In this issue of Blood, Fielding et al demonstrate a significant enhancement of long-term outcomes for a large series of adult patients with Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL), who were prospectively treated in 2 sequential cohorts with an imatinib-containing protocol.1
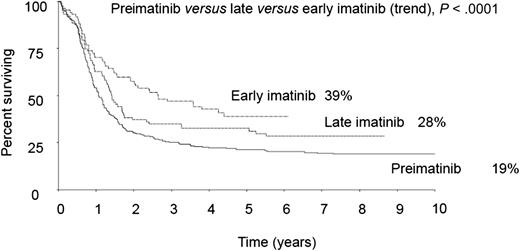
With imatinib, long-term survival of adult patients with Ph+ ALL was substantially improved in trial UKALLXII/ECOG299, to a greater extent in patients receiving the drug since induction phase II (early) rather than consolidation therapy (late). See Figure 2B in the article by Fielding et al that begins on page 843.
With imatinib, long-term survival of adult patients with Ph+ ALL was substantially improved in trial UKALLXII/ECOG299, to a greater extent in patients receiving the drug since induction phase II (early) rather than consolidation therapy (late). See Figure 2B in the article by Fielding et al that begins on page 843.
The results show a 4-year overall survival rate of 38% compared with 22% in the preimatinib cohort (P = .003), with a further beneficial effect observed in the patient cohort receiving early imatinib, that is, starting from induction phase II rather than from postinduction consolidation onward (see figure).
This work was part of the large international United Kingdom (UK) ALL XII/Eastern Cooperative Oncology Group (ECOG) 2993 trial and marks the end of more than a decade of imatinib-based studies in adult Ph+ ALL. There is now sound proof that imatinib, when used together with chemotherapy and allogeneic stem cell transplantation (SCT) like in this study, exerts a favorable therapeutic effect in this very high-risk ALL subset. Applied to the crude daily practice and without the technical jargon and the prognostic analyses, the take home message is that the study policy nearly doubled the proportion of patients alive at 4 years compared with the preimatinib baseline figure.
Moreover, the therapeutic role of allogeneic SCT in transforming remission into cure was rather convincingly documented, with a survival rate of 50% compared with 19% in nontransplanted patients. Even the latter figure is of interest, because it is not too distant from the general outcomes of the pre-imatinib era, inclusive of transplant and nontransplant patients. Allogeneic SCT, however, although ensuring higher cure rates, causes transplantation-related mortality (∼19% calculating the differential between overall survival [50%] and relapse-free survival [69%] in the present study), which, besides ALL recurrence, is another major obstacle toward cure.
The word “cure” is used with some caution but also deliberate precision. In the opinion of the authors, this means being disease free, both clinically and molecularly, after a minimum of 2 years and even better at 3+ years after complete remission, and preferably off therapy. This status is certainly easier to achieve with allogeneic SCT than with any other therapy. This point is rather well illustrated in the UKALLXII/ECOG2993 study (see Figure 4 in the article by Fielding et al)1 and by some other imatinib-based trials reporting long-term outcomes in series with >50 patients. In these studies, totaling several hundred patients, overall survival ranged from 49% to 53% (depending on induction chemotherapy intensity) at 3 years (n = 265)2 to 31% to 50% (depending on imatinib induction schedule) at 4 years (n = 335)3 and 38% at 5 years (n = 65).4 With an allograft, further survival improvement was confirmed by all, ranging between 49% and 58%.
More recently, of great interest, other comparable trials reported good to very good outcomes for many patients treated without allogeneic SCT, particularly in those achieving an early, deep, or complete minimal residual disease (MRD) response on chemotherapy plus either imatinib or dasatinib combinations,5,6 or following autologous rather than allogeneic SCT,2,7 again with post-transplantation maintenance and negligible transplantation-related mortality. These findings raise important questions. If a cure of acute leukemia is defined by a durable remission after cessation of therapy, how do we interpret these results? Is it perhaps time to redefine treatment end points and response definitions in Ph+ ALL, in relation with the MRD course and emerging treatment options with newer tyrosine kinase inhibitors (dasatinib, nilotinib, and ponatinib) and/or autologous SCT? Is transplant-associated mortality an acceptable toll in patients achieving a prompt and durable molecular remission in response to the most active among modern treatment regimens?
Leaving these questions aside, the authoritativeness of the study of Fielding et al lies in the large patient number, the multi-institutional treatment setting, and the long follow-up period, enabling us to see definitive treatment results. Although a 38% 4-year survival rate may not be uniformly perceived as huge therapeutic success, it indeed is if we look carefully at the exact clinical context. For too many years, the whole area of ALL therapy in adults was a source of frustration and skepticism because of the substantial lack of progress in Ph+ ALL because of its high prevalence in older age groups and an intractable nature using standard therapeutics. Imatinib brought targeted therapy in ALL and did it in the worst prognostic subset.
Given the formidable therapeutic task posed by Ph+ ALL, it is no surprise that the ideal treatment strategy has yet to be defined and that imatinib is not the perfect drug. Several new opportunities are under scrutiny. Among them are ponatinib, which is effective against the highly resistant T315I point mutation; other inhibitors of primary metabolic pathways in B-lymphoblasts (hedgehog, PIK3/AKT, Aurora kinase, Btk, JAK2/STAT, etc); and novel immunotherapeutics such as inotuzumab ozogamicin, a calicheamcin-linked monoclonal antibody targeting CD22+ blasts, and blinatumomab, a bispecific construct engaging cytotoxic CD3+ T cells against CD19+ blasts.8 Cases of relapsed/resistant Ph+ ALL responsive to these agents have been reported. Most recently, new remissions lasting 3 and 8+ months, respectively, were induced in 2 patients relapsing after allogeneic SCT using CD19-redirected chimeric antigen receptor-modified T cells.9 The future is thus open to developing more effective single and multitargeted therapies that could eventually relegate chemotherapy and transplants to a secondary role.
However, imatinib has proven powerful, starting a season of progress and allowing many Ph+ ALL patients to grow older along with it. This is a long sought after turning point, which was well caught by this study.
Conflict-of-interest disclosure: The author declares no competing financial interests.