To the editor:
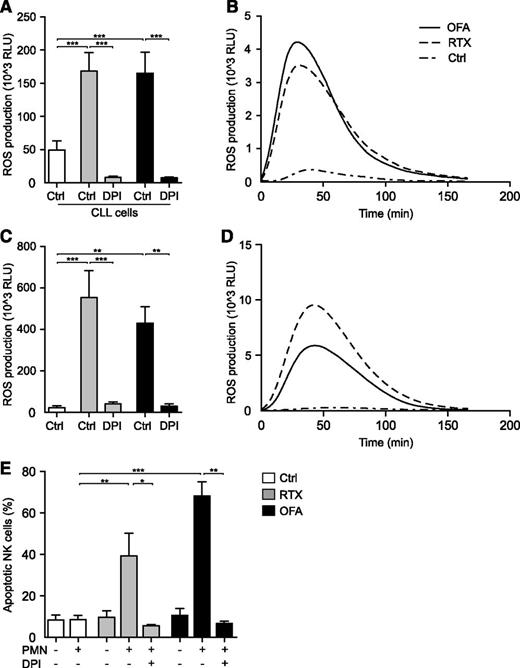
In a recent study, Golay and coworkers address the commonly overlooked role of neutrophils in therapy of chronic lymphocytic leukemia (CLL) with monoclonal antibodies (mAbs).1 Convincingly, they demonstrate that both rituximab (RTX) and, to a greater extent, glycoengineered obinituzumab, trigger neutrophil effector functions via the Fc receptors CD16b and CD32. Moreover, utilizing a 2′,7′-dichlorofluorescein diacetate (H2DCFDA)–based assay, the authors claim that neutrophil activation occurs without concomitant production of reactive oxygen species (ROS; oxygen radicals). However, the experimental approach used to assess ROS production has serious limitations: first, H2DCFDA assays are relatively insensitive and unspecific2 and second, in the study, neutrophils were stained with a fluorescein-conjugated antibody preventing the ability to correctly assess the ROS formation in response to CD20 antibodies with a fluorescein-based assay. Thus, in a series of experiments, we used a sensitive isoluminol-enhanced chemiluminescence method to monitor ROS responses in neutrophils exposed to CD20 mAbs or αCD20-opsonized leukemic cells. These experiments showed that malignant CLL cells in the presence of either RTX or the second-generation agent ofatumumab (OFA), triggered a robust extracellular release of oxygen radicals from neutrophils. ROS production was readily blocked by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor diphenyleneiodonium (DPI). Similar results were obtained in experiments where we exposed CLL patient-derived neutrophils to immobilized CD20 mAbs (Figure 1A-D).
Induction of neutrophil ROS production by αCD20 mAbs. PMNs were isolated by dextran sedimentation followed by standard density gradient centrifugation. (A) PMNs from healthy donors were assessed for ROS production by isoluminol-enhanced chemiluminescence in the presence of purified CLL cells (CLL:PMN ratio, 1:2) and the presence or absence of soluble RTX (10 μg/mL; Roche) or OFA (10 μg/mL; GlaxoSmithKline) and DPI (3μM; Sigma-Aldrich; n = 5). (B) Individual kinetic graph from 1 experiment shown in panel A. (C) PMNs derived from patients with CLL in the presence or absence of plate-bound RTX or OFA (10 μg/mL; n = 4). (D) Individual kinetic graph from 1 experiment shown in panel C. (E) NK cell death in coculture experiments. PMNs and NK cells were added to 96-well plates, previously coated with RTX or OFA, at a ratio of 1:1. After 16 hours at 37°C, cells were washed and stained with the live/dead fixable dead cell stain kit (Invitrogen) and NK cell viability was assessed by flow cytometry (n = 4-9). Error bars represent SEM. Data in panels A and C display total ROS production (area under curve). Statistical analyses were performed using 1-way ANOVAs with Bonferroni post hoc test for panels A and C and the Mann-Whitney U test for panel E. *P ≤ .05, **P ≤ .01, ***P ≤ .001. ANOVA, analysis of variance; PMN, polymorphonuclear cell; RLU, relative light units.
Induction of neutrophil ROS production by αCD20 mAbs. PMNs were isolated by dextran sedimentation followed by standard density gradient centrifugation. (A) PMNs from healthy donors were assessed for ROS production by isoluminol-enhanced chemiluminescence in the presence of purified CLL cells (CLL:PMN ratio, 1:2) and the presence or absence of soluble RTX (10 μg/mL; Roche) or OFA (10 μg/mL; GlaxoSmithKline) and DPI (3μM; Sigma-Aldrich; n = 5). (B) Individual kinetic graph from 1 experiment shown in panel A. (C) PMNs derived from patients with CLL in the presence or absence of plate-bound RTX or OFA (10 μg/mL; n = 4). (D) Individual kinetic graph from 1 experiment shown in panel C. (E) NK cell death in coculture experiments. PMNs and NK cells were added to 96-well plates, previously coated with RTX or OFA, at a ratio of 1:1. After 16 hours at 37°C, cells were washed and stained with the live/dead fixable dead cell stain kit (Invitrogen) and NK cell viability was assessed by flow cytometry (n = 4-9). Error bars represent SEM. Data in panels A and C display total ROS production (area under curve). Statistical analyses were performed using 1-way ANOVAs with Bonferroni post hoc test for panels A and C and the Mann-Whitney U test for panel E. *P ≤ .05, **P ≤ .01, ***P ≤ .001. ANOVA, analysis of variance; PMN, polymorphonuclear cell; RLU, relative light units.
A significant part of the benefit of CD20 mAbs in therapy of CLL is attributed to antibody-dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells.3,4 However, cytotoxic NK cells are also highly sensitive to oxygen radical-mediated inactivation.5,6 Thus, we investigated whether αCD20-induced neutrophil ROS production had an impact on NK-cell viability.5 Indeed, we found that NK cells displayed significant cell death after exposure to neutrophils in the presence of either RTX or OFA, but not to either agent alone. The addition of DPI rescued NK cells, strongly suggesting NADPH oxidase– and ROS-dependent NK cell death (Figure 1E). During the course of these experiments we did not have access to the glycoengineered antibody obinituzumab, but given its profound capacity to stimulate neutrophils, it is likely to share the ROS-triggering characteristics of RTX and OFA.
Collectively, our findings raise the question of whether oxygen radical release from αCD20-exposed neutrophils may inactivate NK cells also in vivo and thus limit the efficacy of therapeutic mAbs in CLL. More studies are warranted to investigate whether neutrophils or neutrophil-derived ROS are important effector arms in antibody treatment of CLL, and whether it may be beneficial to supplement αCD20 therapy with antioxidative strategies to unravel the full effector function of NK cells in CLL.
Approval was obtained from the Ethical Review Board of Gothenburg for these experiments. Informed consent was provided according to the Declaration of Helsinki.
Authorship
Acknowledgments: This work was supported by the Göteborg Medical Society, the Wilhelm and Martina Lundgren Foundation, the Assar Gabrielsson Foundation, and the Swedish Cancer Society.
Contribution: O.W., R.E.R., and M.S. performed experiments; O.W. analyzed results and made the figure; and O.W., J.A., and F.B.T. designed the research and wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olle Werlenius, Sahlgrenska Cancer Center, University of Gothenburg, Box 425, 405 30 Gothenburg, Sweden; e-mail: olle.werlenius@gu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal