To the editor:
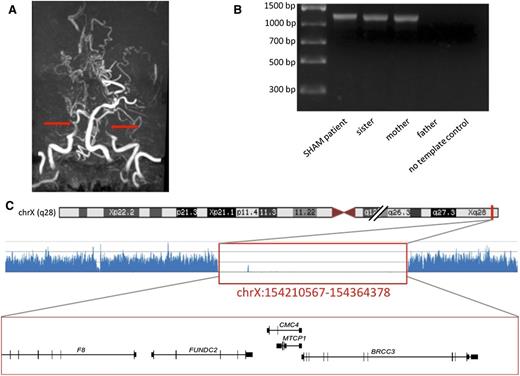
A 10-year-old boy with severe hemophilia A and no other obvious morbidity arrived at the hospital with focal neurological signs and a suspected intracranial hemorrhage. Surprisingly, radiological studies demonstrated an ischemic stroke. Neither active thromboembolism nor genetic predisposition to thrombosis was found. Neuroimaging demonstrated severe narrowing of internal carotid arteries and their branches and development of a collateral vascular network, diagnostic of moyamoya syndrome (Figure 1). Further clinical workup revealed mild facial dysmorphia, hypertension, osteopenia, and duplication of the right renal artery, a phenotype likely caused by a genetic aberration. Next-generation sequencing followed by long-range polymerase chain reaction (Figure 1 and supplemental Materials) demonstrated a large Xq28 deletion of ∼150 kbp encompassing exons 1 to 6 of F8, as well as the FUNDC2, MTCP1NB, MTCP1, and BRCC3 genes. BRCC3 was recently identified as a familial moyamoya gene.1 We demonstrate that both centromeric and telomeric breakage sites of the deletion are located in nearly identical repetitive Alu sequences that could be mutational hotspots. The patient’s sister and mother are heterozygous for the same deletion. At the age of 18, the sister presented a mild phenotype including low levels of factor VIII (22%), aortic coarctation, and hypertension, but she has no signs of moyamoya angiopathy.
The radiological and genetic diagnosis of Xq28 linked moyamoya in hemophilia A patient. (A) MRI angiography, the arrows show truncation of the internal carotid arteries. (B) Long-range PCR products in 4 members of the family. (C) NGS trace (the location shown on chromosome ideogram) below genes located within the region, which corresponds to the long-range PCR-confirmed Xq28 deletion in a SHAM patient (indicated with red box).
The radiological and genetic diagnosis of Xq28 linked moyamoya in hemophilia A patient. (A) MRI angiography, the arrows show truncation of the internal carotid arteries. (B) Long-range PCR products in 4 members of the family. (C) NGS trace (the location shown on chromosome ideogram) below genes located within the region, which corresponds to the long-range PCR-confirmed Xq28 deletion in a SHAM patient (indicated with red box).
A review of the literature yields 3 more likely individuals/families with this novel severe hemophilia and moyamoya (SHAM) syndrome: 1 clinical description in a Japanese patient2 and 2 descriptions of Xq28 rearrangements in hemophilia A patients that disrupt BRCC3 and bear striking clinical similarity to Xq28-linked familial moyamoya, although no neuroimaging data are available to confirm the diagnosis.3,4 There is also a genetic report of BRCC3 deletion in a hemophilia patient without phenotype data.5 The ratio of BRCC3 inactivation in hemophilia A is unknown because the regions telomeric to F8 are rarely subjected to genetic diagnostics. We accessed the Centers for Disease Control Hemophilia A mutation project database that contains >2000 pathological F8 mutations reported worldwide. This reports 5.9% of cases with large structural variation among hemophilia A patients, with a rate of large deletions of 4.7% and a rate of deletions affecting exon 1 of 1.1%. It has not been determined how many of these lesions extend to other genes. It must be considered, however, that in some patients, hemophilia A comorbidity, including well-documented osteopenia, hypertension, and reduced growth velocity,6-8 might be exacerbated by genomic disruption or defective regulation of genes other than F8. We conclude hemophilia A may be associated with moyamoya angiopathy, and the patients with SHAM syndrome are at risk of ischemic stroke.
Because of features of cerebral circulation insufficiency, our patient underwent neurosurgical intervention (indirect bypass revascularization) and is clinically stable with no ischemic episodes at 2.5 years after surgery. The prognosis is poor because of multiorgan abnormalities expected in familial moyamoya linked to Xq28.1
The online version of this article contains a data supplement.
Authorship
Contribution: A.F., M. Koblowska, A.P., D.B., O.W., M. Kostrazewska, P.L., and. M.B. performed experiments and analyzed results; and S.J. and W.M. designed the research, performed experiments, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wojciech Mlynarski, Department of Pediatrics, Oncology, Hematology and Diabetology, Medical University of Lodz, 36/50 Sporna Str, 91-738 Lodz, Poland; e-mail: wojciech.mlynarski@umed.lodz.pl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal