Key Points
Phosphorylated JAM-A associates with resting integrin αIIbβ3.
JAM-A suppresses outside-in signaling by recruiting Csk to the integrin-c–Src complex.
Abstract
Fibrinogen binding to activated integrin induces outside-in signaling that results in stable platelet aggregates and clot retraction. How integrin αIIbβ3 is discouraged from spontaneous activation is not known. We have recently shown that junctional adhesion molecule-A (JAM-A) renders protection from thrombosis by suppressing integrin outside-in signaling. In this study, we show that JAM-A associates with integrin αIIbβ3 in resting platelets and dissociates upon platelet activation by agonists. We also show that integrin-associated JAM-A is tyrosine phosphorylated and is rapidly dephosphorylated upon platelet activation. C-terminal Src kinase (Csk) binds to tyrosine phosphorylated JAM-A through its Src homology 2 domain. Thus, JAM-A recruits Csk to the integrin-c–Src complex in resting platelets. Csk, in turn, keeps integrin-associated c-Src in an inactive state by phosphorylating Y529 in its regulatory domain. Absence of JAM-A results in impaired c-SrcY529 phosphorylation and augmentation of outside-in signaling-dependent c-Src activation. Our results strongly suggest that tyrosine-phosphorylated JAM-A is a Csk-binding protein and functions as an endogenous inhibitor of integrin signaling. JAM-A recruits Csk to the integrin-c–Src complex, where Csk negatively regulates c-Src activation, thereby suppressing the initiation of outside-in signaling. Upon agonist stimulation, JAM-A is dephosphorylated on the tyrosine, allowing the dissociation of Csk from the integrin complex, and thus facilitating outside-in signaling.
Introduction
On circulating platelets, integrin αIIbβ3 is in a resting conformation incapable of binding its ligand fibrinogen (Fg). During platelet activation by physiological agonists, the integrin is rapidly converted to a high-affinity state through a chain of signaling events termed “inside-out” signaling. Engagement of activated integrin by Fg transmits signals within the platelets through a cascade termed as “outside-in” signaling that results in platelet adhesion, spreading, and clot retraction.1 Integrin activation and signaling is important for the process of hemostasis and thrombosis.2,3 Unwanted activation and signaling can result in life-threatening complications. An elaborate mechanism of regulation of agonist-dependent integrin affinity modulation avoids uninstigated activation of integrin. Likewise, signaling through the integrin (outside-in signaling) is also tightly regulated by both positive and negative regulators. Although a significant amount of information is available on the positive regulators, very little is known about the negative regulators.4
The mechanism of integrin outside-in signaling in platelets is just beginning to be understood. It has been shown that c-Src, a member of the Src family kinases (SFKs), plays a significant role in integrin outside-in signaling.3,5-7 Under resting conditions, SFKs and c-Src are maintained in an inactive state through two intramolecular interactions, including the binding of the Src homology 3 (SH3) domain to a polyproline type II helix,8-10 and the subsequent binding through its Src homology 2 (SH2) domain to a phosphotyrosine residue (Y529) in the C-terminal regulatory domain.11 Maintaining SFKs in an inactive state requires phosphorylation of the C-terminal inhibitory Y529 residue in the SFK by cytoplasmic kinases Csk (C-terminal Src kinase) or Csk homologous kinase.12 It has been shown that Csk suppression of c-Src involves the movement of Csk to sites of c-Src activity.13 Csk also has a SH2 domain through which it binds to a phosphotyrosine residue in a Csk-binding protein.14 Previous studies have shown that the adapter protein, Paxillin, can participate in feedback inhibition of nearby SFKs by recruiting Csk.15,16 It has also been shown that Hic-5, a member of the Paxillin family, is expressed in human platelets and is tyrosine phosphorylated upon platelet activation. Csk is shown to bind phosphorylated Hic-5 via its SH2 domain.16 In resting platelets, c-Src is constitutively associated with the integrins,17 and is kept in an inactive state by phosphorylation at c-SrcY529 residue by Csk.7 However, Hic-5 does not associate with the integrin or regulate c-Src activation. What recruits Csk to the integrin-c–Src complex in inactive platelets is currently unknown.
Cell adhesion molecules (CAMs) belonging to the immunoglobulin superfamily have been indicated to exert a negative effect on platelet activation.4 Junctional adhesion molecule A (JAM-A) is a member of the cortical thymocyte marker of the Xenopus (CTX) family of CAMs.18 JAM-A was originally identified as a platelet receptor for a monoclonal antibody, mAb-F11.19 It has been shown that JAM-A is rapidly phosphorylated during platelet activation by physiological agonists in a protein kinase C-dependent manner.19,20 We recently showed that the genetic ablation of murine Jam-A resulted in a shortened tail bleeding time.21 Furthermore, we showed that the loss of Jam-A resulted in a prothrombotic phenotype as observed by a variety of in vivo thrombosis assays. We also showed that in Jam-A null murine platelets, inside-out signaling was unaffected, while outside-in signaling was impaired.21 How JAM-A affects integrin outside-in signaling is currently unknown.
In this study, we describe the molecular mechanism by which JAM-A regulates integrin outside-in signaling. We found that JAM-A recruited Csk to the integrin αIIbβ3 and c-Src complex in resting platelets, helping maintain c-Src in an inactive state. This complex is rapidly dissociated upon platelet activation by agonists, followed by dephosphorylation of c-SrcY529, which allows the activation of c-Src during integrin outside-in signaling.
Materials and methods
Reagents and antibodies
Human Fg and thrombin were purchased from Enzyme Research (South Bend, IN), PP2 from Calbiochem (La Jolla, CA), adenosine 5′-diphosphate (ADP) from Chrono-Log (Havertown, PA), and antibodies for phospho-c-SrcY418 and phospho-c-SrcY529 from Invitrogen (Carlsbad, CA). Polyclonal antibodies against c-Src, Csk, integrin β3, integrin αIIb, or anti-glutathione S-transferase (GST) monoclonal or isotype-specific control immunoglobulin G (IgGs) were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA). SEW8 (anti-αIIb) was a generous gift from Dr Peter J. Newman, the Blood Center of Wisconsin (Milwaukee, WI). Light chain-specific horseradish peroxidase-conjugated mouse anti-rabbit secondary antibody was from the Jackson Immuno Research Laboratories (West Grove, PA). JAM-A monoclonal and anti-CD36 polyclonal antibodies were obtained from BD Pharmingen (San Jose, CA). Polyclonal anti-GPIbα was obtained from R&D Systems (Minneapolis, MN). The Csk-SH2 construct was a generous gift from Dr Debra Newman, the Blood Center of Wisconsin. Arg-Gly-Asp-Ser (RGDS) and all other chemicals, unless otherwise indicated, were of analytical grade purchased from Sigma-Aldrich (St. Louis, MO).
Human and murine platelet preparation
Whole human blood was drawn into acid/citrate/dextrose 6:1 (v/v) or in 3.8% sodium citrate 9:1 (v/v) by venipuncture from healthy, drug-free volunteers ≥18 years of age under informed consent. Approval for these studies was obtained from the University of Delaware Institutional Review Board according to the Declaration of Helsinki. Washed human platelets were obtained as previously described.22,23 A detailed characterization of Jam-A knockout mice (Jam-Agt/gt) used in this study has been previously reported.24,25 Approval for animal experimental studies was received from the University of Delaware Institutional Animal Care and Use Committee. A heparinized syringe was used to draw blood from the posterior vena cava of 10- to 14-week-old mice, and transferred into a tube containing acid/citrate/dextrose or 3.8% sodium citrate as an anticoagulant depending on the experiment. Mouse blood was diluted using Tyrode’s buffer (1:1) in the absence of calcium, and platelet-rich plasma was obtained by centrifugation at 200 × g for 10 minutes at room temperature (RT).26
Immunofluorescence
Immunofluorescence studies were performed as described previously.27 In brief, platelet samples were incubated overnight with anti-αIIb and anti–JAM-A at 4°C. Samples were then washed and incubated with secondary antibodies: Alexa Fluor 647 donkey anti-rabbit–conjugated IgG and Alexa Fluor 568 donkey anti-mouse IgG. Alexa Fluor 488 phalloidin was used to stain filamentous actin. All samples were mounted in phosphate-buffered saline. Using super-resolution structured illumination microscope (SIM) ELYRA PS.1 (Carl Zeiss, Ithaca, NY), SIM z-stacks were acquired with 5 rotations and 5 phase shifts using a ×100 α-Plan-Apochromat (NA = 1.46) oil immersion medium objective lens. Images were captured using an iXon 885 back-thinned EM-CCD camera (Andor Technology PLC, Belfast, Northern Ireland). SIM reconstructions were performed using an experimental point spread function, baseline cutoff processing, and an affine image alignment based on a multispeck bead alignment. Images were acquired and adjusted in Zen 2011 software (Carl Zeiss).
Cell culture and transfection
Immunoprecipitation
Immunoprecipitation studies were performed using human platelets (4 × 108/mL) or mouse platelets (2 × 108/mL) as previously described.29 In brief, nontissue culture petri dishes were coated with Fg (100 μg/mL) or 3% bovine serum albumin (BSA) for 1 hour at 37°C and then blocked with 0.5% heat-inactivated BSA. Washed mouse or human platelets (1 mL per dish) pretreated with apyrase (1 U/mL), and aspirin (1 mM) were allowed to spread on Fg or BSA precoated dishes for 60 minutes (for human platelets) and 90 minutes (for murine platelets) at 37°C. In a separate set of experiments, platelets were pretreated with RGDS or EDTA prior to stimulation with thrombin. For artificial activation of the integrin, platelets in suspension were treated with Mn2+ in the absence or presence of Fg. For time course experiments, platelets in suspensions were activated by 20 μM ADP or 10 μg/mL collagen at 0, 1, 3, and 5 minutes. Platelets were lysed using ice-cold lysis buffer (1% NP-40, 150 mM NaCl, and 50 mM Tris-HCl pH 7.5 containing 10 μg/mL each of leupeptin, levamisole, and aprotinin; 1 mM each of phenylmethylsulfonyl fluoride, NaF, and sodium orthovanadate; and 0.2% deoxycholate). Lysates were precleared by incubating with IgG, followed by protein G-Sepharose beads for 30 minutes. Precleared lysates were incubated with the indicated primary antibody or isotype-specific IgG as a control for 1 hour at RT, followed by overnight incubation with protein G-Sepharose beads at 4°C. In the integrin αIIb or β3 immunoprecipitation experiments, precleared lysates were incubated with the indicated primary antibody or isotype-specific IgG overnight at 4°C and centrifuged for 30 seconds, while the supernatant was incubated with protein A-Sepharose beads (GE Healthcare, Piscataway, NJ) for 1 hour at RT. Immunocomplex beads were washed 3× with lysis buffer in the absence of inhibitors, and immediately boiled in 2× Laemmli-sample buffer and processed for immunoblotting.
Immunoblotting
Proteins from the lysates or immunoprecipitates were separated by 8% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and transferred to polyvinylidene difluoride membrane. After transfer, the blots were blocked with 3% BSA and incubated overnight with primary antibody as indicated, followed by incubation with the appropriate secondary antibody at RT for 1 hour. The bands were visualized by chemiluminescence using LumiGLO substrate (Cell Signaling Technology, Danvers, MA). Blots were stripped and reprobed with the appropriate primary antibody, followed by secondary antibody, to determine the total amount of protein in the immunoprecipitates. Quantification of band intensity was performed using Gel doc software (Bio-Rad, Hercules, CA).
