Key Points
The addition of valproic acid to intensive induction therapy in combination with all-trans retinoic acid did not result in an improvement of clinical outcome.
Valproic acid-related hematologic toxicity and higher death rates were observed when valproic acid and idarubicin were given in parallel.
Abstract
The outcome of patients with acute myeloid leukemia who are older than 60 years has remained poor because of unfavorable disease characteristics and patient-related factors. The randomized German-Austrian AML Study Group 06-04 protocol was designed on the basis of in vitro synergistic effects of valproic acid (VPA) and all-trans retinoic acid with chemotherapy. Between 2004 and 2006, 186 patients were randomly assigned to receive 2 induction cycles with idarubicin, cytarabine, and all-trans retinoic acid either with VPA or without (STANDARD). In all patients, consolidation therapy was intended. Complete remission rates after induction tended to be lower in VPA compared with STANDARD (40% vs 52%; P = .14) as a result of a higher early death rate (26% vs 14%; P = .06). The main toxicities attributed to VPA were delayed hematologic recovery and grade 3/4 infections, observed predominantly during the second induction cycle. After restricting VPA to the first induction cycle and reducing the dose of idarubicin, these toxicities dropped to rates observed in STANDARD. After a median follow-up time of 84 months, event-free and overall survival were not different between the 2 groups (P = .95 and P = .57, respectively). However, relapse-free-survival was significantly superior in VPA compared with STANDARD (24.4% vs 6.4% at 5 years; P = .02). Explorative subset analyses revealed that AML with mutated Nucleophosmin 1 (NPM1) may particularly benefit from VPA. This trial was registered at www.clinicaltrials.gov as #NCT00151255.
Introduction
Acute myeloid leukemia (AML) is predominantly a disease of older patients. Based on the Swedish population-based registry study, the median age at diagnosis is 71 years.1 Although general improvement has been observed in the prognosis of younger adults during the last years, treatment results in older patients have remained poor.2 Patient-specific factors independently predict increased treatment-related mortality, especially during the induction phase of treatment,3,4 whereas leukemia-intrinsic factors, and in particular cytogenetic and molecular factors, are the most powerful prognostic determinants with regard to response to induction therapy and survival.2,5-7 To advance the therapeutic field of older patients with AML, less-toxic and more-specific/more-targeted therapies are urgently needed to overcome resistance to chemotherapy; in the best case, without affecting on toxicity.
Epigenetic therapy in cancer is a relatively recent concept that has produced positive results in some hematologic malignancies. In AML, epigenetic therapy with the demethylating agents azacitidine and decitabine has shown promising effects.8,9 In addition, modification of histone acetylation status may influence the transcription of genes involved in differentiation and cycle-control aberrantly deacetylated in malignant cells, thereby overcoming the differentiation block.10,11
Valproic acid (VPA), a short-chain fatty acid used for decades as an anticonvulsant, has been shown also to be a potent histone deacetylase (HDAC) inhibitor, inducing differentiation and/or apoptosis of AML blasts.12-14 Preclinical studies demonstrated an even more potent induction of differentiation and apoptosis in leukemic cells when HDAC inhibitors (with VPA among them) are combined with the differentiating agent all-trans retinoic acid (ATRA).15
When tested together in phase 2 to 3 studies for the treatment of myelodysplastic syndromes and AML, ATRA and VPA showed interesting results in terms of hematologic improvement and decrease of transfusion needs, even if no significant percentage of complete remission (CR) could be achieved.16-18
With respect to ATRA, the German-Austrian AML Study Group (AMLSG) showed a beneficial effect on outcome of ATRA as adjunct to intensive chemotherapy with a safe toxicity profile.6,19 Thus, on the basis of the positive results of the randomized AMLHD98B study,19 ATRA as adjunct to intensive chemotherapy was included in the standard treatment. The biologic evidence indicating high effectiveness of a combination therapy of VPA and ATRA, together with the previous positive experience with those agents, provided the basis for the AMLSG 06-04 study protocol.
The aim of the study was to evaluate the efficacy and toxicity profile of VPA as adjunct to intensive induction therapy plus ATRA for patients older than 60 years who were candidates for an intensive approach.
Patients and methods
Patients
Patients older than 60 years with newly diagnosed AML, including de novo AML, secondary AML with a preceding history of myelodysplastic or myeloproliferative disorder, and therapy-related AML after treatment of a primary malignancy, as defined by the World Health Organization 2001 classification, were eligible for the trial.20 Patients with acute promyelocytic leukemia as well as patients with concomitant renal (creatinine >1.5× upper normal serum level), liver (bilirubin, aspartate aminotransferase or alcaline phosphatase > 2× upper normal serum level), or cardiac (New York Heart Association III/IV) dysfunction; uncontrolled infectious disease; primary coagulation disturbance; or performance status (Eastern Cooperative Oncology Group) higher than 2 were excluded. Written informed consent was obtained from all patients. The protocol was approved by the local ethics review committees of each participating site and registered at www.clinicaltrials.gov (#NCT00151255). The study was conducted in accordance with the Declaration of Helsinki.
Cyto- and molecular genetics
Chromosome banding analysis was performed centrally in the AMLSG Laboratory for Cytogenetic and Molecular Diagnosis. Karyotypes were designated according to the International System for Human Cytogenetic Nomenclature.21 Leukemia samples were analyzed for mutations in FLT3 (FLT3 internal tandem duplication [ITD] and FLT3 tyrosine kinase domain mutations at codons D835/I836) and Nucleophosmin 1 (NPM1), as previously described.6,22
Study design
Induction therapy.
Patients were randomly assigned to receive induction chemotherapy either with (VPA) or without (STANDARD) VPA. Induction therapy consisted of 2 cycles of idarubicin, 12 mg/m2 intravenously, days 1 to 3; cytarabine, 100 mg/m2 continuously intravenously, days 1 to 5; and ATRA, by mouth, 45 mg/m2, days 3 to 5, and 15 mg/m2, days 6 to 28 (AIC) or by the same chemotherapy plus VPA (V-AIC), started at a dosage of 400 mg twice a day by mouth and then adapted according to the biweekly measured serum level, starting from day 3, to obtain a serum level of 100 mg/L (60-150 mg/L) on days 1 to 28. Patients achieving a CR or partial remission (PR) after the first induction received a second cycle identical to the first one, according to initial randomization. Bone marrow evaluation was scheduled between days 28 and 35 after chemotherapy; the second cycle was started after assessment of the remission status.
After an interim analysis based on the first 77 patients, the protocol was amended because of prolonged hematologic toxicity to reduce the dose intensity of the induction cycles. In both induction cycles, idarubicin was reduced from days 1, 2, and 3 to days 1 and 3, and VPA was given with the same schedule, but only during the first cycle (amendment 1). After a second interim analysis on 186 patients, randomization was stopped because of persistent increased toxicity and inferior CR rates in the V-AIC group.
Patients with refractory disease (RD) after the first cycle or with PR or RD at the end of the second induction cycle terminated study treatment.
Consolidation therapy.
Independent of the initial randomization, all patients in CR after 2 induction cycles received a first consolidation according to the following schema: cytarabine, 0.5 g/m2 per 12 hours intravenously, days 1 to 3; mitoxantrone, 10 mg/m2 intravenously, days 2 and 3; ATRA, 15 mg/m2 by mouth, days 4 to 28. This was followed by a second cycle of idarubicin, 12 mg/m2 intravenously, days 1 and 3; etoposide, 100 mg/m2 intravenously, days 1 to 5; and ATRA, 15 mg/m2 boy mouth, days 4 to 28, as previously described.23 Both cycles were applied at an interval of 43 to 50 days from the previous cycle after confirming CR status with a bone marrow evaluation.
Allogeneic transplantation was allowed for patients with a suitable donor at the discretion of the local investigator after completion of at least the induction phase.24
Definition of response criteria, survival endpoints, and hematologic recovery
In accordance with standard criteria, CR was defined as less than 5% bone marrow blasts, an absolute neutrophil count of 1000/µL or more, a platelet count of 100 000/µL or more, no blasts in the peripheral blood, and no extramedullary leukemia; CR with incomplete blood count recovery was characterized as CR except for residual neutropenia (neutrophils <1000/µL) or thrombocytopenia (platelets <100 000/µL). PR was defined as a blast count reduction in the bone marrow of at least 50%, and in case of initial blast counts, above 50% to at least 25%; an absolute neutrophil count of 1000/µL or more; a platelet count of 100 000/µL or more; no blasts in the peripheral blood; and no extramedullary leukemia.25 Therapeutic failures were classified as either RD or early/hypoplastic death (ED/HD), which was death during double-induction therapy. Relapse was defined as more than 5% bone marrow blasts unrelated to recovery from the preceding course of chemotherapy or new extramedullary leukemia in patients with previously documented CR.
Event-free survival (EFS), overall survival (OS), relapse-free survival (RFS), cumulative incidence of relapse, and cumulative incidence of death in CR were defined as recommended.2,25 OS times of patients being alive at last follow-up or at the date of allogeneic transplantation were censored. Times to leukocyte, neutrophil, and platelet recovery were measured from the first day of chemotherapy of each cycle until the first day with values more than or equal to 1000/µL, 500/µL, and 20 000/µL for leukocytes, neutrophils, and platelets, respectively. Toxicities were defined and graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0.
Statistical analysis
The primary endpoint of the study was EFS; secondary endpoints were OS, RFS, cumulative incidence of relapse, cumulative incidence of death in CR, therapy-related toxicity, and their correlation with the study drug. The median duration of follow-up was calculated according to the method of Korn26 ; the Kaplan-Meier method was used to estimate the distribution of EFS, RFS, and OS. Survival distributions were compared using the log-rank test. Cumulative incidences and differences between groups were estimated using the method described by Gray.27
A Cox model was used to evaluate prognostic variables. The following variables were evaluated: log10 (white blood cell count), age, cytogenetic risk according to current European LeukemiaNet recommendations,2 type of AML (de novo vs secondary AML/tAML), randomization group (STANDARD vs VPA), and FLT3 and NPM1 mutational status. All statistical analyses were performed with the statistical software environment R, version 2.14.0, using the R packages rms, version 3.3-1, and cmprsk, version 2.2-2.
The initial sample size planning was based on the results obtained within the experimental therapy of the AML HD98-B study, which was set as the STANDARD group of the current study.19 According to available data at the time of treatment planning, the 2-year EFS for the standard group was estimated to be 15%. Success of the experimental group was defined as an increase of the 2-year EFS by 10% to 25%, resulting in a hazard ratio (HR) of 0.73. A required study sample size of 500 patients (250 in each group) was calculated on a stipulated 2-side level of significance of 5% and a supposed drop-out rate of overall 5%, which was detectable with a statistical power of 90%.
Results
Demographics and clinical baseline characteristics of the study population
Between August 2004 and February 2006 a total of 195 patients from 25 institutions were screened. Nine registered patients were ineligible for the following reasons: diagnosis different from AML (n = 3) or inclusion criteria not fulfilled (n = 6).
Of the 186 eligible patients, 93 were randomly assigned to VPA and 93 to STANDARD (before amendment: VPA, n = 40; STANDARD, n = 37; after amendment: VPA, n = 53; STANDARD, n = 56). Table 1 shows the distribution of clinical parameters as well as cytogenetic and molecular-genetic characteristics of the patients by up-front randomization. Patients in VPA were characterized by a significantly lower white blood cell count (P = .05) and an in-trend lower percentage of bone marrow blasts (P = .06) compared with patients in STANDARD.
Patient characteristics
| . | Standard group, n = 93 . | VPA group, n = 93 . | P . |
|---|---|---|---|
| Sex, male/female | 52/41 | 46/47 | .46 |
| Age, years, median (range) | 67.6 (61.0-83.7) | 68.8 (61.5-77.6) | .45 |
| Leukocytes, 109/L | |||
| Median (range) | 13.7 (0.5-439.5) | 9.4 (0.5-196.1) | .05 |
| Missing | 1 | 1 | |
| Hemoglobin, g/dL | |||
| Median (range) | 9.4 (5.7-13.4) | 9.4 (4.4-14.5) | .53 |
| Missing | 1 | 1 | |
| Platelets, 109/L | |||
| Median (range) | 54.5 (2-294) | 47 (7-490) | .49 |
| Missing | 1 | 1 | |
| Bone marrow blasts, %* | |||
| Median (range) | 75 (15-99) | 60 (4-99) | .06 |
| Missing | 8 | 13 | |
| Peripheral blood blasts, % | |||
| Median (range) | 28 (0-99) | 22.5 (0-99) | .33 |
| Missing | 3 | 1 | |
| Hepatomegaly | |||
| n (%) | 13 (14.6) | 14 (15.7) | .99 |
| Missing | 4 | 4 | |
| Splenomegaly | |||
| n (%) | 21 (23.1) | 19 (21.1) | .85 |
| Missing | 2 | 3 | |
| Lactate dehydrogenase, U/L | |||
| Median (range) | 372 (91-3710) | 382 (102-5760) | .80 |
| Missing | 1 | 1 | |
| AML type | |||
| De novo AML, n (%) | 71 (76.3) | 69 (74.2) | |
| Secondary AML/therapy-related AML, n (%) | 21 (22.6) | 24 (25.8) | .73 |
| Missing | 1 | 0 | |
| Karyotype† | |||
| Favorable, n (%) | 5 (5.4) | 3 (3.2) | .67 |
| Intermediate, n (%) | 60 (64.5) | 54 (58.1) | |
| Adverse, n (%) | 23 (27.4) | 24 (25.8) | |
| Normal, n (%) | 41 (48.8) | 42 (51.9) | |
| Missing | 7 | 12 | |
| Nucleophosmin 1 | |||
| n (%) | 22 (25.9) | 18 (22.5) | .46 |
| Missing | 8 | 13 | |
| FLT3 internal tandem duplication | |||
| n (%) | 11 (13.1) | 12 (15.0) | .79 |
| Missing | 9 | 13 | |
| FLT3 tyrosine kinase domain mutation | |||
| n (%) | 3 (5.3) | 4 (5.1) | .30 |
| Missing | 8 | 15 |
| . | Standard group, n = 93 . | VPA group, n = 93 . | P . |
|---|---|---|---|
| Sex, male/female | 52/41 | 46/47 | .46 |
| Age, years, median (range) | 67.6 (61.0-83.7) | 68.8 (61.5-77.6) | .45 |
| Leukocytes, 109/L | |||
| Median (range) | 13.7 (0.5-439.5) | 9.4 (0.5-196.1) | .05 |
| Missing | 1 | 1 | |
| Hemoglobin, g/dL | |||
| Median (range) | 9.4 (5.7-13.4) | 9.4 (4.4-14.5) | .53 |
| Missing | 1 | 1 | |
| Platelets, 109/L | |||
| Median (range) | 54.5 (2-294) | 47 (7-490) | .49 |
| Missing | 1 | 1 | |
| Bone marrow blasts, %* | |||
| Median (range) | 75 (15-99) | 60 (4-99) | .06 |
| Missing | 8 | 13 | |
| Peripheral blood blasts, % | |||
| Median (range) | 28 (0-99) | 22.5 (0-99) | .33 |
| Missing | 3 | 1 | |
| Hepatomegaly | |||
| n (%) | 13 (14.6) | 14 (15.7) | .99 |
| Missing | 4 | 4 | |
| Splenomegaly | |||
| n (%) | 21 (23.1) | 19 (21.1) | .85 |
| Missing | 2 | 3 | |
| Lactate dehydrogenase, U/L | |||
| Median (range) | 372 (91-3710) | 382 (102-5760) | .80 |
| Missing | 1 | 1 | |
| AML type | |||
| De novo AML, n (%) | 71 (76.3) | 69 (74.2) | |
| Secondary AML/therapy-related AML, n (%) | 21 (22.6) | 24 (25.8) | .73 |
| Missing | 1 | 0 | |
| Karyotype† | |||
| Favorable, n (%) | 5 (5.4) | 3 (3.2) | .67 |
| Intermediate, n (%) | 60 (64.5) | 54 (58.1) | |
| Adverse, n (%) | 23 (27.4) | 24 (25.8) | |
| Normal, n (%) | 41 (48.8) | 42 (51.9) | |
| Missing | 7 | 12 | |
| Nucleophosmin 1 | |||
| n (%) | 22 (25.9) | 18 (22.5) | .46 |
| Missing | 8 | 13 | |
| FLT3 internal tandem duplication | |||
| n (%) | 11 (13.1) | 12 (15.0) | .79 |
| Missing | 9 | 13 | |
| FLT3 tyrosine kinase domain mutation | |||
| n (%) | 3 (5.3) | 4 (5.1) | .30 |
| Missing | 8 | 15 |
n = 7 patients presented with bone marrow blast counts below 20%: 5 with peripheral blast counts above 20% and 2 with extramedullary disease.
According to European LeukemiaNet classification, based on cytogenetics.2
Eight randomized patients did not receive the scheduled therapy because of death before the start of induction therapy (n = 8). These patients were included into the intention-to-treat EFS analysis and excluded from toxicity analyses.
In spite of the initial randomization for VPA, 7 patients did not receive VPA during the first induction (5 received AIC, 2 receiving IC), according to the local clinicians’ judgment. For the intention-to-treat-based survival analysis, those patients were included in VPA; for the toxicity analysis, they were grouped together with the patients belonging to STANDARD.
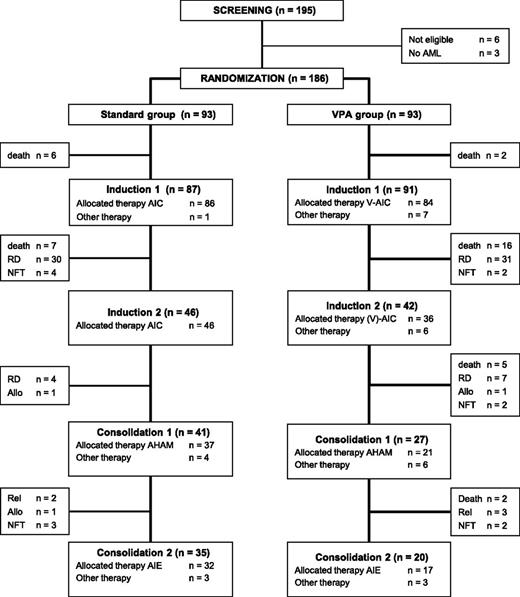
The trial is summarized in the flow diagram according to Consolidated Standards of Reporting Trials (CONSORT) state in Figure 1.
Flowchart on study conduct. Flowchart showing enrollment, program completion, and/or drop-out according to the randomization result. AIC, idarubicin, 12 mg/m2 intravenously, days 1 to 3; cytarabine, 100 mg/m2 continuously intravenously, days 1 to 5; and ATRA, by mouth, 45 mg/m2, days 3 to 5, and 15 mg/m2, days 6 to 28; A-HAM, cytarabine, 0.5 g/m2 per 12 hours intravenously, days 1 to 3; mitoxantrone, 10 mg/m2 intravenously, days 2 and 3; A-IE, idarubicin, 12 mg/m2 intravenously, days 1 and 3; etoposide, 100 mg/m2 intravenously, days 1 to 5; ATRA, 15 mg/m2 by mouth, days 4 to 28; Allo, allogeneic HSCT; NFT, no further treatment; RD, refractory disease; Rel, relapse; V-AIC, VPA, AIC.
Flowchart on study conduct. Flowchart showing enrollment, program completion, and/or drop-out according to the randomization result. AIC, idarubicin, 12 mg/m2 intravenously, days 1 to 3; cytarabine, 100 mg/m2 continuously intravenously, days 1 to 5; and ATRA, by mouth, 45 mg/m2, days 3 to 5, and 15 mg/m2, days 6 to 28; A-HAM, cytarabine, 0.5 g/m2 per 12 hours intravenously, days 1 to 3; mitoxantrone, 10 mg/m2 intravenously, days 2 and 3; A-IE, idarubicin, 12 mg/m2 intravenously, days 1 and 3; etoposide, 100 mg/m2 intravenously, days 1 to 5; ATRA, 15 mg/m2 by mouth, days 4 to 28; Allo, allogeneic HSCT; NFT, no further treatment; RD, refractory disease; Rel, relapse; V-AIC, VPA, AIC.
Response to induction therapy
After the first induction cycle, a CR was achieved in 61 patients: 31% in VPA (n = 29) and 34% in STANDARD (n = 32) (Table 2). The overall response rate including CR, CR with incomplete blood count recovery, and PR was 47.3% (44 patients) and 52.7% (49 patients) in VPA and STANDARD, respectively. In both groups, 31 patients (33.3%) had RD. No statistically significant difference in rates of CR, PR, RD, and ED was observed between the 2 therapy groups when evaluating the response rate separately both before and after amendment (supplemental Table 1, available on the Blood Web site).
Response to induction chemotherapy
| . | Standard group, n = 93 . | VPA group, n = 93 . | P . |
|---|---|---|---|
| After the first induction cycle | |||
| CR, n (%) | 32 (34.4) | 29 (31.2) | .75 |
| Cri, n (%) | 10 (10.8) | 7 (7.5) | |
| PR, n (%) | 7 (7.5) | 8 (8.6) | |
| RD, n (%) | 31 (33.3) | 31 (33.3) | .99 |
| Death, n (%) | 13 (13.9) | 18 (19.4) | .43 |
| After 2 induction cycles | |||
| CR, n (%) | 48 (51.6) | 37 (39.8) | .14 |
| RD, n (%) | 32 (34.4) | 32 (34.4) | .99 |
| Death, n (%) | 13 (13.9) | 24 (25.8) | .06 |
| . | Standard group, n = 93 . | VPA group, n = 93 . | P . |
|---|---|---|---|
| After the first induction cycle | |||
| CR, n (%) | 32 (34.4) | 29 (31.2) | .75 |
| Cri, n (%) | 10 (10.8) | 7 (7.5) | |
| PR, n (%) | 7 (7.5) | 8 (8.6) | |
| RD, n (%) | 31 (33.3) | 31 (33.3) | .99 |
| Death, n (%) | 13 (13.9) | 18 (19.4) | .43 |
| After 2 induction cycles | |||
| CR, n (%) | 48 (51.6) | 37 (39.8) | .14 |
| RD, n (%) | 32 (34.4) | 32 (34.4) | .99 |
| Death, n (%) | 13 (13.9) | 24 (25.8) | .06 |
Forty-two patients in VPA received a second induction (n = 18 before amendment and n = 24 after amendment); 2 patients had no further therapy and 5 of 18 patients treated before amendment did not receive the scheduled VPA for second induction therapy. Of 50 patients in STANDARD, 46 received the scheduled therapy and 4 had no further treatment (Figure 1).
After 2 cycles of induction, CR rates tended to be higher in STANDARD (52% vs 40%; P = .14; odds ratio [OR], 1.61; 95% confidence interval [CI], 0.86-3.0). When evaluating response to induction therapy separately, before and after amendment, no difference in CR rate was evident, even if a higher percentage of CR was consistently observed in STANDARD. Rates of RD after induction therapy did not differ between the 2 study groups, both when analyzing the whole cohort or separately before and after amendment. A tendency to a higher ED rate was found in VPA (26% vs 14%; P = .06; OR, 0.47; 95% CI, 0.26-1.0). This effect was predominantly evident before the amendment (35% vs 19%; P = .09; OR, 0.4; 95% CI, 0.12-1.1), but not after the amendment (19% vs 11%; P = .28; OR, 0.52; 95% CI, 0.14-1.72). Irrespective of the random assignment to VPA or STANDARD, the ED rate was significantly higher in patients treated before the amendment compared with those treated after the amendment (27.3% vs 14.7%; P = .04; OR, 0.46; 95% CI, 0.2-1.0). This indicates that the decrease of toxicity was a result not only of the omission of VPA in the second induction cycle but also of dose reduction of idarubicin after the amendment.
Consolidation therapy
Sixty-eight patients received the first consolidation chemotherapy, 2 patients received an allogeneic transplantation, and 2 patients had no further treatment. Fifty-five patients received the second consolidation cycle, and 1 patient proceeded to an allogeneic transplantation after the first consolidation cycle. The intended treatment including all induction and consolidation cycles was completed in 20 (22%) of 93 patients in VPA and in 35 (38%) of 93 patients in STANDARD (P = .02).
Nonhematologic toxicity
Toxicities during the first induction cycle are summarized in Table 3. Clinically relevant higher frequencies of neurologic toxicity grade 3/4 (P = .007) and pulmonary toxicity (P = .06) were observed in VPA. No significant differences were observed for gastrointestinal, cardiologic, hemorrhagic, and infectious complications.
Nonhematologic toxicity: induction cycle 1
| Toxicity . | Standard group, n = 93 . | VPA group, n= 83 . | Grade 3/4 . | ||||
|---|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 3/4 . | Grade 3 . | Grade 4 . | Grade 3/4 . | P . | |
| Cardiac, n (%) | 2 (2.2) | 5 (5.4) | 7 (7.5) | 1 (1.2) | 2 (2.4) | 3 (3.6) | .34 |
| Gastrointestinal, n (%) | 10 (10.8) | 0 | 10 (10.8) | 10 (12.0) | 2 (2.4) | 12 (14.5) | .50 |
| Hemorrhage, n (%) | 2 (2.2) | 4 (4.3) | 6 (6.4) | 1 (1.2) | 2 (2.4) | 3 (3.6) | .50 |
| Infections, n (%) | 55 (59.1) | 13 (14.0) | 68 (73.1) | 33 (39.8) | 20 (24.1) | 53 (63.9) | .20 |
| Neurology, n (%) | 0 (0) | 1 (1.1) | 1 (1.1) | 6 (7.2) | 3 (3.6) | 9 (10.8) | .007 |
| Pulmonary, n (%) | 2 (2.1) | 8 (8.6) | 10 (10.7) | 4 (4.8) | 14 (16.9) | 18 (21.7) | .06 |
| Other, n (%) | 21 (22.6) | 6 (6.4) | 27 (29.0) | 13 (15.7) | 6 (7.2) | 19 (22.9) | .50 |
| Toxicity . | Standard group, n = 93 . | VPA group, n= 83 . | Grade 3/4 . | ||||
|---|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 3/4 . | Grade 3 . | Grade 4 . | Grade 3/4 . | P . | |
| Cardiac, n (%) | 2 (2.2) | 5 (5.4) | 7 (7.5) | 1 (1.2) | 2 (2.4) | 3 (3.6) | .34 |
| Gastrointestinal, n (%) | 10 (10.8) | 0 | 10 (10.8) | 10 (12.0) | 2 (2.4) | 12 (14.5) | .50 |
| Hemorrhage, n (%) | 2 (2.2) | 4 (4.3) | 6 (6.4) | 1 (1.2) | 2 (2.4) | 3 (3.6) | .50 |
| Infections, n (%) | 55 (59.1) | 13 (14.0) | 68 (73.1) | 33 (39.8) | 20 (24.1) | 53 (63.9) | .20 |
| Neurology, n (%) | 0 (0) | 1 (1.1) | 1 (1.1) | 6 (7.2) | 3 (3.6) | 9 (10.8) | .007 |
| Pulmonary, n (%) | 2 (2.1) | 8 (8.6) | 10 (10.7) | 4 (4.8) | 14 (16.9) | 18 (21.7) | .06 |
| Other, n (%) | 21 (22.6) | 6 (6.4) | 27 (29.0) | 13 (15.7) | 6 (7.2) | 19 (22.9) | .50 |
During the second induction therapy, a significant higher rate of infections was observed in VPA (19/36 vs 16/48; 52.8% vs 33.3%; P = .0001). Gastrointestinal, cardiac, hemorrhagic, and pulmonary toxicities were equally distributed between the 2 groups (data not shown).
Hematologic toxicity
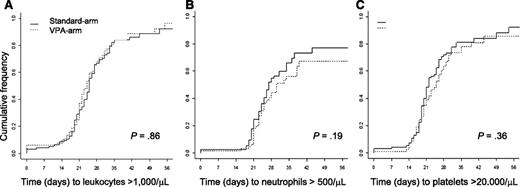
The median time to leukocyte, neutrophil, and platelet recovery after the first induction therapy was measured from the first day of chemotherapy and did not differ between VPA and STANDARD (median time to leukocytes, >1000 /µL, 25 vs 24 days [P = .86]; neutrophils, >500/µL, 27 vs 30 days [P = .19]; platelets, >20 000/µL, 22 vs 25 days [P = .36]; Figure 2). There were also no differences in hematologic recovery when analyzing the cohorts before and after amendment separately (before amendment: median time to leukocytes, >1000 /µL, 25 vs 24 days [P = .31]; neutrophils, >500/µL, 31 vs 30 days [P = .90]; platelets, >20 000/µL, 22 vs 27 days [P = .42]; after amendment: median time to leukocytes, >1000/µL, 22 vs 22 days [P = .59]; neutrophils, >500/µL, 27 vs 34 days [P = .10]; platelets, >20 000/µL, 22 vs 23 days [P = .97])
Hematologic recovery after the first induction cycle. (A) Cumulative incidence of leukocyte recovery. (B) Cumulative incidence of neutrophil recovery. (C) Cumulative incidence of platelet recovery.
Hematologic recovery after the first induction cycle. (A) Cumulative incidence of leukocyte recovery. (B) Cumulative incidence of neutrophil recovery. (C) Cumulative incidence of platelet recovery.
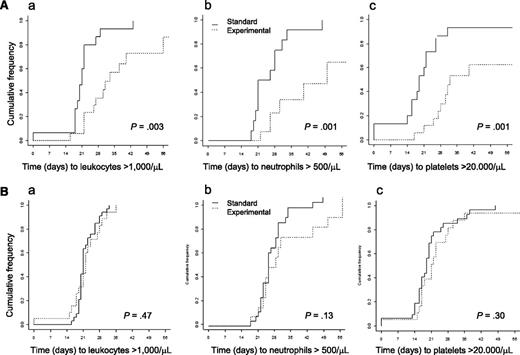
In contrast, for second induction therapy, a longer time to leukocyte, neutrophil, and platelet recovery for VPA compared with STANDARD was observed (median time to leukocytes, >1000 /µL, 21 vs 26 days [P = .02]; neutrophils, >500/µL, 24 vs 30 days [P = .002]; platelets, >20 000/µL, 19 vs 28 days [P = .009]). However, this difference between VPA and STANDARD was observed only in patients treated before the amendment (median time to leukocytes, >1000/µL, 21 vs 33 days [P = .003]; neutrophils, >500/µL, 21 vs 50 days [P = .001]; platelets, >20 000/µL, 19 vs 32 days [P = .001]), but not for patients treated after the amendment, when VPA was restricted to the first induction cycle (median time to leukocytes, >1000/µL, 21 vs 22 days [P = .47]; neutrophils, >500/µL, 24 vs 27 days [P = .13]; platelets, >20 000/µL, 19 vs 21 days [P = .30]; Figure 3A-B).
Hematologic recovery after the second induction cycle. (A) before amendment; (a) cumulative incidence of leukocyte recovery; (b) cumulative incidence of neutrophil recovery; (c) cumulative incidence of platelet recovery. (B) After amendment; (a) cumulative incidence of leukocyte recovery; (b) cumulative incidence of neutrophil recovery; (c) cumulative incidence of platelet recovery
Hematologic recovery after the second induction cycle. (A) before amendment; (a) cumulative incidence of leukocyte recovery; (b) cumulative incidence of neutrophil recovery; (c) cumulative incidence of platelet recovery. (B) After amendment; (a) cumulative incidence of leukocyte recovery; (b) cumulative incidence of neutrophil recovery; (c) cumulative incidence of platelet recovery
VPA dosage and serum levels
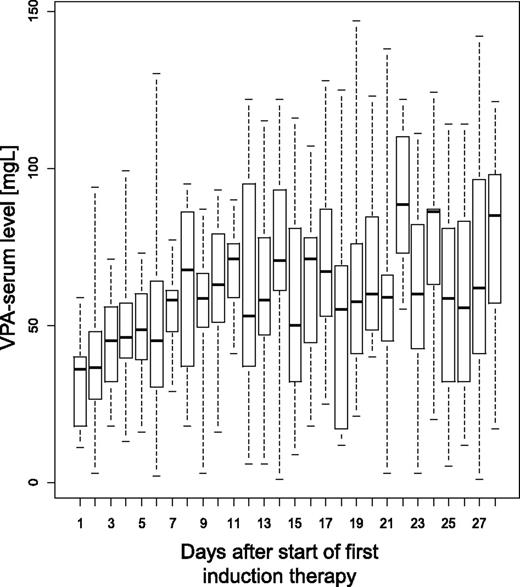
During first induction therapy, the dose and corresponding serum VPA levels were available as repetitive measurements in 81 patients (median per patient, n = 6; range, 1-28). VPA was started in all patients with a cumulative dose per day of 800 mg. At steady state between day 11 and day 21, the median cumulative daily dose ranged from 1200 to 1800 mg. The corresponding median VPA-serum levels were 36 mg/L at day 1 and ranged between 52 and 71 mg/L at steady state between day 11 and day 21 (Figure 4). A VPA-serum level above the lower target level of 60 mg/L was documented in 40% of the measurements. During second induction therapy, the median VPA-serum levels ranged between 40 and 96 mg/L, with VPA-serum levels above the lower target level of 60 mg/L in 58% of the measurements.
VPA serum levels during first induction therapy. Box and whisker plots of VPA serum levels measured during first induction therapy
VPA serum levels during first induction therapy. Box and whisker plots of VPA serum levels measured during first induction therapy
In a subset of 8 patients, the free drug fraction was determined by ultracentrifugation and correlated to VPA-serum levels. There was a strong correlation between free- and total VPA-serum levels (Spearman correlation coefficient, 0.89). Factors associated in a mixed model with free VPA-serum levels were total VPA serum levels (P < .0001) and albumin serum levels (P < .0001), whereas total protein and creatinine serum levels as well as age were not significantly associated.
Survival analysis
After a median follow-up of 84.4 months, 164 patients died and 22 were alive, 11 of whom were randomly assigned to STANDARD and 11 to VPA. Twenty-five had received an allogeneic transplantation, of whom 9 were still alive (16 in STANDARD and 9 in VPA; 19 matched unrelated donors, 6 matched family donors). Of 84 patients who achieved a CR after induction therapy, 66 relapsed and 7 died in CR.
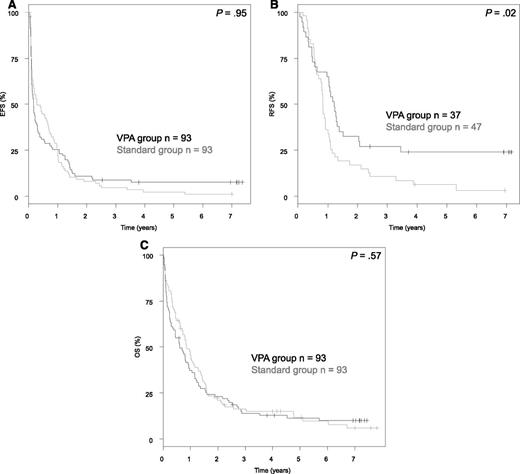
Analysis of the primary endpoint EFS revealed no difference between the 2 treatment groups (P = .95; Figure 5), with an EFS at 5 years of 2.3% (95% CI, 0.6%-9.9%) in STANDARD and 7.6% (95% CI, 3.7%-15.6%) in VPA. In contrast, a significant difference was observed for the endpoint RFS in favor for VPA (P = .02), with a RFS at 5 years of 6.4% (95% CI, 2.1%-19.1%) in STANDARD and 24.0% (95% CI, 13.5%-42.8%) in VPA. However, this did not translate into a difference in OS (P = .57), with an OS at 5 years of 11.7% (95% CI, 6.3%-21.5%) in STANDARD and 11.4% (95% CI, 6.3%-20.5%) in VPA.
Survival analyses according to randomization. (A) EFS. (B) RFS. (C) OS.
In addition, we grouped patients according to VPA serum levels. Patients with VPA serum levels above 60 mg/L (the lower boundary of the predefined target serum level) measured in 50% or more points during cycle 1 were categorized as patients with high VPA levels. In 28 of 93 patients randomly assigned to the VPA group, this was the case. According to the VPA levels, no significant difference in CR rates (low VPA levels, 45%; high VPA levels, 29%; P = .17) and early death rates (low VPA levels, 31%; high VPA levels, 14%; P = .12) were present, whereas a significantly higher proportion of patients with refractory disease after induction therapy was seen in the high-VPA group (low VPA levels, 25%; high VPA levels, 57%; P = .004). Furthermore, no differences in survival endpoints were present (EFS, P = .44; RFS, P = .87; OS, P = .75).
