Key Points
Imatinib improves outcomes for adults with Ph+ ALL at least in part by facilitating allogeneic stem cell transplant.
Allogeneic hematopoietic stem cell transplant is not dispensible in Ph+ ALL in the imatinib era.
Abstract
The Philadelphia chromosome positive arm of the UKALLXII/ECOG2993 study for adult acute lymphoblastic leukemia (ALL) enrolled 266 patients between 1993 and 2003 (pre-imatinib cohort). In 2003 imatinib was introduced as a single-agent course following induction (N = 86, late imatinib). In 2005 imatinib was added to the second phase of induction (N = 89, early imatinib). The complete remission (CR) rate was 92% in the imatinib cohort vs 82% in the preimatinib cohort (P = .004). At 4 years, the overall survival (OS) of all patients in the imatinib cohort was 38% vs 22% in the preimatinib cohort (P = .003). The magnitude of the difference between the preimatinib and imatinib cohorts in event-free survival (EFS), OS, and relapse-free survival (RFS) seen in univariate analysis was even greater in the multivariate analysis. In the preimatinib cohort, 31% of those starting treatment achieved hematopoietic stem cell transplant (alloHSCT) compared with 46% in the imatinib cohort. A Cox multivariate analysis taking alloHSCT into account showed a modest additional benefit to imatinib (hazard ratio for EFS = 0.64, 95% confidence interval 0.44-0.93, P = .02), but no significant benefit for OS and RFS. Adding imatinib to standard therapy improves CR rate and long-term OS for adults with ALL. A proportion of the OS benefit derives from the fact that imatinib facilitates alloHSCT. This trial was registered at clinicaltrials.gov as NCT00002514.
Introduction
Adults with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) constitute the largest defined subgroup of ∼25% of patients.1 Due to the poor prognosis with chemotherapy treatment alone, allogeneic hematopoietic stem cell transplant (alloHSCT) is recommended for all adults in first complete remission (CR1) following induction chemotherapy.2 The tyrosine kinase inhibitor (TKI) imatinib has been widely studied as an addition to initial therapy, and several studies have now reported higher rates of CR with the potential for improved long-term outcomes.3-7
The “imatinib cohort” of the Philadelphia positive arm of the adult ALL trial UKALLXII/Eastern Cooperative Oncology Group (ECOG)2993 evaluated the hypothesis that the addition of imatinib to therapy would improve CR rate and enhance overall survival (OS). The imatinib cohort was open between May 2004 and December 2006 (US) and between March 2003 and October 2008 (United Kingdom [UK]).
Initially, a 1-month block of single-agent imatinib was given as a consolidation therapy after 2 cycles of induction, hereafter referred to as “late imatinib.” An amendment in 2005 to 2006 mandated that imatinib be given in conjunction with the second phase of induction chemotherapy, hereafter referred to as “early imatinib.” Following achievement of CR, all patients underwent alloHSCT if there was a suitable sibling or unrelated donor. The outcome of 267 patients with Ph+ ALL from the preimatinib cohort (1993-2004) has already been published.8 The current report comprises the final analysis of the 175 patients in the imatinib-treated cohort. It is the largest prospective study of the use of imatinib in Ph+ ALL. The patients treated on the same protocol in the preimatinib cohort provide an important comparator group to assess the role of imatinib in Ph+ ALL in long-term outcome, with the advantage that the only change in protocol treatment was the addition of imatinib.
Methods
Eligibility and diagnosis
Patients with newly diagnosed Ph+ ALL aged 15 to 65 (for changes in the age limits over time, see supplemental Methods, available on the Blood Web site) established by documenting >25% bone marrow lymphoblasts were eligible; t(9;22)(q34;q11.2) or BCR-ABL fusion was detected and checked as previously described.8
Treatment and response evaluation
The Ethics Committee (UK) or Institutional Review Board of participating centers (US) gave approval. All subjects gave written informed consent in accordance with the Declaration of Helsinki. Phase I and II of induction chemotherapy were administered as published and are described in the supplemental Methods.9 In the late imatinib cohort, patients received a postinduction cycle of 1 month of imatinib at 400 mg/day, intensified to 600 mg wherever possible. An amendment introduced imatinib earlier, to be co-administered with the second phase of induction chemotherapy, hereafter termed early imatinib. Patients in CR after induction were offered myeloablative alloHSCT etoposide and total body irradiation, although other total body irradiation-containing regimens were acceptable. Those who lacked a suitable allogeneic donor or had contraindications to alloHSCT could receive either autologous HSCT or continue consolidation and maintenance chemotherapy. The definition of a “suitable allogeneic donor” was amended during the lifetime of the protocol. Sibling and fully matched (8 of 8) unrelated donors (MUD) were permitted from the outset. Mismatched, haploidentical, and umbilical cord blood alloHSCT were not part of the preimatinib cohort protocol, and hence those few that were carried out were recorded as deviations and considered “nonprotocol alloHSCT” in the published pre-imatinib cohort analysis.8 However, an amendment allowed these in the imatinib cohorts. Nonmyeloablative, reduced intensity conditioning (RIC) regimens were not part of the protocol for either cohort but were recorded as deviations and analyzed as “nonprotocol alloHSCT.” Imatinib was scheduled to be restarted as soon as possible following HSCT and to be continued for 2 years, as tolerated. Continuous administration of imatinib with consolidation and maintenance chemotherapy was also permitted.
Response was evaluated by conventional morphological criteria. CR was defined as <5% marrow lymphoblasts with trilineage hematopoiesis and adequate blood count recovery. The “on protocol” CR rate is the proportion of the total population who achieved remission after 2 phases of protocol induction. The overall CR rate is the proportion of patients who achieved CR at any point during induction/consolidation. Where patients’ therapy deviated from the protocol, outcome data collection was continued to allow analysis by intention to treat. Patients did not receive any TKI other than imatinib except in 2 cases; in one, a switch to dasatinib occurred due to toxicity of imatinib and in another a short period of dasatinib was administered pretransplant. Both of these patients relapsed, so we can confirm that the administration of dasatinib did not account for any potential benefit.
Statistical analysis
Detailed methods for statistical analysis are given in the supplemental Methods.
Results
Patients
Figure 1 details the progress of all patients through the study; 175 eligible patients were enrolled and started induction treatment in the imatinib cohort, 86 in the late imatinib cohort and 89 in the early imatinib cohort. Three patients were lost to follow-up before 6 months and were censored at the date last seen. Median follow-up for the remaining imatinib cohort was 4 years 9 months (range: 18 months to 8 years, 8 months). The outcome of 267 preimatinib cohort patients has been previously reported; one misdiagnosis means that a preimatinib cohort of N = 266 patients, median follow-up at 10.4 years is presented here. Patient characteristics are summarized in Table 1. No differences in white blood count (WBC) or gender were seen between the treatment cohorts (P > .1). There is a slightly (but statistically significantly) older median age in the imatinib cohort due to an increase in the upper age limit for enrolment from 55 years to 65 years in 2003.
A flowchart of all patients entered into the Ph+ arm of UKALLXII/E2993.
Patient characteristics at diagnosis
| . | Number (%) . | P value . | |||||
|---|---|---|---|---|---|---|---|
| Preimatinib cohort (N = 266) . | Imatinib cohort . | ||||||
| Any imatinib (N = 175) . | Late imatinib (N = 86) . | Early imatinib (N = 89) . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre vs late vs early imatinib (trend) . | ||
| Sex | |||||||
| Male | 149 (56) | 110 (63) | 54 (63) | 56 (63) | P = .2 | P = .9 | P = .2 |
| Female | 117 (44) | 65 (37) | 32 (37) | 33 (37) | |||
| WBC ×109/l | |||||||
| Unknown | 2 (1) | 0 | 0 | 0 | |||
| <30 | 140 (52) | 96 (55) | 46 (53) | 50 (56) | P = .7 | P = .7 | P = .6 |
| ≥30 | 124 (47) | 79 (45) | 40 (47) | 39 (44) | |||
| Median (range) | 26.8 (1.5-438) | 21.0 (0.5-491) | 22.1 (0.5-491) | 21.0 (0.8-372.3) | P = .4 | P = .8 | P = .4 |
| Age (years) | |||||||
| <30 | 65 (24) | 38 (22) | 17 (20) | 21 (24) | P = .0007 | P = .8 | P = .002 |
| 30-49 | 155 (58) | 75 (43) | 39 (45) | 36 (40) | |||
| ≥50 | 46 (17) | 62 (35) | 30 (35) | 32 (36) | |||
| Median (range) | 40 (15-60) | 42 (16-64) | 43 (16-63) | 42 (16-64) | P = .002 | P = .9 | P = .003 |
| CNS disease | |||||||
| Yes | 14 (5) | 3 (2) | 3 (3) | 0 (0) | P = .06 | P = .08 | P = .03 |
| No/unknown | 252 (95) | 172 (98) | 83 (97) | 89 (100) | |||
| . | Number (%) . | P value . | |||||
|---|---|---|---|---|---|---|---|
| Preimatinib cohort (N = 266) . | Imatinib cohort . | ||||||
| Any imatinib (N = 175) . | Late imatinib (N = 86) . | Early imatinib (N = 89) . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre vs late vs early imatinib (trend) . | ||
| Sex | |||||||
| Male | 149 (56) | 110 (63) | 54 (63) | 56 (63) | P = .2 | P = .9 | P = .2 |
| Female | 117 (44) | 65 (37) | 32 (37) | 33 (37) | |||
| WBC ×109/l | |||||||
| Unknown | 2 (1) | 0 | 0 | 0 | |||
| <30 | 140 (52) | 96 (55) | 46 (53) | 50 (56) | P = .7 | P = .7 | P = .6 |
| ≥30 | 124 (47) | 79 (45) | 40 (47) | 39 (44) | |||
| Median (range) | 26.8 (1.5-438) | 21.0 (0.5-491) | 22.1 (0.5-491) | 21.0 (0.8-372.3) | P = .4 | P = .8 | P = .4 |
| Age (years) | |||||||
| <30 | 65 (24) | 38 (22) | 17 (20) | 21 (24) | P = .0007 | P = .8 | P = .002 |
| 30-49 | 155 (58) | 75 (43) | 39 (45) | 36 (40) | |||
| ≥50 | 46 (17) | 62 (35) | 30 (35) | 32 (36) | |||
| Median (range) | 40 (15-60) | 42 (16-64) | 43 (16-63) | 42 (16-64) | P = .002 | P = .9 | P = .003 |
| CNS disease | |||||||
| Yes | 14 (5) | 3 (2) | 3 (3) | 0 (0) | P = .06 | P = .08 | P = .03 |
| No/unknown | 252 (95) | 172 (98) | 83 (97) | 89 (100) | |||
CNS, central nervous system.
Response to induction therapy: imatinib vs preimatinib cohort
Table 2 details the outcome following induction therapy. “Clinically significant” infection was reported in between 40% and 60% of patients for each induction course and did not differ between the cohorts. The induction death rate was similar in the preimatinib and imatinib cohorts (13/266, 5% vs 9/175, 5%; P = .9).
Response to induction therapy
| . | Number (%) . | P value . | |||||
|---|---|---|---|---|---|---|---|
| Preimatinib cohort (N = 266) . | Imatinib cohort . | ||||||
| Any imatinib (N = 175) . | Late imatinib (N = 86) . | Early imatinib (N = 89) . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre vs late vs early imatinib (trend) . | ||
| Significant infection phase I induction | 112/254 (44%) | 84/169 (50%) | 43/83 (52%) | 41/86 (48%) | P = .3 | P = .6 | P = .4 |
| Significant infection phase II induction | 130/217 (60%) | 80/144 (56%) | 41/72 (57%) | 39/72 (54%) | P = .4 | P = .7 | P = .4 |
| Died in induction (<day 56) | 13 (5%) | 9 (5%) | 3 (3%) | 6 (7%) | P = .9 | P = .5 | P = .6 |
| Survived induction, but no CR | 34/253 (13%) | 5/166 (3%) | 4/83 (5%) | 1/83 (1%) | P = .0003 | P = .4 | P = .0003 |
| CR achieved after 2 phases of protocol induction | 177 (67%) | 135 (77%) | 65 (76%) | 70 (79%) | P = .02 | P = .6 | P = .05 |
| CR achieved, but a “protocol deviation” occurred in induction** | 19 (7%) | 19 (11%) | 9 (10%) | 10 (11%) | |||
| No CR following induction, but achieved CR after induction/consolidation | 20 (8%) | 5 (3%) | 5 (6%) | 0 (0%) | |||
| CR assumed, but no date available* | 3 (1%) | 2 (1%) | 0 (0%) | 2 (2%) | |||
| Overall CR | 219 (82%) | 161 (92%) | 79 (92%) | 82 (92%) | P = .004 | P = .9 | P = .008 |
| . | Number (%) . | P value . | |||||
|---|---|---|---|---|---|---|---|
| Preimatinib cohort (N = 266) . | Imatinib cohort . | ||||||
| Any imatinib (N = 175) . | Late imatinib (N = 86) . | Early imatinib (N = 89) . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre vs late vs early imatinib (trend) . | ||
| Significant infection phase I induction | 112/254 (44%) | 84/169 (50%) | 43/83 (52%) | 41/86 (48%) | P = .3 | P = .6 | P = .4 |
| Significant infection phase II induction | 130/217 (60%) | 80/144 (56%) | 41/72 (57%) | 39/72 (54%) | P = .4 | P = .7 | P = .4 |
| Died in induction (<day 56) | 13 (5%) | 9 (5%) | 3 (3%) | 6 (7%) | P = .9 | P = .5 | P = .6 |
| Survived induction, but no CR | 34/253 (13%) | 5/166 (3%) | 4/83 (5%) | 1/83 (1%) | P = .0003 | P = .4 | P = .0003 |
| CR achieved after 2 phases of protocol induction | 177 (67%) | 135 (77%) | 65 (76%) | 70 (79%) | P = .02 | P = .6 | P = .05 |
| CR achieved, but a “protocol deviation” occurred in induction** | 19 (7%) | 19 (11%) | 9 (10%) | 10 (11%) | |||
| No CR following induction, but achieved CR after induction/consolidation | 20 (8%) | 5 (3%) | 5 (6%) | 0 (0%) | |||
| CR assumed, but no date available* | 3 (1%) | 2 (1%) | 0 (0%) | 2 (2%) | |||
| Overall CR | 219 (82%) | 161 (92%) | 79 (92%) | 82 (92%) | P = .004 | P = .9 | P = .008 |
Preimatinib cohort patients: 2 died (at 13 months and 2 years, 3 months), 1 alive at 9 years, 1 month. Imatinib cohort patients: 1 emigrated day 75, and 1 alive at 4 years, 8 months.
NB, no patient received dasatinib during initial therapy.
Regarding response to treatment, 177/266 (67%) patients in the preimatinib cohort achieved CR following 2 phases of protocol induction compared with 135/175 (76%) in the imatinib cohort (P = .02). At the time-point of formal CR assessment, the late imatinib cohort had not yet received the drug. There was a significant decrease in the proportion of patients surviving induction but never achieving CR between the preimatinib and the imatinib cohorts (34/253, 13% vs 5/166, 3%; P = .0003). The overall CR rate, which includes all patients who achieved CR at any time, was 10% higher, 161/175 (92%) in patients who received any imatinib compared with 219/266 (82%) in the preimatinib cohort, and this was highly statistically significant (P = .004).
The difference in the on-protocol CR rate was not statistically significant between the preimatinib and late imatinib cohorts (67% vs 76%, P = .1) or between the late and early imatinib cohorts (76% vs 79%, P = .6). However, the trend to improved CR rate from the preimatinib cohort, through the addition of late imatinib to the introduction of early imatinib, was marginally significant (P = .05).
Outcome: imatinib cohort vs preimatinib cohort
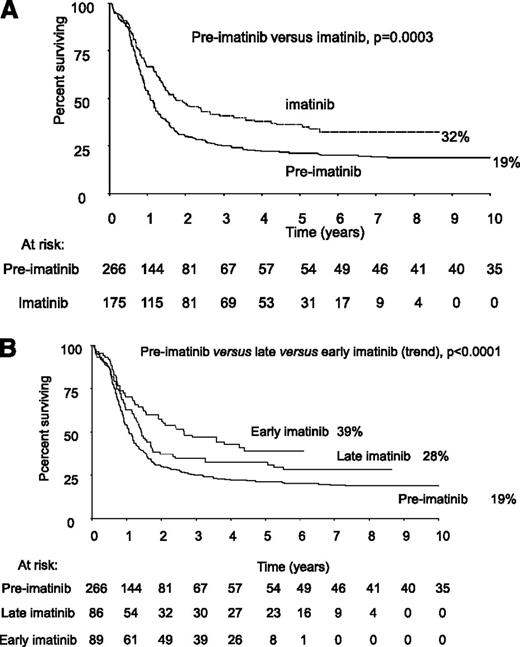
Median follow-up in the imatinib cohort was 4 years, 9 months and for the early imatinib cohort it was 4.5 years; hence, we present the main outcome measures at 4 years, as shown in Table 3. At 4 years, the OS of all patients in the imatinib cohort was 38% (95% CI = 31% to 45%), event-free survival (EFS) was 33% (95% CI = 26% to 40%), and relapse-free survival (RFS) was 50% (95% CI = 41% to 58%). The numerical data including odds ratios and confidence intervals for all end points are given in Table 3. For OS, EFS, and RFS, the addition of imatinib at any time during therapy was associated with a significantly better outcome than when no imatinib was given. In particular, there was a considerable reduction in relapse risk in the imatinib cohort (preimatinib vs any imatinib OR = 0.59 [0.46-0.79], P = .0003). A comparison of outcome between the early and late imatinib cohorts did not show any statistical significance, although the P value for the trend across the 3 cohorts was ≤.0001 for each of OS, EFS, and RFS, suggesting that earlier exposure to imatinib during therapy was likely to be beneficial. To show the longest possible outcome data, Figure 2A gives the 10-year Kaplan Meier plot for OS for all patients in the report and Figure 2B shows the 10-year Kaplan Meier plot for OS by imatinib cohort.
Univariate analysis of outcome
| . | Preimatinib cohort (N = 266) . | Imatinib cohort . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre- vs late vs early imatinib (trend) . | ||
|---|---|---|---|---|---|---|---|
| Any imatinib (N = 175) . | Late imatinib (N = 86) . | Early imatinib (N = 89) . | |||||
| Percent at 4 y (95% CI) . | |||||||
| OS | 22 (17-27) | 38 (31-45) | 33 (23-43) | 43 (32-53) | OR = 0.67 (0.53-0.83) P = .0003 | OR=0.75 (0.52-1.09) P = .1 | OR=0.77 (0.67-0.88) P < .0001 |
| EFS | 18 (13-22) | 33 (26-40) | 28 (18-37) | 37 (27-48) | OR = 0.65 (0.52-0.80) P = .0001 | OR=0.74 (0.51-1.06) P = .1 | OR=0.75 (0.66-0.85) P < .0001 |
| RFS | 33 (26-41) | 50 (41-58) | 46 (33-58) | 53 (41-66) | OR = 0.59 (0.46-0.79) P = .0003 | OR=0.67 (0.42-1.09) P = .1 | OR=0.72 (0.61-0.85) P = .0001 |
| Survival free from death in remission | 64 (55-73) | 72 (64-80) | 66 (53-80) | 76 (66-86) | OR = 0.81 (0.53-1.24) P = .3 | OR=0.79 (0.41-1.52) P = .5 | OR=0.86 (0.67-1.11) P = .2 |
| . | Preimatinib cohort (N = 266) . | Imatinib cohort . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre- vs late vs early imatinib (trend) . | ||
|---|---|---|---|---|---|---|---|
| Any imatinib (N = 175) . | Late imatinib (N = 86) . | Early imatinib (N = 89) . | |||||
| Percent at 4 y (95% CI) . | |||||||
| OS | 22 (17-27) | 38 (31-45) | 33 (23-43) | 43 (32-53) | OR = 0.67 (0.53-0.83) P = .0003 | OR=0.75 (0.52-1.09) P = .1 | OR=0.77 (0.67-0.88) P < .0001 |
| EFS | 18 (13-22) | 33 (26-40) | 28 (18-37) | 37 (27-48) | OR = 0.65 (0.52-0.80) P = .0001 | OR=0.74 (0.51-1.06) P = .1 | OR=0.75 (0.66-0.85) P < .0001 |
| RFS | 33 (26-41) | 50 (41-58) | 46 (33-58) | 53 (41-66) | OR = 0.59 (0.46-0.79) P = .0003 | OR=0.67 (0.42-1.09) P = .1 | OR=0.72 (0.61-0.85) P = .0001 |
| Survival free from death in remission | 64 (55-73) | 72 (64-80) | 66 (53-80) | 76 (66-86) | OR = 0.81 (0.53-1.24) P = .3 | OR=0.79 (0.41-1.52) P = .5 | OR=0.86 (0.67-1.11) P = .2 |
Overall ratio (brackets denote 95% CI). OR <1 indicates better outcome in the later comparator(s) (ie, better outcome in the any imatinib compared with preimatinib cohort, better outcome in early imatinib compared with the late imatinib cohort, and better outcome in early imatinib compared with the late imatinib compared with the preimatinib cohort). P values that are significant between the cohorts are shown in bold.
Kaplan Meier plot of 10-year OS of all patients with Ph+ ALL by cohort. (A) Preimatinib vs imatinib. (B) Preimatinib vs late imatinib vs early imatinib.
Kaplan Meier plot of 10-year OS of all patients with Ph+ ALL by cohort. (A) Preimatinib vs imatinib. (B) Preimatinib vs late imatinib vs early imatinib.
Postinduction therapy; rate of alloHSCT, imatinib vs preimatinib cohort
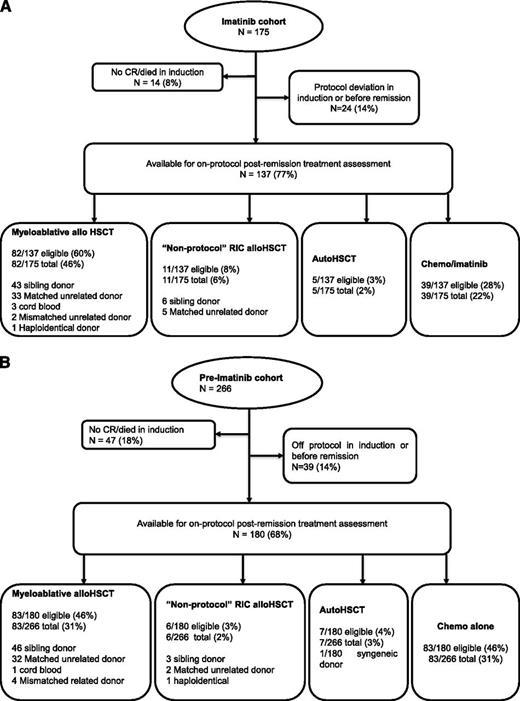
A flowchart of the progress of imatinib cohort patients during postinduction therapy is shown in Figure 3A. Fourteen patients in the imatinib cohort did not achieve CR or died during induction, and 24 had a protocol deviation in induction or before remission, leaving 137 (77%) eligible for protocol postinduction therapy. Eighty-two patients (60%) received a myeloablative alloHSCT (43 sibling donor, 33 MUD, 3 cord blood, 2 mismatched unrelated donor, 1 haploidentical donor) and 5 patients (3%) received a per-protocol autologous HSCT. Eleven (8%) patients received nonprotocol RIC alloHSCT (5 MUD and 6 sibling donor). The remaining 39 patients did not receive a transplant.
A flowchart of postremission treatment. (A) Treatment received in the imatinib cohort. (B) Treatment received in the preimatinib cohort.
A flowchart of postremission treatment. (A) Treatment received in the imatinib cohort. (B) Treatment received in the preimatinib cohort.
The detailed postinduction therapy of the patients in the pre-imatinib cohort has already been published,8 but the updated data are also shown in Figure 3B: 47 patients did not achieve CR or died during induction, and 39 had a significant protocol deviation in induction or before CR, leaving 180 (68%) eligible for protocol postinduction therapy. Eighty-three patients (46% of those eligible) received a myeloablative alloHSCT (46 sibling donor, 32 MUD, 1 cord blood, 4 mismatched related donor) and 1 patient received a syngeneic donor HSCT. Seven patients (4%) received a per protocol autologous HSCT. Six (3%) patients received nonprotocol RIC alloHSCT (2 RIC MUD and 3 RIC sibling donor, 1 RIC haploidentical donor). The remaining 83 patients did not receive alloHSCT in CR1. Eighty-three of 180 pre-imatinib cohort patients who achieved CR on protocol (46%) received protocol type myeloablative sibling donor or MUD alloHSCT; this was 31% of the total population starting therapy. In the imatinib cohort, 82 of 137 patients who achieved CR on protocol (60%) received a myeloablative sibling donor or MUD alloHSCT; this was 46% of the total population. These data suggest that imatinib has had an impact on the most common reasons for not receiving an alloHSCT in the preimatinib cohort,8 namely failure to remit or relapse prior to alloHSCT. To analyze this more carefully, we quantified the number of alloHSCTs in patients reaching 84 days (12 weeks) in CR, because this was the earliest possible time of alloHSCT on protocol. In the imatinib cohort, 136 of 175 (78%) patients reached the 84-day point in CR compared with only 176 of 266 (66%) in the preimatinib cohort (P = .009), strongly suggesting that the increased alloHSCT rate was at least in part due to a higher proportion of patients being eligible for alloHSCT in the imatinib cohort. However, the median time from diagnosis to alloHSCT was significantly shorter in the imatinib cohort than in the preimatinib cohort (135 days compared with 160 days, P < .0001). Furthermore, a higher proportion of eligible patients who reached 84 days in CR received alloHSCT in the imatinib cohort compared with the preimatinib cohort (98 of 136 [71%] vs 97 of 176 [55%], P = .002). Taken together, these data suggest that both imatinib and a shorter time from diagnosis to transplant have contributed to the improved alloHSCT rate in the imatinib cohort.
Relationship between alloHSCT and outcome in the imatinib cohort
Data within the imatinib cohort were analyzed by postremission treatment received. Figure 4 shows the OS at 4 years (the 10-year OS curves are shown in supplemental Figure 1) for all patients who achieved CR on protocol and survived in CR1 to day 84 who went on to receive a myeloablative MUD or sibling donor alloHSCT (N = 76), a nonprotocol RIC alloHSCT (N = 11), or no alloHSCT (N = 38). The “nonprotocol” types of myeloablative alloHSCT were excluded from the alloHSCT curve. Data from patients receiving RIC alloHSCT are also shown on the figure in a separate curve to make the results comparable with analyses done for the preimatinib cohort. The Kaplan Meier plot shows a significantly superior OS for patients who received a sibling donor or MUD myeloablative alloHSCT (50%) over those who received chemotherapy alone (19%). EFS (46% vs 14%) and RFS (69% vs 18%), respectively, were also significantly superior (curves not shown) in patients receiving myeloablative alloHSCT. When the 6 patients who received mismatched, unrelated cord blood and haploidentical transplants were included with the MUD and sibling donor transplants, the outcome (4-year OS: 52%, EFS: 49%, RFS: 72%) was still superior to the no-alloHSCT group. However, the 39 patients who did not receive a transplant were significantly older than those who received a myeloablative alloHSCT (median age 52 years [range 20-64] vs median age 37 years [range 16-59]; P < .0001), and there are likely to be other selection biases. An improved outcome compared with chemotherapy and imatinib alone was also apparent for those who received a nonprotocol RIC alloHSCT (median age 54 years, range 44-63 years). Of the 5 patients who received autologous HSCT, 1 died of toxicity 4 days after transplant, 1 relapsed 25 months from trial entry and died 4 months later, and the other 3 are known to have survived in CR for 3.5, 8, and 8 years, respectively.
Kaplan Meier plot of 4-year OS in the imatinib cohort. Represents receipt of protocol myeloablative sibling/MUD alloHSCT, nonprotocol RIC alloHSCT, or no alloHSCT in patients who achieved CR on protocol and survived to day 84 (12 weeks), the earliest possible time of alloHSCT per protocol.
Kaplan Meier plot of 4-year OS in the imatinib cohort. Represents receipt of protocol myeloablative sibling/MUD alloHSCT, nonprotocol RIC alloHSCT, or no alloHSCT in patients who achieved CR on protocol and survived to day 84 (12 weeks), the earliest possible time of alloHSCT per protocol.
Multivariate analysis: impact of imatinib cohort on outcome
Cox multivariate analyses of the data from the entire Ph+ ALL cohort were performed to discover whether the benefit of imatinib was still apparent when prognostic characteristics (gender, age, initial WBC, and/or CNS disease at presentation) were taken into account. Age and presenting WBC were highly significant prognostic variables for OS, EFS, and RFS, with increasing age and higher presenting WBC engendering an increasing risk of poor outcome. Gender and CNS disease at presentation were not prognostic for any end point. Table 4 shows the Cox univariate models and models from multivariate analyses of the entire Ph+ study population, both with and without taking these prognostic factors into account. When prognostic characteristics were taken into account, the improvement in OS, EFS, and RFS in the imatinib cohorts over the preimatinib cohort was even greater than in univariate analysis. Outcomes continuously improved through the pre-, late, and early imatinib cohorts.
Cox analysis: effect of inclusion of imatinib and use of alloHSCT on outcome
| . | . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre- vs late vs early imatinib (trend) . |
|---|---|---|---|---|
| . | . | . | HR (95% CI), P value . | . |
| OS | Univariate Cox model | 0.66 (0.53-0.83), P = .0004 | 0.77 (0.53-1.12), P = .2 | 0.76 (0.66-0.88), P = .0002 |
| Multivariate Cox model* | 0.57 (0.45-0.73), P < .0001 | 0.72 (0.49-1.05), P = .09 | 0.69 (0.59-0.80), P < .0001 | |
| Multivariate Cox allowing for alloHSCT | 0.69 (0.47-1.01), P = .06 | 0.78 (0.41-1.48), P = .4 | 0.77 (0.60-1.00), P = .05 | |
| EFS | Univariate Cox model | 0.64 (0.51-0.80), P < .0001 | 0.76 (0.53-1.08), P = .1 | 0.74 (0.65-0.86), P < .0001 |
| Multivariate Cox model* | 0.58 (0.46-0.73), P < .0001 | 0.67 (0.46-0.98), P = .04 | 0.69 (0.60-0.80), P < .0001 | |
| Multivariate Cox allowing for alloHSCT | 0.64 (0.44-0.93), P = .02 | 0.62 (0.33-1.18), P = .1 | 0.73 (0.57-0.93), P = .01 | |
| RFS | Univariate Cox model | 0.58 (0.43-0.78), P = .003 | 0.68 (0.42-1.09), P = .1 | 0.69 (0.57-0.84), P = .0001 |
| Multivariate Cox model* | 0.50 (0.37-0.68), P < .0001 | 0.57 (0.35-0.95), P = .03 | 0.63 (0.52-0.76), P < .0001 | |
| Multivariate Cox allowing for alloHSCT | 0.68 (0.45-1.02), P = .06 | 0.45 (0.22-0.94), P = .03 | 0.74 (0.56-0.96), P = .03 | |
| Survival free from death in remission | Univariate Cox model | 0.83 (0.55-1.27), P = .4 | 0.85 (0.45-1.61), P = .6 | 0.87 (0.67-1.14), P = .3 |
| Multivariate Cox model* | 0.76 (0.49-1.17), P = .2 | 0.76 (0.40-1.46), P = .4 | 0.82 (0.63-1.07), P = .1 | |
| Multivariate Cox allowing for alloHSCT | 0.68 (0.24-1.97), P = .5 | 2.04 (0.36-11.5), P = .4 | 0.89 (0.47-1.68), P = .7 |
| . | . | Preimatinib vs any imatinib . | Late vs early imatinib . | Pre- vs late vs early imatinib (trend) . |
|---|---|---|---|---|
| . | . | . | HR (95% CI), P value . | . |
| OS | Univariate Cox model | 0.66 (0.53-0.83), P = .0004 | 0.77 (0.53-1.12), P = .2 | 0.76 (0.66-0.88), P = .0002 |
| Multivariate Cox model* | 0.57 (0.45-0.73), P < .0001 | 0.72 (0.49-1.05), P = .09 | 0.69 (0.59-0.80), P < .0001 | |
| Multivariate Cox allowing for alloHSCT | 0.69 (0.47-1.01), P = .06 | 0.78 (0.41-1.48), P = .4 | 0.77 (0.60-1.00), P = .05 | |
| EFS | Univariate Cox model | 0.64 (0.51-0.80), P < .0001 | 0.76 (0.53-1.08), P = .1 | 0.74 (0.65-0.86), P < .0001 |
| Multivariate Cox model* | 0.58 (0.46-0.73), P < .0001 | 0.67 (0.46-0.98), P = .04 | 0.69 (0.60-0.80), P < .0001 | |
| Multivariate Cox allowing for alloHSCT | 0.64 (0.44-0.93), P = .02 | 0.62 (0.33-1.18), P = .1 | 0.73 (0.57-0.93), P = .01 | |
| RFS | Univariate Cox model | 0.58 (0.43-0.78), P = .003 | 0.68 (0.42-1.09), P = .1 | 0.69 (0.57-0.84), P = .0001 |
| Multivariate Cox model* | 0.50 (0.37-0.68), P < .0001 | 0.57 (0.35-0.95), P = .03 | 0.63 (0.52-0.76), P < .0001 | |
| Multivariate Cox allowing for alloHSCT | 0.68 (0.45-1.02), P = .06 | 0.45 (0.22-0.94), P = .03 | 0.74 (0.56-0.96), P = .03 | |
| Survival free from death in remission | Univariate Cox model | 0.83 (0.55-1.27), P = .4 | 0.85 (0.45-1.61), P = .6 | 0.87 (0.67-1.14), P = .3 |
| Multivariate Cox model* | 0.76 (0.49-1.17), P = .2 | 0.76 (0.40-1.46), P = .4 | 0.82 (0.63-1.07), P = .1 | |
| Multivariate Cox allowing for alloHSCT | 0.68 (0.24-1.97), P = .5 | 2.04 (0.36-11.5), P = .4 | 0.89 (0.47-1.68), P = .7 |
Adjusting for gender, age at diagnosis, initial WBC, CNS disease at presentation, and an interaction between WBC and CNS disease at presentation and in “allowing for alloHSCT,” treatment (MUD/sibling alloHSCT vs chemotherapy). Preimatinib vs late imatinib vs early imatinib (treated as an ordinal variable). HR, hazard ratio. HR <1 indicates better outcome with increasing values of the variable (ie, outcome improves with cohort: any imatinib better than preimatinib; early better than late imatinib; early imatinib better than late imatinib better than preimatinib cohort).