GST pull-down assay
GST pull-down assay was performed as previously described.16 In brief, a complementary DNA insert corresponding to Csk-SH2 domain was subcloned into the pGEX4T-1 vector (GE). The in-frame ligation was confirmed by DNA sequencing. For expression of GST-fusion proteins, IPTG-induced bacterial cells were lysed using French press. The lysates were incubated with glutathione beads and the thoroughly washed beads were used as needed. Lysates of washed human platelets (2 × 108/mL) stimulated with AYPGKF (PAR4 peptide) or unstimulated were incubated with GST beads or GST-Csk-SH2 domain fusion protein beads overnight at 4°C. In order to avoid the distortion of JAM-A band by the GST-SH2 fusion protein (which runs at the same place as JAM-A on sodium dodecyl sulfate-polyacrylamide gel electrophoresis), the washed beads were further incubated with thrombin (1 U) for 10 minutes at RT to cleave fusion protein, and immediately boiled in the presence of 2× Laemmli-sample buffer and processed for immunoblotting.
Platelet aggregation
Platelet aggregation was performed using a Chrono-Log lumi-aggregometer as previously described.26 Aggregation curves were recorded using AGGRO/LINK software (Chrono-Log).
Statistical analysis
Statistical analysis of the data was performed using Student t test (mean + standard error of the mean value). P < .05 was regarded as statistically significant. Each experiment was repeated at least 3 times independently using different blood donors or set of mice.
Results
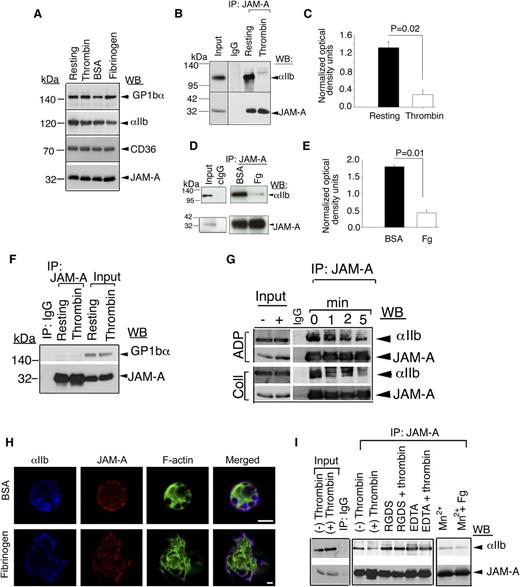
JAM-A associates with resting integrin αIIbβ3
We have recently shown that JAM-A protects from thrombosis by suppressing integrin αIIbβ3 outside-in signaling.21 It is therefore possible that JAM-A may exert this inhibitory effect by physically associating with the integrin. To test this possibility, we immunoprecipitated JAM-A from lysates of resting- and thrombin-stimulated human platelets and tested for the presence of the integrin αIIbβ3 in the immunoprecipitate by immunoblotting. Isotype-specific IgG was used as a negative control. We first tested if the activation of platelets by thrombin affected the protein levels in the lysates. We found an equal amount of candidate proteins present in both resting and activated platelet lysates (Figure 1A). We also found that a significant amount of integrin αIIbβ3 was coimmunoprecipitated with JAM-A from resting-platelet lysates, while it was absent from thrombin-stimulated lysates (Figure 1B-C). Similar results were obtained when washed platelets were exposed to BSA or immobilized Fg. A significant amount of integrin αIIbβ3 was coimmunoprecipitated with JAM-A from platelet lysates exposed to BSA, but only a negligible amount from the lysates of platelets exposed to Fg (Figure 1D-E). In order to determine if this association was specific, we evaluated if GPIb, a major platelet surface protein, was present in the JAM-A immunoprecipitate. We found a complete absence of GPIb in JAM-A immunoprecipitate from both resting- and thrombin-activated platelet lysates, suggesting that the observed interaction between JAM-A and integrin αIIbβ3 is highly specific (Figure 1F). In order to confirm the association of JAM-A from the integrin and its dissociation upon platelet activation, we performed the time course of dissociation upon activation of platelets by other physiological agonists such as ADP and collagen (Figure 1G). We found that, JAM-A time-dependently dissociated from the integrin upon activation of platelets by both strong and mild agonists. When analyzed by super resolution immunofluorescence microscopy, we found that integrin αIIbβ3 and JAM-A co-localized on the membrane of resting platelets exposed to BSA. Upon platelet spreading on immobilized Fg, a significant amount of JAM-A moved toward the center of the platelet, thereby reducing the amount of co-localization with the integrin at the membrane (Figure 1H). These results suggest that JAM-A associates with the integrin αIIbβ3 in the resting confirmation and dissociates from the integrin once the integrin is activated. In order to determine whether the dissociation of JAM-A from the integrin is dependent on integrin activation (inside-out signaling) or is ligand-dependent (outside-in signaling), we performed association studies in the absence of ligand binding (presence of RGDS or ethylene diamine tetraacetic acid). We found no dissociation of JAM-A from the integrin when ligand binding was blocked (Figure 1I). Furthermore, we performed artificial activation of the integrin using Mn+2. We found that artificial activation and ligand occupancy of the integrin is enough to dissociate JAM-A from the integrin (Figure 1I).
JAM-A interacts with the integrin αIIbβ3. (A) Western blot showing the expression levels of several platelet membrane proteins in resting and stimulated platelet lysates. (B) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of resting- or thrombin-activated human platelets. (C) Quantitation of normalized optical density of “B” from 3 independent experiments (P = .02). (D) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of human platelets exposed to immobilized Fg or BSA for 60 minutes. (E) Quantitation of normalized optical density of “D” from 3 independent experiments (P = .01). (F) Representative anti-GPIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of resting- or thrombin-activated human platelets. (G) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of human platelets activated by ADP or collagen at indicated time points. (H) Super resolution SIM images of human platelets exposed to BSA or Fg and stained for JAM-A (Alexa 568, red); integrin αIIb (Alexa 647, blue); and filamentous actin (Alexa 488, green). Scale bar: 1 μm. Original magnification ×1000. (I) Anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of human platelets incubated with 2 mg/mL RGDS, or 5 mM EDTA, and treated with or without thrombin, or incubated with 5 mM Mn2+ in the presence or absence of Fg. In (B,D,F,G,I), blots were reprobed with anti–JAM-A to ensure equal loading. Shown is a representative blot of at least 3 separate experiments.
JAM-A interacts with the integrin αIIbβ3. (A) Western blot showing the expression levels of several platelet membrane proteins in resting and stimulated platelet lysates. (B) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of resting- or thrombin-activated human platelets. (C) Quantitation of normalized optical density of “B” from 3 independent experiments (P = .02). (D) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of human platelets exposed to immobilized Fg or BSA for 60 minutes. (E) Quantitation of normalized optical density of “D” from 3 independent experiments (P = .01). (F) Representative anti-GPIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of resting- or thrombin-activated human platelets. (G) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of human platelets activated by ADP or collagen at indicated time points. (H) Super resolution SIM images of human platelets exposed to BSA or Fg and stained for JAM-A (Alexa 568, red); integrin αIIb (Alexa 647, blue); and filamentous actin (Alexa 488, green). Scale bar: 1 μm. Original magnification ×1000. (I) Anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A from lysates of human platelets incubated with 2 mg/mL RGDS, or 5 mM EDTA, and treated with or without thrombin, or incubated with 5 mM Mn2+ in the presence or absence of Fg. In (B,D,F,G,I), blots were reprobed with anti–JAM-A to ensure equal loading. Shown is a representative blot of at least 3 separate experiments.
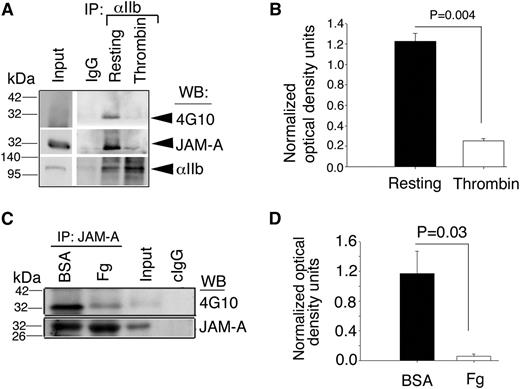
Integrin αIIbβ3-associated JAM-A is tyrosine phosphorylated
JAM-A is phosphorylated on tyrosine residue in its cytoplasmic domain30 and this phosphorylation is important for its function in endothelial cells.31 To test if the tyrosine phosphorylation is altered during its dissociation from the integrin, we immunoprecipitated integrin αIIbβ3 and determined the tyrosine phosphorylation state of the coimmunoprecipitated JAM-A by immunoblotting using 4G10, a phosphotyrosine-specific antibody. As expected, we found that JAM-A was associated with the integrin αIIbβ3 only under resting conditions; and this integrin-associated JAM-A was tyrosine phosphorylated and absent in the integrin immunoprecipitate from activated platelet lysates (Figure 2A-B). However, it does not prove that JAM-A is dephosphorylated upon platelet activation. To determine if JAM-A that is dissociated from the integrin is tyrosine phosphorylated, we immunoprecipitated JAM-A from platelet lysates exposed to Fg or BSA, and determined its tyrosine phosphorylated status. We found that in platelets exposed to BSA, JAM-A was phosphorylated on the tyrosine residue. However, JAM-A was almost completely dephosphorylated upon exposure to Fg (Figure 2C-D). These results suggest that tyrosine phosphorylated JAM-A and resting integrin αIIbβ3 form a complex, which is dissociated upon ligand binding to the activated integrin and possibly leads to the dephosphorylation of JAM-A.
Interaction of JAM-A with the integrin αIIbβ3is dependent upon tyrosine phosphorylation of JAM-A. (A) Representative immunoblots of antiphosphotyrosine (4G10) of proteins immunoprecipitated with anti-αIIb from lysates of resting- or thrombin-activated human platelets. Blots were reprobed with anti–JAM-A, and anti-αIIb to ensure equal loading. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .004). (C) Representative 4G10 immunoblots of immunoprecipitated proteins with anti–JAM-A from lysates of human platelets exposed to immobilized Fg or BSA for 60 minutes. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (P = .03).
Interaction of JAM-A with the integrin αIIbβ3is dependent upon tyrosine phosphorylation of JAM-A. (A) Representative immunoblots of antiphosphotyrosine (4G10) of proteins immunoprecipitated with anti-αIIb from lysates of resting- or thrombin-activated human platelets. Blots were reprobed with anti–JAM-A, and anti-αIIb to ensure equal loading. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .004). (C) Representative 4G10 immunoblots of immunoprecipitated proteins with anti–JAM-A from lysates of human platelets exposed to immobilized Fg or BSA for 60 minutes. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (P = .03).
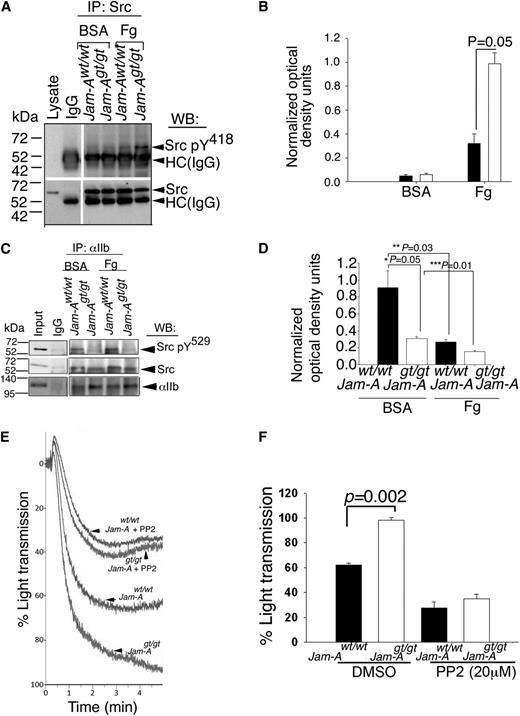
Loss of Jam-A results in activation-dependent, enhanced c-Src activation in murine platelets
Since loss of Jam-A augments integrin αIIbβ3 signaling in platelets,21 we hypothesized that c-Src (a downstream tyrosine kinase activated during outside-in signaling) may be activated in the absence of Jam-A. To test this, we immunoprecipitated c-Src from lysates of Jam-Awt/wt and Jam-Agt/gt platelets exposed to immobilized BSA or Fg, and assessed the Y418 phosphorylation of c-Src, a measure of c-Src activation. We found that c-Src Y418 phosphorylation was minimal in both Jam-Awt/wt and Jam-Agt/gt mouse platelets exposed to BSA (Figure 3A-B). However, c-Src was phosphorylated on Y418 in Jam-Awt/wt platelets exposed to Fg and this phosphorylation was significantly augmented in Jam-Agt/gt platelets exposed to Fg (Figure 3A-B) suggesting that adhesion-dependent integrin outside-in signaling is required for c-Src activation and that the presence of Jam-A suppresses c-Src activation as indicated by increased Y418 phosphorylation in its absence.
Ablation of Jam-A results in enhanced c-Src activation. (A) Representative anti-Src pY418 immunoblots of proteins immunoprecipitated with anti-Src or control IgG from lysates of Jam-Awt/wt and Jam-Agt/gt mouse platelets exposed to immobilized Fg or BSA. Blots were reprobed with anti-Src to ensure equal loading. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .05). (C) Representative anti-Src pY529 immunoblots of proteins immunoprecipitated with anti-αIIb or control IgG from lysates of Jam-Awt/wt and Jam-Agt/gt mouse platelets exposed to immobilized Fg or BSA. Blots were reprobed with anti-Src or anti-αIIb to ensure equal loading. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (*P = .05; **P = .03; ***P = .01). (E) Representative aggregation tracings of Jam-Awt/wt and Jam-Agt/gt mouse platelets pretreated with or without PP2 (20 μM) and stimulated with ADP (10 μM). (F) Quantitation of extent of aggregation from E, performed 3 times independently (P = .002).
Ablation of Jam-A results in enhanced c-Src activation. (A) Representative anti-Src pY418 immunoblots of proteins immunoprecipitated with anti-Src or control IgG from lysates of Jam-Awt/wt and Jam-Agt/gt mouse platelets exposed to immobilized Fg or BSA. Blots were reprobed with anti-Src to ensure equal loading. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .05). (C) Representative anti-Src pY529 immunoblots of proteins immunoprecipitated with anti-αIIb or control IgG from lysates of Jam-Awt/wt and Jam-Agt/gt mouse platelets exposed to immobilized Fg or BSA. Blots were reprobed with anti-Src or anti-αIIb to ensure equal loading. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (*P = .05; **P = .03; ***P = .01). (E) Representative aggregation tracings of Jam-Awt/wt and Jam-Agt/gt mouse platelets pretreated with or without PP2 (20 μM) and stimulated with ADP (10 μM). (F) Quantitation of extent of aggregation from E, performed 3 times independently (P = .002).
Phosphorylation of c-SrcY529 does not occur in the absence of Jam-A
It is known that c-Src is maintained in an unactivated state by multiple intramolecular interactions such as C-terminal Y529 phosphorylation, as well as SH2- and SH3-domain interaction with the catalytic domain.32 Since phosphorylation of Y529 residue in the regulatory domain of c-Src is involved in maintaining the activation of c-Src under check, it is possible that JAM-A somehow enhances this phosphorylation state of c-Src.12 We reasoned that if Jam-A supports Y529 phosphorylation, then the loss of Jam-A should reduce the level of Y529 phosphorylation. To test this, we immunoprecipitated integrin αIIbβ3 from lysates of Jam-Awt/wt and Jam-Agt/gt platelets exposed to BSA or Fg to obtain integrin-associated c-Src. We next analyzed the state of Y529 phosphorylation of this integrin-associated c-Src. We found that a comparable amount of c-Src was coimmunoprecipitated from each of the lysates (Figure 3C). As expected, in Jam-Awt/wt platelets exposed to BSA, almost all integrin-associated c-Src was phosphorylated on Y529, which was significantly reduced upon exposure of Jam-Awt/wt platelets to Fg (Figure 3C-D). Interestingly, Y529 phosphorylation in Jam-Agt/gt platelets exposed to both BSA and Fg was significantly attenuated compared with Jam-Awt/wt platelets (Figure 3C-D). These results suggest that Jam-A is needed for the efficient phosphorylation of c-Src on Y529 residue.
We have previously reported that loss of Jam-A on platelets results in a gain of function as indicated by hyper-aggregation of platelets to physiological agonists.21 Our results presented thus far suggest that the loss of Jam-A promotes c-Src activation. It is, therefore, plausible that observed gain of function could be due to enhanced c-Src activation and that pharmacological inhibition of c-Src activation should rescue this phenotype in Jam-Agt/gt platelets. To test this, we performed ADP-induced platelet aggregation assays using Jam-Awt/wt and Jam-Agt/gt platelets pretreated with PP2, a well-characterized inhibitor of c-Src.33 Dimethylsulfoxide was used as a vehicle control. As previously reported, we found hyper-aggregation in the Jam-Agt/gt platelets compared with Jam-Awt/wt (Figure 3E-F). Pretreatment with PP2 attenuated ADP-induced aggregation response in both Jam-Awt/wt and Jam-Agt/gt platelets. Interestingly, the gain of function seen in Jam-Agt/gt platelets was completely rescued by PP2 (Figure 3E-F). These results suggest that Jam-A exerts its pro-aggregatory effect by suppressing c-Src activation.
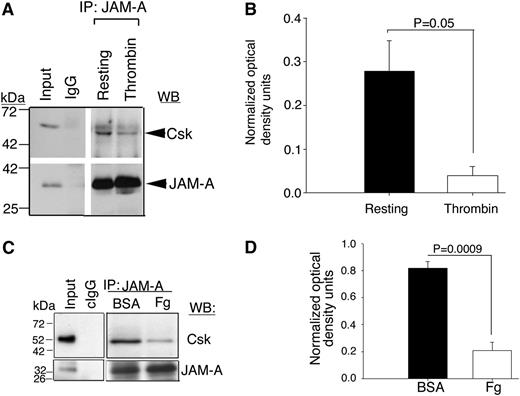
JAM-A associates with Csk in the resting platelets
It has been well documented that Csk is responsible for the Y529 phosphorylation of c-Src in resting platelets.7 Furthermore, Csk has been shown to be associated with the integrin-c–Src complex in resting platelets.7 What recruits Csk to this complex is however, not known. We reasoned that JAM-A may be a Csk-binding protein and is responsible for the recruitment of Csk to the integrin complex. To test this, we immunoprecipitated JAM-A from the lysates of resting- or thrombin-activated human platelets and immunoblotted for Csk. We found that a significant amount of Csk was associated with JAM-A in resting platelets, which dissociated upon platelet activation (Figure 4A-B). To test if this dissociation is outside-in signaling-dependent, we immunoprecipitated JAM-A from the lysates of human platelets pretreated with aspirin, to block TxA2 generation and apyrase to quench any secreted ADP, followed by exposure of these platelets to immobilized BSA or Fg. We found that JAM-A coimmunoprecipitated Csk from BSA-exposed platelet lysates, but not from Fg-exposed platelet lysates (Figure 4C-D). These results suggest that Csk associates with JAM-A in resting platelets and dissociates during outside-in signaling.
JAM-A is a Csk-binding protein. (A) Representative anti-Csk immunoblots of proteins immunoprecipitated with anti–JAM-A or IgG1 from lysates of human platelets exposed to immobilized Fg or BSA. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .05). (C) Representative anti-Csk immunoblots of immunoprecipitated proteins with anti–JAM-A or control IgG1 from lysates of human platelets exposed to immobilized BSA or Fg for 60 minutes. In (A,C), blots were reprobed with anti–JAM-A to ensure equal loading. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (P = .0009).
JAM-A is a Csk-binding protein. (A) Representative anti-Csk immunoblots of proteins immunoprecipitated with anti–JAM-A or IgG1 from lysates of human platelets exposed to immobilized Fg or BSA. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .05). (C) Representative anti-Csk immunoblots of immunoprecipitated proteins with anti–JAM-A or control IgG1 from lysates of human platelets exposed to immobilized BSA or Fg for 60 minutes. In (A,C), blots were reprobed with anti–JAM-A to ensure equal loading. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (P = .0009).
Csk associates with tyrosine-phosphorylated JAM-A through its SH2-domain
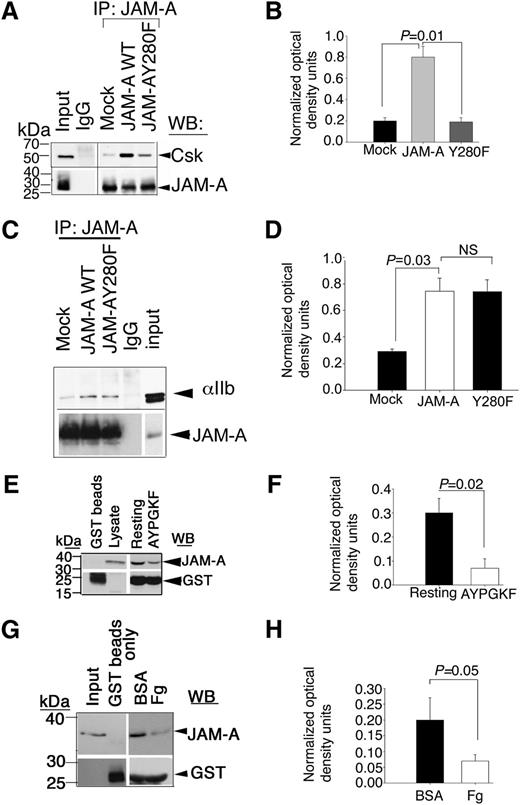
In resting platelets, JAM-A is tyrosine phosphorylated and binds to Csk. Therefore it is reasonable to speculate that tyrosine phosphorylation of JAM-A may be necessary for its ability to bind Csk. We, therefore, determined if tyrosine phosphorylation of JAM-A was necessary for this interaction. We transfected wild-type and Y280F-mutant JAM-A in Dami cells, and coimmunoprecipitation of Csk with JAM-A was assessed in resting cells. We found that a significant amount of Csk was associated with wild-type JAM-A, but not with the Y280F mutant of JAM-A, although similar amounts of JAM-A was immunoprecipitated in each condition (Figure 5A-B). These results suggested that phosphorylated Y280 residue in JAM-A is important for the association of Csk. We next asked if this tyrosine residue was also important for the interaction of JAM-A with the integrin since dephosphorylated JAM-A was not seen associating with the integrin. Interestingly, the Y280F mutation did not affect the ability of JAM-A to associate with the integrin in Dami cells, suggesting that tyrosine phosphorylation was not required for the interaction of JAM-A with the integrin (Figure 5C-D).
Csk binds tyrosine phosphorylated JAM-A through its SH2 domain. (A) Representative anti-Csk immunoblots of proteins immunoprecipitated with anti–JAM-A or control IgG1 from lysates of Dami cells transfected with mock or JAM-A–WT or JAM-A–Y280F constructs. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .01). (C) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A or control IgG1 from lysates of Dami cells transfected with mock or JAM-A–WT or JAM-A–Y280F constructs. In (A,C), blots were reprobed with anti–JAM-A to ensure equal loading. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (P = .03). (E) Representative anti–JAM-A immunoblots of GST-SH2-Csk pull-down proteins from lysates of resting or AYPGKY (100 μM) activated human platelets. (F) Quantitation of normalized optical density of “E” from 3 independent experiments (P = .02). (G) Representative anti–JAM-A immunoblots of GST-SH2-Csk pull-down proteins from lysates of human platelets exposed to immobilized BSA or Fg for 60 minutes. In (E and G), blots were reprobed with anti-GST to ensure equal loading. (H) Quantitation of normalized optical density of “G” from 3 independent experiments (P = .05).
Csk binds tyrosine phosphorylated JAM-A through its SH2 domain. (A) Representative anti-Csk immunoblots of proteins immunoprecipitated with anti–JAM-A or control IgG1 from lysates of Dami cells transfected with mock or JAM-A–WT or JAM-A–Y280F constructs. (B) Quantitation of normalized optical density of “A” from 3 independent experiments (P = .01). (C) Representative anti-αIIb immunoblots of proteins immunoprecipitated with anti–JAM-A or control IgG1 from lysates of Dami cells transfected with mock or JAM-A–WT or JAM-A–Y280F constructs. In (A,C), blots were reprobed with anti–JAM-A to ensure equal loading. (D) Quantitation of normalized optical density of “C” from 3 independent experiments (P = .03). (E) Representative anti–JAM-A immunoblots of GST-SH2-Csk pull-down proteins from lysates of resting or AYPGKY (100 μM) activated human platelets. (F) Quantitation of normalized optical density of “E” from 3 independent experiments (P = .02). (G) Representative anti–JAM-A immunoblots of GST-SH2-Csk pull-down proteins from lysates of human platelets exposed to immobilized BSA or Fg for 60 minutes. In (E and G), blots were reprobed with anti-GST to ensure equal loading. (H) Quantitation of normalized optical density of “G” from 3 independent experiments (P = .05).
Csk has a SH2 domain through which it can bind to phosphotyrosine residues of the binding proteins.14 To test if the SH2 domain of Csk is capable of interacting with JAM-A, we used GST-fusion protein expressing SH2 domain of Csk to pull down JAM-A from lysates of resting platelets, or platelets stimulated with PAR4-peptide. We found that a significantly higher amount of JAM-A was pulled down by SH2-GST fusion protein from resting platelets as compared with activated platelets (Figure 5E-F). GST beads alone did not pull down any JAM-A, suggesting the specificity of the pull-down assay. To test if outside-in signaling will affect this association, we repeated the pull-down assay using lysates of aspirin- and apyrase-treated platelets exposed to immobilized BSA or Fg. We found that a substantial amount of JAM-A was specifically pulled down by SH2-fusion protein from lysates of platelets exposed to BSA (Figure 5G). GST without the SH2 domain did not pull down JAM-A. Furthermore, SH2-fusion protein failed to pull down significant amounts of JAM-A from platelets exposed to Fg (Figure 5H). These results collectively suggest that Csk associates with Y280 phosphorylated JAM-A through its SH2 domain in resting platelets, and platelet activation dissociates this complex probably due to dephosphorylation of JAM-A.
JAM-A recruits Csk to the integrin complex
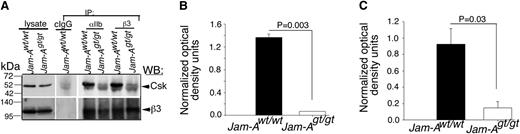
In order to conclusively determine if JAM-A is the only protein that is responsible for recruiting Csk to the integrin αIIbβ3 complex in platelets, we immunoprecipitated integrin αIIbβ3 from lysates of resting Jam-Awt/wt and Jam-Agt/gt mouse platelets using both anti-αIIb and anti-β3 antibodies. Isotype-specific IgG was used as control. Immunoprecipitates were analyzed for the presence of Csk by immunoblotting. We found that both the integrin subunit-specific antibodies coimmunoprecipitated significant amounts of Csk from Jam-Awt/wt platelet lysates (Figure 6A-C). However, Csk was completely absent in the immunoprecipitates from Jam-Agt/gt platelets. The faint ghost band seen in Jam-Agt/gt platelet lysates was the IgG heavy chain, which was also evident in the control IgG lane (Figure 6A). These results strongly suggest that Jam-A is the protein responsible for the recruitment of Csk to the integrin αIIbβ3-c-Src complex in resting platelets.
JAM-A recruits Csk to the integrin complex. (A) Representative anti-Csk immunoblots of proteins immunoprecipitated with anti-αIIb or β3 or isotype-specific IgG (cIgG) from lysates of Jam-Awt/wt and Jam-Agt/gt mouse platelets. Blots were reprobed with anti-β3 to ensure equal loading. Note: The mobility of the Csk band is the same as the IgG heavy chain. Therefore, light chain-specific horseradish peroxidase-conjugated secondary antibody was used to detect Csk. (B,C) Quantitation of normalized band intensities of “A” anti-β3 (B; P = .003) and anti-αIIb (C; P = .03) from 3 independent experiments.
JAM-A recruits Csk to the integrin complex. (A) Representative anti-Csk immunoblots of proteins immunoprecipitated with anti-αIIb or β3 or isotype-specific IgG (cIgG) from lysates of Jam-Awt/wt and Jam-Agt/gt mouse platelets. Blots were reprobed with anti-β3 to ensure equal loading. Note: The mobility of the Csk band is the same as the IgG heavy chain. Therefore, light chain-specific horseradish peroxidase-conjugated secondary antibody was used to detect Csk. (B,C) Quantitation of normalized band intensities of “A” anti-β3 (B; P = .003) and anti-αIIb (C; P = .03) from 3 independent experiments.
We concluded that JAM-A discourages sporadically activated integrin from propagating outside-in signaling by attenuating c-Src activation. This is achieved by the association with the integrin and by recruiting a signaling molecule, Csk, which is known to exert an inhibitory constrain on outside-in signaling. In unactivated, circulating platelets, a subpopulation of integrin αIIbβ3 is associated with tyrosine phosphorylated JAM-A. Csk, a tyrosine kinase, is bound to this phosphotyrosine through its SH2 domain. JAM-A, therefore, brings Csk within close proximity of integrin-bound c-Src. Csk in turn, applies an inhibitory constraint on c-Src activation by phosphorylation of Y529 residue. During platelet activation, agonist-induced inside-out signaling through talin-dependent affinity modulation and subsequent ligand binding to the integrin, rapidly dissociates JAM-A from the integrin complex. In effect, JAM-A takes away Csk from c-Src. The c-Src is then dephosphorylated on Y529 (removing one of the negative constrains on its activation), and primes it for activation during outside-in signaling (achieved by integrin clustering and trans-autophosphorylation of the Y418 residue).34
Discussion
It has been well documented that affinity modulation of integrin is carefully regulated in platelets.3,35,36 Our results show that there also exists an intricate control of integrin signaling. We show that JAM-A is responsible for suppressing integrin outside-in signaling. Similar to JAM-A, several other CAM family members notably platelet endothelial CAM-1 (PECAM-1), carcinoembryonic antigen-related CAM 1, and endothelial CAM (ESAM) have also been known to suppress platelet activation albeit through different mechanisms. PECAM-1, which contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an immunoreceptor tyrosine-based switch motif, is rapidly tyrosine phosphorylated upon platelet activation.37 Phosphorylated PECAM-1 binds phosphatase SHP1 and SHP2, and inhibits inside-out signaling induced by glycoprotein IV (GPVI).37,38 However, ablation of PECAM-1 impairs integrin outside-in signaling such as clot retraction, platelet spreading on immobilized Fg, and tyrosine phosphorylation, suggesting that PECAM-1 supports integrin outside-in signaling.39-41 Carcinoembryonic antigen-related CAM 1, also an ITIM-containing protein, negatively regulates platelet functions induced through GPVI signaling.42 Additionally, G6B, another ITIM containing orphan receptor expressed on platelets, was also shown to exert an inhibitory effect on GPVI-induced platelet function, probably via the recruitment of SHP1 and SHP2.43,44 In contrast, JAM-A did not contain ITIM or immunoreceptor tyrosine-based switch motifs, was constitutively phosphorylated on tyrosine, and rapidly dephosphorylated upon platelet activation. Furthermore, ablation of JAM-A resulted in faster clot retraction and platelet spreading, as well as increased integrin tyrosine phosphorylation.21
ESAM, another CAM family member closely related to JAM-A (both part of the CTX subfamily), has also been shown to be a suppressor of platelet function with a difference. Although ablation of ESAM results in a gain-of-function similar to JAM-A, integrin outside-in signaling as assessed by clot retraction is impaired in the absence of ESAM, which is the opposite of JAM-A.45
Our finding that JAM-A associates with integrin αIIbβ3 in resting platelets and its dissociation from the integrin upon platelet activation provides a mechanistic role for its function. Interestingly, we as well as others, have shown that JAM-A associate with integrin αvβ3 on quiescent endothelial cells, and similar to platelets, it dissociates from the integrin upon activation of endothelial cells by growth factors or cytokines.28,31,46 Furthermore, it has recently been shown that the association of JAM-A with the integrin αvβ3 on endothelial cells is via CD9.46 Since platelets abundantly express CD9, it is possible that JAM-A associates with integrin αIIbβ3 via CD9 in platelets as well.
Our identification of JAM-A as the Csk-binding protein provides a molecular mechanism of regulation of integrin-dependent c-Src activation in platelets. The identity of JAM-A as the Csk-binding protein is supported by the study of Ford et al, where it showed an unknown 36 kilodalton protein being the Csk-binding protein in the human megakaryocytic cell line.47 This molecular size matches well with JAM-A and is also expressed abundantly in these cells. Paxillin and its family member, Hic-5, have been shown to function as Csk-binding proteins.16 However, they bind SFKs other than c-Src. Also, they function to restrict feedback inhibition of SFKs by sequestering Csk away from c-Src.
It is known that c-Src can become partially activated (or primed) simply by binding to the β3 integrin tail through its SH3 domain.34 However, JAM-A via the recruitment of Csk might suppress this activation. Dissociation of JAM-A from integrin alone may not be sufficient for the full activation of c-Src. It has been shown that ligand-dependent clustering of the integrin allows trans-autophosphorylation of activation loop Y418 residue, which is necessary for its full activation.34 This explains why c-Src is not constitutively active in Jam-A knockout platelets.
To our knowledge, our results, for the first time, demonstrate that JAM-A suppresses integrin outside-in signaling by recruiting Csk to the integrin-c–Src complex. Thus, reduced or dysfunctional expression of JAM-A could enhance the risk of developing cardiovascular diseases and cancer. In fact, recent reports highlighting that misregulation of JAM-A or single nucleotide polymorphisms correlate to risks of breast cancer and venous thromboembolism, support this notion.48-50
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kushal U. Naik for editing the manuscript.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (2R01 HL63960-10, 1R01 HL113188-01, and R01 HL119374-01) and the National Center for Research Resources (5P20 RR015588-10 and 2P20 RR016472-11) to U.P.N.
Authorship
Contribution: M.U.N. performed experiments, analyzed the data, and wrote the manuscript; J.L.C. performed experiments; and U.P.N. planned research, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulhas P. Naik, 309 Wolf Hall, University of Delaware, Newark, DE 19716; e-mail: unaik@udel.edu.