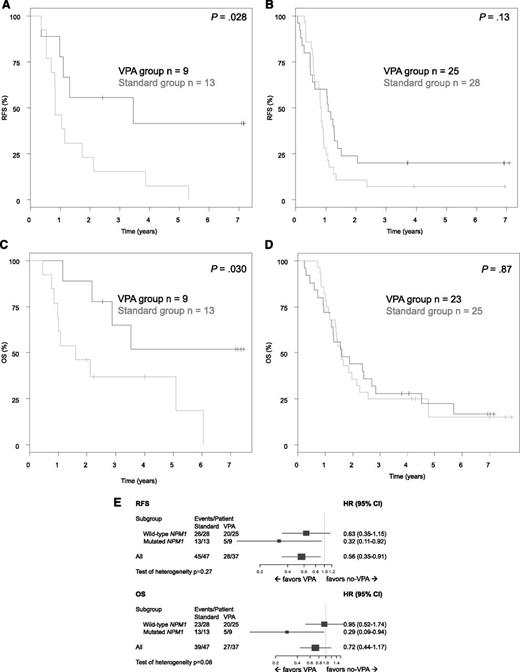
In univariable explorative subset analyses for patients in CR after induction therapy (Figure 6), significant superior RFS (P = .03) and OS (P = .03) were observed in patients with mutated NPM1 randomly assigned to VPA (RFS at 5 years: STANDARD, 8%; VPA, 42%; OS at 5 years: STANDARD, 37%; VPA, 52%). In contrast, no such a difference was seen in patients with NPM1 wild-type (RFS at 5 years: STANDARD, 7%; VPA, 20% [P = .13]; OS at 5 years: STANDARD, 15%; VPA, 22% [P = .87]). However, these analyses were based on a small sample size, and thus the tests on heterogeneity were not significant, but revealed a trend for OS (P = .08). Furthermore, because of the small numbers, a subgroup analysis based on the combined genotype mutated NPM1 in the absence of FLT3-ITD was not possible.
Explorative subset analyses according the NPM1 mutational status. (A) RFS in AML with mutated NPM1. (B) RFS in AML with NPM1 wild-type. (C) OS in AML with mutated NPM1 in first CR. (D) OS in AML with NPM1 wild-type in first CR. (E) Forest plots showing hazard ratios for death or relapse (relapse-free survival), as well as death (OS) and 95% confidence intervals (bars) for 75 patients with AML in first CR.
Explorative subset analyses according the NPM1 mutational status. (A) RFS in AML with mutated NPM1. (B) RFS in AML with NPM1 wild-type. (C) OS in AML with mutated NPM1 in first CR. (D) OS in AML with NPM1 wild-type in first CR. (E) Forest plots showing hazard ratios for death or relapse (relapse-free survival), as well as death (OS) and 95% confidence intervals (bars) for 75 patients with AML in first CR.
Multivariate analysis on the endpoint RFS after limited backward selection revealed random assignment to VPA (HR, 0.56; P = .02) as a significantly favorable variable, whereas multivariate analysis on the endpoint OS revealed adverse cytogenetics according to European LeukemiaNet classification (HR, 2.37; P < .0001), higher age (HR for 5 years difference, 1.56; P < .0001), and logarithm of white blood cell count (HR, 1.43; P = .0014) as significant variables.
Discussion
The aim of our randomized phase III study was to evaluate the efficacy and toxicity of VPA in combination with ATRA and chemotherapy as induction treatment of older patients with AML considered fit for intensive chemotherapy.
The induction therapy backbone consisted of idarubicin and cytarabine in combination with ATRA, which was started at day 3 and administered as reported.19 Different from our previously published study,19 the dose of idarubicin was intensified by 33%, and etoposide was omitted. The idea of adding VPA was driven by in vitro evidence of VPA as a potent histondeacetylase-inhibitor12,13,15 and by a favorable efficacy and toxicity profile in combination with ATRA in the treatment of myelodysplastic syndromes and secondary AML.16-18 Our results show that the addition of VPA to ATRA and intensive chemotherapy did not increase the CR rate but was associated with clinically relevant hematologic toxicity. The hematologic toxicity with significantly prolonged duration of neutropenia and thrombocytopenia was, in particular, observed after the second induction cycle, when VPA already had been given continuously from course 1 on and in parallel to chemotherapy during course 2. Thus, continuous parallel administration of VPA with idarubicin and cytarabine resulted in severe hematopoietic progenitor cell toxicity. Hematologic toxicity has been reported to rarely occur during anticonvulsive therapy with VPA.28,29 The hematologic toxicity observed in our study was addressed by an amendment restricting VPA treatment to the first induction cycle, as well a dose reduction of idarubicin. This resulted in a significant reduction of hematologic toxicity after course 2 with values comparable to in the standard group of the study (Figure 3).
However, the study was terminated early after the planned interim analysis after a recruitment of 186 patients because of lack of efficacy. After 2 cycles of induction therapy, CR rates were lower in VPA compared with STANDARD, both before and after the amendment. As rates of RD were comparable and not significantly different between the 2 groups, the lower response rate was mainly attributable to a higher rate of ED during induction therapy. The addition of VPA led to an increased rate of pulmonary toxicity, which manifested mainly as pneumonia, and not unexpectedly, to a higher rate of neurological toxicity (Table 3).
The way HDAC inhibitors are combined with conventional cytotoxic agents may affect toxicity and efficacy; however, there are only scarce data providing a rationale for a specific schedule. In a phase 1 trial, vorinostat given before cytarabine and etoposide did not prolong hematologic recovery.30 In vitro data with primary AML cells indicate a synergistic activity of the anthracycline doxorubicin and panobinostat.31 In a phase 1 trial of panobinostat with cytarabine and mitoxantrone, parallel administration did not lead to an increase in hematologic toxicity.32 Although in our study the ED rate was highest in VPA before amendment, the reduction of idarubicin dosage from 3 to 2 days (33% dose reduction) in both induction cycles further reduced the ED rate in both groups without increasing the rate of RD. In contrast, a dose escalation of daunorubicin from 45 to 90 mg/m2 for 3 days within a 7+3 induction therapy in older patients (>60 years) was feasible and resulted in a subgroup of patients aged between 60 and 65 years with significantly improved survival.33
In contrast, in a French study, dose escalation of idarubicin in middle-aged patients (aged 50-70 years) did not result in an improvement of outcome but, rather, in an increase rate of toxicity (mucositis in particular).34 These data highlight that older patients with AML are a vulnerable patient population and that escalation of anthracycline dosages, and in particular idarubicin, should be performed cautiously with a continuous safety assessment. In addition, the combination of idarubicin with HDAC inhibitors has to be carefully monitored as performed in an ongoing phase 1 trial evaluating the maximal tolerated dose of panobinostat in combination with idarubicin and cytarabine (www.clinicaltrials.gov #NCT01242774). One reason we did not observe VPA-related hematologic toxicity in course 1 may be the slow increase in serum VPA levels during induction course 1 (Figure 4). However, a serum level at the lower target level (60 mg/L) was sufficient to cause the observed hematologic toxicity. Furthermore, patients with VPA levels mostly below the lower target level over time showed a significantly lower RD rate after induction therapy compared with those with VPA levels within the target range, indicating more complex pharmacodynamics, as anticipated.
In addition, the close correlation of total serum VPA levels to the biological active free VPA levels argues strongly for the use of total VPA serum levels for treatment adaptations. As expected, as per the response data, we did not observe a significant difference in EFS and OS between the 2 treatment groups. However, our treatment results with regard to OS were comparable with others in the same age group.35 Of note, for the survival endpoint RFS, a significantly better outcome was observed in VPA compared with STANDARD (Figure 4B). In exploratory subgroup analysis, this benefit in RFS for VPA was only present in patients with NPM1-mutated AML (Figure 5) and seen for RFS and OS of patients achieving a first CR. Because of a limited patient number after early stopping of the trial, we were not able to evaluate the subgroups within the NPM1-mutated AML, according to FLT3-ITD status. Thus, our previously reported effect of ATRA on survival endpoints in older patients, especially with the genotype mutated NPM1 in the absence of a FLT3-ITD, could not further be explored with regard to VPA.
In summary, the addition of VPA to intensive induction therapy and ATRA did not result in an improvement of CR rates, EFS, or OS. This was mainly a result of an increased VPA-related hematologic toxicity and higher death rates during the second induction cycle, when VPA was given in parallel with idarubicin and cytarabine. For patients achieving a CR after induction therapy, a significantly better RFS was observed in the VPA group, which could be attributed to the subset of patients with NPM1 mutation. On the basis of these data, further exploration of VPA or other HDAC inhibitors in combination with intensive chemotherapy should be envisaged within clinical trials, paying specific attention to scheduling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Axel Benner (German Cancer Research Center, Heidelberg, Germany) for his statistical review and Daniela Späth (Department of Internal Medicine III, University Hospital Ulm, Ulm, Germany) for her support in the clinical trials office.
This work was supported by a grant from the Deutsche José Carreras Leukämie-Stiftung (DJCLS R 06/32).
Authorship
Contribution: M.T., K.D., M.G., H.D., and R.F.S. conceived and designed the study; K.D., P.B., G.H., K.G., H.-A.H., M.R., C.-H.K., S.K., A.R., G.W., H.K., D.N., H.G.D., M.W., E.K., W.B., A.M., R.G., G.H., P.P., V.I.G., M.G., H.D., and R.F.S. provided the study materials or patients; M.T. and R.F.S. collected and assembled data; M.T., H.D., and R.F.S. analyzed and interpreted data; and M.T., K.D., P.B., G.H., K.G., H.-A.H., M.R., C.-H.K., S.K., A.R., G.W., H.K., D.N., H.G.D., M.W., E.K., W.B., A.M., R.G., G.H., P.P., V.I.G., M.G., H.D., and R.F.S approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of additional members of the German-Austrian Acute Myelogenous Leukemia Study Group appears in “Appendix.”
Correspondence: Richard F. Schlenk, University Hospital Ulm, Department of Internal Medicine III, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail richard.schlenk@uniklinik-ulm.de.
Appendix
Additional members of the German-Austrian Acute Myelogenous Leukemia Study Group are: David Nachbaur (Innsbruck, Austria); Günter Gastl (Innsbruck, Austria); Andreas Petzer (Linz, Austria); Elisabeth Koller (Vienna, Austria); Günter Schlimok (Augsburg, Germany); Jörg Westermann (Berlin, Germany); Peter Brossart (Bonn, Germany); Marie von Lilienfeld-Toal (Bonn, Germany); Jürgen Krauter (Braunschweig Germany); Jens Kersten (Braunschweig, Germany); Rainer Haas (Düsseldorf, Germany); Andrea Kündgen (Düsseldorf, Germany); Peter Reimer (Essen, Germany); Mohammed Wattad (Essen, Germany); Michael Lübbert (Freiburg, Germany); Alexander Burchardt (Gießen, Germany); Matthias Rummel (Gießen, Germany); Gerald Wulf (Göttingen, Germany); Lorenz Trümper (Göttingen, Germany); Hans Salwender (Hamburg, Germany); Arnold Ganser (Hannover, Germany); Michael Heuser (Hannover, Germany); Brigitte Schlegelberger (Hannover, Germany); Michael Pfreundschuh (Homburg, Germany); Gerhard Held (Homburg, Germany); Mark Ringhoffer (Karlsruhe, Germany); Martin Bentz (Karlsruhe, Germany); Heinz-A. Horst (Kiel, Germany); Michael Kneba (Kiel, Germany); Stephan Kremers (Lebach, Germany); Gerhard Heil (Lüdenscheid, Germany); Thomas Kindler (Mainz, Germany); Matthias Theobald (Mainz, Germany); Katharina Götze (München, Germany); Christian Peschel (München, Germany); Sabine Struve (München, Germany); Clemens Wendtner (München, Germany); Claus-Henning Köhne (Oldenburg, Germany); Axel Matzdorff (Saarbrücken, Germany); Hans-Günther Mergenthaler (Stuttgart, Germany); Heinz Kirchen (Trier, Germany); Helmut R. Salih (Tübingen, Germany); Lothar Kanz (Tübingen, Germany); Hartmut Döhner (Ulm, Germany); Konstanze Döhner (Ulm, Germany); Richard F. Schlenk (Ulm, Germany); Aruna Raghavachar (Wuppertal, Germany).