Effect of imatinib on outcome, allowing for the effect of HSCT
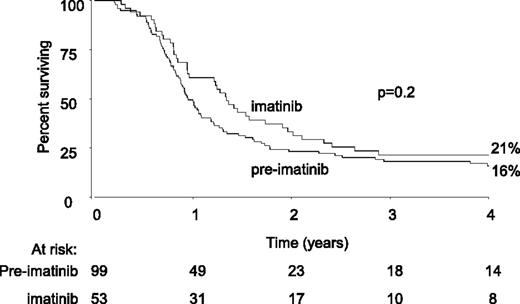
We showed that adding imatinib to induction treatment increases the rate of alloHSCT. To discover whether imatinib has an effect on outcome in addition to increasing the number of patients surviving to alloHSCT, the Cox multivariate analysis was repeated on the entire preimatinib and imatinib dataset, also allowing for treatment (MUD or sibling alloHSCT vs no alloHSCT) as well as other known prognostic characteristics. The data are shown in Table 4. Of the 380 patients who achieved CR (either on protocol or following any element of nonprotocol treatment), 175 patients received a myeloablative MUD or sibling alloHSCT in CR1, whereas 152 patients achieved CR but did not receive a transplant. The 9 patients in the preimatinib cohort who only achieved CR after alloHSCT were excluded from the analysis, as were the 12 patients who received autograft, 1 who received syngeneic transplant, and the 17 who received RIC. The 14 patients who received a myeloablative alloHSCT from a mismatched, cord, or haploidentical donor were also excluded from the main analysis so as not to bias the comparison, because they were not considered “protocol” in the preimatinib cohort. As a check, we re-analyzed the data to include them, but the results did not change. The analysis showed a modest beneficial effect of adding imatinib to treatment in all end points except survival free from death in remission. That benefit was over and above the beneficial effect of alloHSCT. The magnitude of the benefit reached statistical significance for EFS (hazard ratio = 0.64, 95% CI = 0.44-0.93, P = .02) but was not significant for OS and RFS. Further modeling indicated the size of the beneficial effect of imatinib was similar in transplanted and nontransplanted patients (interaction P values P > .2 for OS, EFS, and RFS). The analysis was repeated, excluding patients who received any protocol deviation treatment prior to achievement of CR. With fewer patients, the estimates of the hazard ratios were similar but did not reach statistical significance (data not shown). Figure 5 shows a 4-year Kaplan Meier plot of OS in the 152 patients who achieved CR but did not receive a transplant, by imatinib cohort. The 10-year OS curves can be found in supplemental Figure 2.
Kaplan Meier plot of 4-year OS in all patients with Ph+ ALL who did not receive alloHSCT, by preimatinib vs imatinib cohort.
Kaplan Meier plot of 4-year OS in all patients with Ph+ ALL who did not receive alloHSCT, by preimatinib vs imatinib cohort.
Discussion
This is the largest prospective trial evaluating the role of imatinib in patients with Ph+ ALL. The outcome for patients treated with imatinib has been compared with the outcome of a large cohort of patients from the preimatinib era treated on the same trial. Our first finding, that the addition of imatinib increases the overall rate of CR by 10% from 82% to 92% in patients between 15 and 65 years of age, concurs with previously published findings from smaller studies.3-7 In our study, the addition of imatinib to the second phase of induction chemotherapy regimen appeared safe and without added toxicity. When imatinib was first added to UKALLXII/ECOG2993 as an additional course following conventional chemotherapy induction, the on-protocol CR rate after 2 phases of induction treatment improved over cohorts: 67% preimatinib vs 76% late imatinib vs 79% early imatinib, which was statistically significant (P value for trend = .05). Because the late imatinib cohort had not received any imatinib by the time of initial CR assessment, our data indicate that an element of improvement in outcome over time may be due to factors that the trial was not designed to assess, such as improvements in supportive care. Nonetheless, our finding is clear: imatinib therapy improves CR rate in Ph+ ALL.
Does the improved CR rate translate to long-term survival benefit? Most previously published studies of imatinib have reported at a time of short follow-up and have included only historical controls. A recent Northern Italian Leukaemia Group study10 included a total of 94 patients, 35 of whom were a (nonrandomized) historical cohort who did not receive imatinib. The 59 patients who received imatinib had a statistically significant improvement in 5-year OS: 38% vs 23% for those who did not receive the drug. Our study confirms this survival benefit with a benefit of a similar magnitude seen in a much larger patient population; at 4 years, the OS of all 175 patients in our imatinib cohort was 38% vs 22% in our 266 patient preimatinib cohort. Similarly, EFS was 33% vs 18% and RFS 50% vs 33%, respectively, all highly significant differences. Furthermore, the Cox multivariate analysis confirmed the benefit of imatinib when prognostic characteristics were taken into account. Taken together, even allowing for the potential flaw of studies with historical cohort controls, our data support a firm conclusion that adding imatinib to a treatment regimen for Ph+ ALL reduces the risk of relapse and improves survival.
Myeloablative alloHSCT remains the post-remission treatment of choice for patients with Ph+ ALL. Our data strongly suggest that one potential way in which imatinib confers long-term benefits is to increase the rate of alloHSCT. Our data show a significant increase in the proportion of patients reaching alloHSCT in the imatinib cohort as a proportion both of those starting treatment and those who reached CR. However, the median time to alloHSCT was shorter in the imatinib cohort, so improvements in transplant practice over time cannot be ruled out as contributing benefit.
Historically, in the preimatinib era, there were few long-term survivors among patients who did not receive alloHSCT. However, the central role of alloHSCT has been questioned in the imatinib era, particularly in children. In the US Children’s’ Oncology Group study,11 patients aged 21 or younger were treated with imatinib added to chemotherapy, a final cohort receiving continuous imatinib. Only sibling alloHSCT was permitted, allowing a donor vs no donor analysis, which was unfortunately confounded by the high nonprotocol MUD alloHSCT rate. At 3 years, the outcomes were not significantly different for those treated with chemotherapy plus imatinib compared with those assigned to alloHSCT. However, there were 25 or fewer patients per arm. The ALL202 trial from the Japanese Adult Leukemia Study Group5 reported the outcome of 31 patients of “transplantable age” who received chemotherapy and imatinib, but not alloHSCT, in an 80-patient trial. When compared with no-imatinib, chemotherapy-alone historical controls, the 2-year estimated EFS was significantly better for those receiving imatinib. Given these previous studies and the strong interaction between imatinib and alloHSCT in improving the outcome for patients, a key aim of our analyses was to determine the outcome for patients who were unable to receive alloHSCT. This is a difficult question to address statistically, because there are numerous potential confounding differences between the cohorts. However, using a Cox multivariate analysis, taking into account all known prognostic variables as well as receipt of alloHSCT, we demonstrated a modest benefit for the addition of imatinib. OS was marginally, but not statistically, significantly better in the imatinib cohort (P = .06), with the hazard of death in the imatinib cohort being 70% of that in the preimatinib cohort. However, there was an additional improvement in outcome with the trial cohort (pre-imatinib vs late vs early imatinib) over any effect of alloHSCT. This implies that imatinib treatment can modestly affect outcome in the absence of alloHSCT. However, factors such as improvements in supportive care cannot be ruled out as having benefit. We found no evidence that the magnitude of the benefit of transplant differs between cohorts.
Our study has some limitations. First, we did not perform an exhaustive analysis of the relationship between outcome and BCR-ABL1 molecular response and are not able to show useful data on that point.
A second limitation was that the optimal use and tolerance of imatinib post-alloHSCT could not be addressed; although all patients were assigned to receive imatinib, we did not collect sufficiently detailed information on toxicity to draw conclusions. Ribera et al6 reported that 62% of patients could not tolerate imatinib after alloHSCT in a Programa para el Estudio de la Terapeutica en Hemopatıa Maligna study. A MD Anderson retrospective study of 113 patients showed that TKI use post-HSCT did not significantly impact outcome.12 A randomized trial from the German Multicentre Adult ALL Group showed that post-transplant imatinib results in a low relapse rate and excellent long-term outcome irrespective of whether it is given prophylactically or only based on persistence or re-appearance of BCR-ABL1 on molecular monitoring.13 Ram et al14 reported better tolerability with imatinib when given following RIC alloHSCT with only 3 of 18 patients discontinuing, though its effect on relapse was not statistically significant.
Third, the optimum duration of imatinib is uncertain, particularly without myeloablative alloHSCT. In our study, imatinib duration was 2 years, but we do not know whether patients continued the drug after completing the study.
In summary, the UKALLXII/ECOG2993 imatinib study conclusively demonstrates that adding imatinib to the therapy of patients with Ph+ ALL improves overall outcome compared with that observed in the historical UKALLXII/ECOG2993 preimatinib study, with over one-half of patients surviving disease free at 3 years. Our data do not support the omission of alloHSCT from the treatment of Ph+ ALL. Our limited study data on RIC alloHSCT, which have hitherto only been explored in a retrospective manner,15,16 also suggest that there is potential benefit to this approach, which is currently being explored in the UK Neurological Clinical Research Institute trial UKALL14 trial (NCT010185617).
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Novartis for providing imatinib free of charge. We wish to thank Susan M. Richards for her continued support of the trial, and we thank all participating centers, physicians, and patients.
This work was supported by the Medical Research Council (UK) and Leukaemia and Lymphoma Research UK (clinical and laboratory aspects, respectively) in the UK. In the US, we acknowledge grants to ECOG (CA21115, CA17145, CA13650, CA14548, CA15488, and CA14958).
Authorship
Contribution: A.K.F. took part in running the trial, analyzed trial data, and wrote the manuscript; A.H.G. and J.M.R. were Chief Investigators for the trial in the UK and the US, respectively; G.B. was the trial statistician and analyzed trial data; D.I.M., A.K.M., S.M.L., B.P., M.R.L., and M.S.T. took part in running the trial and helped analyze trial data; A.V.M., L.F., G.G., and E.P. organized and oversaw laboratory aspects of the study; and all authors contributed to writing the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adele K. Fielding, University College London, Royal Free Campus, Rowland Hill St, London NW3 2PF, UK; e-mail: a.fielding@ucl.ac.uk.