. participated in interpretation and designing the experiments; B.H. and K.H. provided MMP-9 KO mice and actively participated in designing the experiments; and T.K. conceived the project, secured funding, and participated in manuscript writing.
Conflict-of-interest disclosure: T.K. serves as a consultant for R&D Systems and Rigel Pharmaceuticals. The remaining authors declare no competing financial interests.
The current affiliation for Y.H. and H.H. is Department of Hematology, Juntendo University School of Medicine, Tokyo, Japan. The current affiliation for F.N. is Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine Research, Departments of Cell Biology and Medicine, Albert Einstein Cancer Center, Albert Einstein College of Medicine, Bronx, NY.
Correspondence: Toshio Kitamura, Division of Stem Cell Signaling, Center for Stem Cell Therapy, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail: kitamura@ims.u-tokyo.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal