Key Points
Marrow CD8+ T-cell infiltrates may be a novel predictor of response to donor lymphocyte infusions in patients with relapsed CML.
Reversal of T-cell exhaustion is tightly linked to effective antileukemia responses to donor lymphocyte infusions.
Abstract
Increasing evidence across malignancies suggests that infiltrating T cells at the site of disease are crucial to tumor control. We hypothesized that marrow-infiltrating immune populations play a critical role in response to donor lymphocyte infusion (DLI), an established and potentially curative immune therapy whose precise mechanism remains unknown. We therefore analyzed marrow-infiltrating immune populations in 29 patients (22 responders, 7 nonresponders) with relapsed chronic myelogenous leukemia who received CD4+ DLI in the pre–tyrosine kinase inhibitor era. Immunohistochemical analysis of pretreatment marrow revealed that the presence of >4% marrow-infiltrating CD8+ (but not CD4+) T cells predicted DLI response, even in the setting of high leukemia burden. Furthermore, mRNA expression profiling of marrow-infiltrating T cells of a subset of responders compared with nonresponders revealed enrichment of T-cell exhaustion–specific genes in pretreatment T cells of DLI responders and significant downregulation of gene components in the same pathway in responders in conjunction with clinical response. Our data demonstrate that response to DLI is associated with quantity of preexisting marrow CD8+ T cells and local reversal of T-cell exhaustion. Our studies implicate T-cell exhaustion as a therapeutic target of DLI and support the potential use of novel anti-PD1/PDL1 agents in lieu of DLI.
Introduction
Donor lymphocyte infusion (DLI) is a highly effective immunotherapy for relapsed hematologic malignancies after allogeneic hematopoietic stem cell transplant (HSCT). Since 1990,1 DLI has convincingly demonstrated the potency of the graft-vs-leukemia (GvL) effect. The ensuing 2 decades have revealed DLI response to vary considerably depending on the hematologic malignancy, with chronic myelogenous leukemia (CML) exhibiting the highest sensitivity rates.2,3 Strategies to optimize DLI therapy have not been standardized because of variability across institutions in numerous factors including cell dose, timing, and cell type of the infused donor lymphocyte population.4-9 Thus although DLI is established as a potentially remission-inducing therapy, the identification of biological response predictors and understanding of the precise basis of its effectiveness remain elusive.
The high frequency of durable DLI responses for the treatment of CML suggests that dissecting the mechanism of efficacy in this setting may yield important information that contributes to the development of more efficacious immunotherapeutic approaches. A growing body of evidence across solid malignancies suggests that tumor-infiltrating T cells are critical to tumor control.10-12 In CML, the primary tumor site is the bone marrow, a microenvironment that also serves as a major immunologic priming site13 and reservoir for memory T cells.14 Consistent with the idea that bone marrow can harbor T cells that control malignant disease, myeloid leukemia–specific T cells have been found to reside preferentially in the marrow rather than in the peripheral blood.15-17 We recently demonstrated that CD8-depleted (CD4+) DLI for CML rapidly expanded preexisting marrow-resident CD8+ T cells specific for CML66, an immunogenic antigen expressed on myeloid progenitor cells.18
To explore the hypothesis that marrow-infiltrating immune populations contribute to effective DLI responses, we examined a cohort of CML patients treated primarily with CD4+ DLI, which we previously reported as a promising approach for reducing the risk of graft-vs-host-disease without sacrificing GvL potency.19-22 Herein we investigate whether immune subset profiles in peripheral blood differ from those at the site of disease, whether specific marrow-infiltrating immune populations predict DLI response, and whether transcriptional profiling of infiltrating T cells reveals insight into the mechanisms of response to DLI therapy. Our studies unveiled both quantitative (higher CD8+ T-cell infiltrates) and qualitative (T-cell exhaustion signatures) features of pretreatment immune cell infiltrates associated with effective clinical response. The implications of these findings on the generation of effective human antitumor immunity will be discussed.
Methods
Patients
Blood and marrow biopsies were obtained before and after DLI from patients enrolled in clinical trials of DLI treatment with relapse after HSCT at the Dana-Farber Cancer Institute (DFCI) between 1994-2001.21 The DFCI Human Subjects Protection Committee approved these protocols. This study was conducted in accordance with the Declaration of Helsinki. Peripheral blood (PBMC) and marrow mononuclear cells (BMMC) were isolated by Ficoll-Hypaque density gradient centrifugation, cryopreserved with 10% dimethyl sulfoxide, and stored in vapor-phase liquid nitrogen. Marrow core biopsy specimens were fixed in Zenker solution and embedded in paraffin.
Clinical response definitions
Clinical response was defined as complete hematologic (normalization of blood counts and marrow cellularity) and cytogenetic response (the absence of marrow Philadelphia [Ph+] chromosome by fluorescence in situ hybridization [FISH]) after DLI.
Immunophenotyping
Patients’ PBMCs before and after DLI were incubated at 4°C (30 min) with a panel of mouse monoclonal antibodies. Cells were washed and incubated at 4°C with fluorescein isothiocyanate goat anti-mouse conjugate (TAGO). Ten thousand cells within the lymphocyte gate were analyzed in each sample using automated flow cytometry (EPICS-V, Coulter Electronics). Absolute cell numbers were calculated from clinical leukocyte counts and differentials.
Immunohistochemistry
Staining of 5-μm-thick marrow tissue sections was performed using monoclonal antibodies (DAKO USA, Carpinteria, CA) by standard immunohistochemical methods. PD-1 antibody (EH33) was a gift from Gordon Freeman (DFCI). Deparaffinized slides were pretreated with 1.0 mmol/L ethylenediaminetetraacetic acid (pH 8.0; Zymed, South San Francisco, CA) or citrate (pH 6.0; Invitrogen; PD1 staining) in a steam pressure cooker (Decloaking Chamber, BioCare Medical, Walnut Creek, CA) followed by washing in distilled water. All further steps occurred at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (DAKO USA; 5 minutes), followed by blocking goat serum (1:5, in 50 mmol/L Tris-Cl [pH 7.4]; 20 minutes) or serum-free protein block (DAKO; PD1 staining). Monoclonal anti-human antibodies were applied with 3% goat serum (1:50 dilution in 50 mmol/L Tris-Cl [pH 7.4]; 1 hour). Slides were washed in 50 mmol/L Tris-Cl, 0.05% Tween 20 (pH 7.4), and goat anti-mouse horseradish peroxidase–conjugated antibody (Envision detection kit, DAKO) was applied (30 minutes). Immunoperoxidase staining was developed using a diamino benzidine chromogen kit (DAKO) per the manufacturer. Slides were placed in a chromogenic-enhancing solution (Zymed, 5 minutes) at room temperature and counterstained with hematoxylin. A hematopathologist reviewed specimens for cellularity, density, and composition of the lymphoid infiltrates as percent cells per high-power field. PD-1 positivity was defined as >3% positive cells per high-power field.
Gene expression microarray analysis
Patient-derived BMMCs were thawed; CD3+ T cells were enriched using magnetic bead sorting (Miltenyi Biotec). Total RNA was isolated from CD3+ cells using TRIzol reagent (Invitrogen), followed by column purification (RNeasy Mini Kit, Qiagen, Valencia CA). cDNA was synthesized and amplified from total RNA (Ovation Pico WTA System V2, NuGEN, San Carlos, CA), and samples were hybridized to Affymetrix U133A+ 2.0 arrays (Santa Cruz, CA) and scanned at the DFCI Microarray Core Facility (The Affymetrix GeneChip Scanner 3000). Expression profiles were processed using RMA, implemented by the R package (www.r-project.org)23 and Affymetrix probes were collapsed to unique genes (Gene Symbol) by averaging. The resulting values were log2-transformed for downstream analysis and deposited in the NCBI Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/info/linking.html), accession number GSE49067.
The limma package24 for R identified differentially expressed genes and created rank-ordered lists using a cutoff of an unadjusted P value of < .001. We compared expression values of pooled responders to pooled nonresponders before DLI (“pretreatment” analysis) as well as the change in expression in responders after DLI to that in nonresponders after DLI (“response to treatment” analysis). The results were visualized using GENE-E.25 Rank-ordered gene lists from both “pretreatment” and “response to treatment” comparisons were further assessed with gene set enrichment analysis (GSEA) (www.broadinstitute.org/gsea). Gene sets were considered significantly enriched if P < .05. Exhaustion gene sets were specifically curated from literature review of published reports.26,27
Expression of microarray target genes was validated using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) and cDNA amplification was performed using the TaqMan Gene Expression Master Mix (Applied Biosystems) on an Applied Biosystems 7500HT Fast real-time polymerase chain reaction (PCR) System (10-minute enzyme activation and 40 cycles of 15 s at 95°C, 1 minute at 60°C). Samples were measured in triplicate. GAPDH served as a housekeeping gene to calculate relative expression values.
Statistical analysis
Comparisons of continuous and categorical variables were analyzed using the 2-sided exact Wilcoxon rank-sum test and the 2-sided Fisher exact test, respectively. The 2-sided Wilcoxon signed-rank test was used to compare pre/post-DLI percent cellularity with immunophenotypic markers (significance level of .05). To establish a cutoff value for predictive pre-DLI percent cellularity and CD8+ T cells, receiver operator characteristic (ROC) curve analysis was performed. To assess whether the cutoff value predicts the response to DLI in the presence of other prognostic factors, exact logistic regression analysis was performed, adjusting for age, patient-donor sex mismatch, and donor type. All tests were 2-sided at the significance level of .05. Testing multiple biomarkers is not adjusted in the level of significance. Locally weighted scatterplot smoothing (Lowess)28 was used to describe the data in Figure 1. All graphic assessments were performed using R package (v.2.10.1) or GraphPad software.
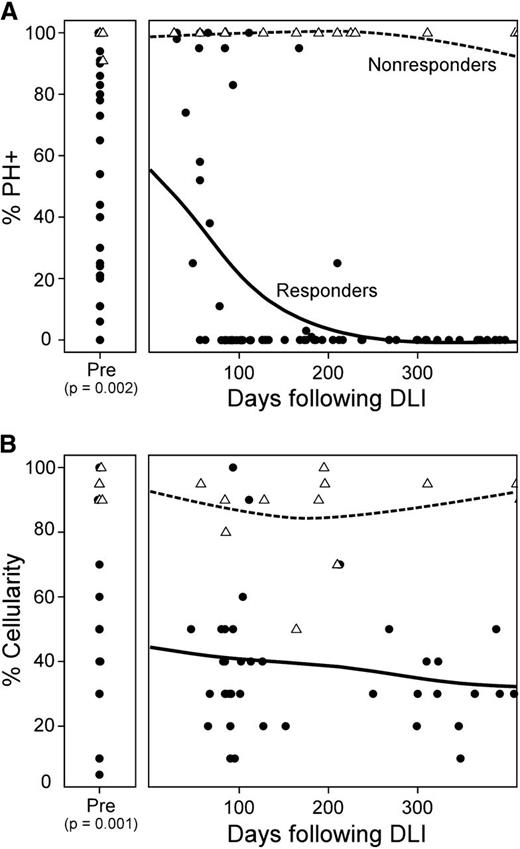
Preexisting leukemic burden in the bone marrow inversely correlates with clinical response to DLI. (A) Disease burden in patients with relapsed CML before and at serial time points after DLI therapy demonstrated by both percent of bone marrow cells expressing the Philadelphia chromosome detected by FISH as well as (B) percent marrow cellularity. P < .01 for (A) and (B), Responders (circles) vs Nonresponders (triangles) using the exact Wilcoxon rank-sum test.
Preexisting leukemic burden in the bone marrow inversely correlates with clinical response to DLI. (A) Disease burden in patients with relapsed CML before and at serial time points after DLI therapy demonstrated by both percent of bone marrow cells expressing the Philadelphia chromosome detected by FISH as well as (B) percent marrow cellularity. P < .01 for (A) and (B), Responders (circles) vs Nonresponders (triangles) using the exact Wilcoxon rank-sum test.
Results
DLI response associates with disease burden in pretreatment marrow
We analyzed pre- and post-DLI treatment marrow biopsies and peripheral blood collected from 29 patients with relapsed CML. Twenty-eight of 29 (97%) patients received myeloablative conditioning regimens and matched related bone marrow as the source of hematopoietic progenitor cells for their transplants (supplemental Table 1, available on the Blood Web site). At a median of 26.9 months from transplant, patients received 3 to 10 × 107 CD4+ T cells per kilogram as DLI for relapsed disease. The observed clinical response rate was consistent with previous reports.21 Overall survival and progression-free survival at 2 years after DLI was 93% (range 75-98) and 92% (range 73-98), respectively. Twenty-two patients demonstrated normalization of marrow cellularity within 12 months associated with the disappearance of marrow BCR-ABL+ cells, detected through FISH cytogenetics, and were termed “responders.” In contrast, 7 patients revealing persistent marrow hypercellularity and BCR-ABL positivity were considered to be “nonresponders” (Figure 1). The median follow-up among survivors was 7.4 years.
No significant differences in age, gender, sex mismatch, cell source for transplant, or donor type (related or unrelated) were observed between responders and nonresponders (supplemental Table 1). However, responders presented with variable but significantly lower marrow cellularity at the time of DLI (median 50%, range 5-100) compared with nonresponders (median 92.5%, range 90-100) (Figure 1B; P = .001). Consistent with prior reports,29 these results suggest that increasing tumor burden is strongly associated with poor DLI response in relapsed CML after HSCT.
Temporal characterization of marrow immune cell subsets after DLI
We previously observed increased peripheral blood B cells developing only in responders, peaking at 6 to 9 months after DLI.30 Therefore we characterized different immune cell subsets (CD3+, CD4+, CD8+, and CD20+) in the peripheral blood by flow cytometry before and at serial time points after DLI (supplemental Figure 1). As was shown previously, CD20+ B-cell lymphocytosis was observed in responders at 6 and 9 months post-DLI compared with pre-DLI levels (P = .03 and P = .04, respectively), but not in nonresponders at any time point post-DLI. We noted the absence of significant differences by flow cytometry in the numbers of peripheral blood CD3+, CD4+, or CD8+ T-cell populations over time (supplemental Figure 1). Because recent studies suggest that peripheral blood does not always reflect activity in local tumor sites,31,32 we sought to examine whether DLI instead resulted in changes in immune effector populations within the local tumor microenvironment of the marrow.
Marrow sections were stained with CD3-, CD8-, CD4-, and CD20-specific monoclonal antibodies. Eighty-five marrow specimens from 28 patients were analyzed at 3 distinct time points: baseline, 3 to 6 months, and 9 to 12 months after DLI. We enumerated the positively stained cells at these intervals as a fraction of total marrow cellularity. Paired pre- and posttreatment samples were available in 22 of 28 patients.
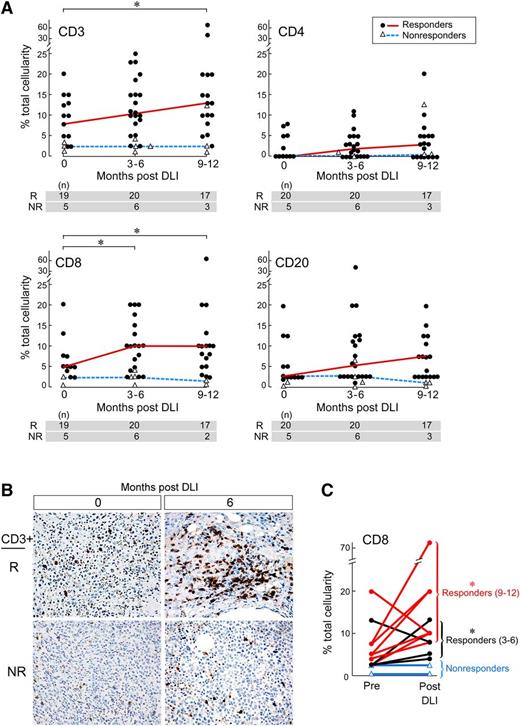
We observed distinct temporal patterns of immune cell populations within the marrow tumor microenvironment compared with peripheral blood after DLI. In the nonresponder cohort, no significant changes in CD3+, CD8+, and CD4+ T cells, or CD20+ B cells, were apparent from the time before DLI to either 3 to 6 months or 9 to 12 months after DLI (Figure 2A and Table 1). In the responder cohort, nonsignificant increases in CD20+ B cells were found at both 3 to 6 months (median 5.38 vs 2.5, P = .08) and 9 to 12 months (median 7.5 vs 2.5, P = .4) pre-DLI. More strikingly, we observed a significant increase in CD3+ T cells from pre-DLI (median 8%) to 9 to 12 months post-DLI (median 13%, P = .02) (Figure 2B). Although no significant changes in CD4+ T cells were noted, CD8+ T cell numbers rose significantly at 3 to 6 months (median 5% vs 10%, P = .03), persisting at similar levels to 9 to 12 months post-DLI (median 5% vs 10%, P = .03, Table 1). This expansion of marrow-infiltrating CD8+ T cells after DLI was confirmed within individuals (Figure 2C). In paired pre- and post-DLI marrow samples from 18 responders and 4 nonresponders, we detected significant increases of CD8+ T cells only in responders (P = .01). Fifteen of 18 paired responders demonstrated at least a twofold increase in marrow CD8+ T cells, with 8 exhibiting at least a fourfold increase. In contrast, this twofold increase post-DLI was observed in only 1 of 4 nonresponders. Together, these data demonstrate that DLI clinical response associates with T-cell responses at the site of disease that are not apparent in the peripheral blood.
Characterization of immune cell subsets in marrow over time after DLI. (A) IHC staining analysis of marrow immune populations from responders and nonresponders before and 3 to 6 and 9 to 12 months after DLI. For each immune cell subset, significant differences between 0 and 3 to 6- or 9 to 12-month time points are noted. (B) Representative IHC of marrow samples from one responder and one nonresponder demonstrates robust marrow infiltration by CD3+ T cells in the responder before and subsequent increase after DLI. Images were captured with an Olympus QColor 5 camera on an Olympus BX41 microscope with a x40/0.75 objective using Adobe Photoshop Software (vCS5). (C) Pairwise comparison of individual patients’ marrow before and after DLI therapy revealed significant upregulation of marrow CD8+ T cells in the responder cohort after treatment. *P < .05 using the Wilcoxon signed-rank test.
Characterization of immune cell subsets in marrow over time after DLI. (A) IHC staining analysis of marrow immune populations from responders and nonresponders before and 3 to 6 and 9 to 12 months after DLI. For each immune cell subset, significant differences between 0 and 3 to 6- or 9 to 12-month time points are noted. (B) Representative IHC of marrow samples from one responder and one nonresponder demonstrates robust marrow infiltration by CD3+ T cells in the responder before and subsequent increase after DLI. Images were captured with an Olympus QColor 5 camera on an Olympus BX41 microscope with a x40/0.75 objective using Adobe Photoshop Software (vCS5). (C) Pairwise comparison of individual patients’ marrow before and after DLI therapy revealed significant upregulation of marrow CD8+ T cells in the responder cohort after treatment. *P < .05 using the Wilcoxon signed-rank test.
Percentages of marrow infiltration by immune effectors before and after DLI
| . | . | Responders . | Nonresponders . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
| CD3 | 0 | 19 | 8 | 2.5-20 | 5 | 2.5 | 1-3.5 | .009 |
| 3-6 | 20 | 10.5 | 2.5-25 | 6 | 2.5 | 1-5 | .002 | |
| 9-12 | 17 | 13 | 2.5-70 | 3 | 2.5 | 1-12.5 | .09 | |
| P | P | |||||||
| 0 vs 3-6 | .07 | NS | ||||||
| 0 vs 9-12 | .02 | NS | ||||||
| . | . | Responders . | Nonresponders . | . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
| CD3 | 0 | 19 | 8 | 2.5-20 | 5 | 2.5 | 1-3.5 | .009 |
| 3-6 | 20 | 10.5 | 2.5-25 | 6 | 2.5 | 1-5 | .002 | |
| 9-12 | 17 | 13 | 2.5-70 | 3 | 2.5 | 1-12.5 | .09 | |
| P | P | |||||||
| 0 vs 3-6 | .07 | NS | ||||||
| 0 vs 9-12 | .02 | NS | ||||||
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
|---|---|---|---|---|---|---|---|---|
| CD8 | 0 | 19 | 5 | 2.5-20 | 5 | 2.5 | 0.5-3.75 | .009 |
| 3-6 | 20 | 10 | 2.5-20 | 6 | 2.5 | 0.5-4.38 | .002 | |
| 9-12 | 17 | 10 | 2.5-70 | 2 | 1.5 | 0.5-2.5 | .03 | |
| P | P | |||||||
| 0 vs 3-6 | .03 | NS | ||||||
| 0 vs 9-12 | .03 | NS |
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
|---|---|---|---|---|---|---|---|---|
| CD8 | 0 | 19 | 5 | 2.5-20 | 5 | 2.5 | 0.5-3.75 | .009 |
| 3-6 | 20 | 10 | 2.5-20 | 6 | 2.5 | 0.5-4.38 | .002 | |
| 9-12 | 17 | 10 | 2.5-70 | 2 | 1.5 | 0.5-2.5 | .03 | |
| P | P | |||||||
| 0 vs 3-6 | .03 | NS | ||||||
| 0 vs 9-12 | .03 | NS |
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
|---|---|---|---|---|---|---|---|---|
| CD4 | 0 | 19 | 0 | 0-8 | 5 | 0 | 0-0.5 | .24 |
| 3-6 | 20 | 1.88 | 0-11 | 6 | 0 | 0-2.5 | .11 | |
| 9-12 | 17 | 3 | 0-20 | 3 | 0.5 | 0-12.5 | .99 | |
| P | P | |||||||
| 0 vs 3-6 | .96 | NS | ||||||
| 0 vs 9-12 | .32 | NS |
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
|---|---|---|---|---|---|---|---|---|
| CD4 | 0 | 19 | 0 | 0-8 | 5 | 0 | 0-0.5 | .24 |
| 3-6 | 20 | 1.88 | 0-11 | 6 | 0 | 0-2.5 | .11 | |
| 9-12 | 17 | 3 | 0-20 | 3 | 0.5 | 0-12.5 | .99 | |
| P | P | |||||||
| 0 vs 3-6 | .96 | NS | ||||||
| 0 vs 9-12 | .32 | NS |
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
|---|---|---|---|---|---|---|---|---|
| CD20 | 0 | 19 | 2.5 | 2-20 | 5 | 2.5 | 0-2.5 | .02 |
| 3-6 | 20 | 5.38 | 1-40 | 6 | 2.5 | 0-6.25 | .04 | |
| 9-12 | 17 | 7.5 | 2.5-20 | 3 | 1 | 0.5-2.5 | .01 | |
| P | P | |||||||
| 0 vs 3-6 | .08 | NS | ||||||
| 0 vs 9-12 | .4 | NS |
| . | Months . | n . | Median (%) . | Range (%) . | n . | Median (%) . | Range (%) . | P (R vs NR) . |
|---|---|---|---|---|---|---|---|---|
| CD20 | 0 | 19 | 2.5 | 2-20 | 5 | 2.5 | 0-2.5 | .02 |
| 3-6 | 20 | 5.38 | 1-40 | 6 | 2.5 | 0-6.25 | .04 | |
| 9-12 | 17 | 7.5 | 2.5-20 | 3 | 1 | 0.5-2.5 | .01 | |
| P | P | |||||||
| 0 vs 3-6 | .08 | NS | ||||||
| 0 vs 9-12 | .4 | NS |
NS, not significant.
Comparisons between patients within response cohorts were analyzed by the Wilcoxon signed-rank test and between responders (R) and nonresponders (NR) by the Wilcoxon exact rank-sum test.
Bold values indicate statistical significance of P < .05.
Significantly increased CD8+ T-cell infiltrates in pre-DLI marrow in responders
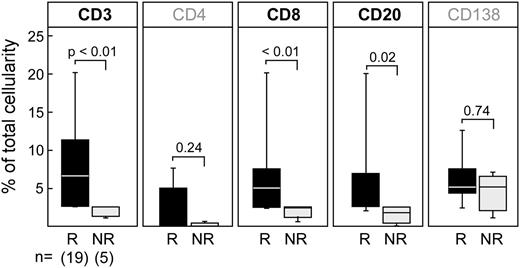
Between responders and nonresponders, we observed no differences in the temporal kinetics of T cells in peripheral blood (supplemental Figure 1). In contrast, we noted significantly different temporal patterns of these immune effectors infiltrating the marrow before and after DLI between the 2 cohorts, suggesting immunologic differences before immunomodulatory therapy. We compared pre-DLI marrow infiltrates of CD4+, CD8+, CD20+, and CD138+ lymphocytes of responders and nonresponders (Figure 3). We found significantly higher proportions of CD3+ and CD8+ T cells (P < .01) and also B cells (P = .02) in DLI responders. CD4+ T cells and CD138+ plasma cells were not significantly different, although our study may be underpowered to detect such differences. Thus the pretreatment immunologic profile at the site of disease of responders differs markedly from that of nonresponders, as evidenced by significantly increased T- (CD3+CD8+) and B- (CD20+) lymphocyte marrow infiltration.
CD8+T-cell infiltrates are significantly increased in pretreatment marrow in responders. Quantification of immune cell subsets (CD3+, CD8+, CD4+, CD20+, CD138+) performed on marrow from responders and nonresponders before DLI therapy. Whiskers in box plots indicate maximum and minimum values. The line indicates median value; P values refer to responders (R) vs nonresponders (NR) comparison using the exact Wilcoxon rank-sum test.
CD8+T-cell infiltrates are significantly increased in pretreatment marrow in responders. Quantification of immune cell subsets (CD3+, CD8+, CD4+, CD20+, CD138+) performed on marrow from responders and nonresponders before DLI therapy. Whiskers in box plots indicate maximum and minimum values. The line indicates median value; P values refer to responders (R) vs nonresponders (NR) comparison using the exact Wilcoxon rank-sum test.
CD8+ T-cell marrow infiltrates sensitively predict clinical response
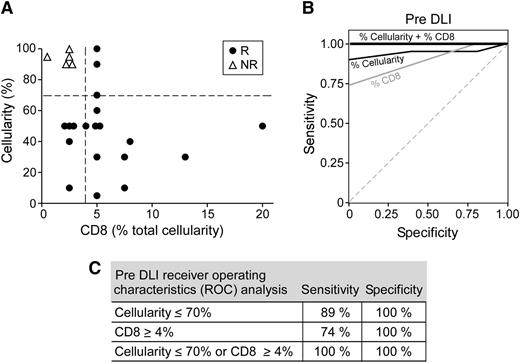
Because disease burden is a known risk factor for ineffectual DLI response, we evaluated the interaction between disease burden and pre-DLI CD8+ T-cell infiltrate through 8 patients with high (≥70%) pre-DLI marrow cellularity. Three of the 8 had ≥4% CD8+ T-cell marrow infiltrates before DLI, and these infiltrates significantly increased two- to fourfold after DLI treatment (Figure 4A). All 3 patients subsequently achieved cytogenetic remission. In contrast, 5 of 8 had <4% CD8+ T-cell infiltrates and all failed to show a cytogenetic response.
Preexisting infiltrating CD8+T cells and normal marrow cellularity are sensitive predictors of clinical response to DLI therapy. (A) Correlation of marrow cellularity and CD8+ T-cell infiltrates for all patients before DLI. (B) ROC analysis for stratifying clinical response (responders and nonresponders) by either preexisting marrow cellularity ≤70% or CD8+ T-cell infiltrate ≥4%. (C) Combining criteria results in 100% sensitivity and specificity.
Preexisting infiltrating CD8+T cells and normal marrow cellularity are sensitive predictors of clinical response to DLI therapy. (A) Correlation of marrow cellularity and CD8+ T-cell infiltrates for all patients before DLI. (B) ROC analysis for stratifying clinical response (responders and nonresponders) by either preexisting marrow cellularity ≤70% or CD8+ T-cell infiltrate ≥4%. (C) Combining criteria results in 100% sensitivity and specificity.
These observations suggest that marrow cellularity and CD8+ T cells together predict DLI response. We thus assessed the relative importance of these 2 factors in response prediction by applying ROC analysis using the criteria of pre-DLI cellularity ≤70% or CD8+ T-cell infiltrate ≥4%. This analysis revealed a sensitivity and specificity of 100% in predicting responsiveness to DLI (Figure 4B-C). Taken together, these findings support the idea that immune status can overcome the adverse influence of high disease burden in therapeutic response.
Expression profiling reveals reversal of T-cell exhaustion during response to DLI
Because preexisting CD8+ T-cell infiltrates proved to strongly predict DLI response, we hypothesized that gene expression patterns of T-cell infiltrates would differ between responders and nonresponders before and after DLI and possibly provide mechanistic insights into DLI response. We therefore used cDNA microarrays to analyze gene expression patterns of CD3+ T cells isolated from BMMCs collected from 4 responders and 2 nonresponders before and after DLI (clinical characteristics are shown in supplemental Table 2).
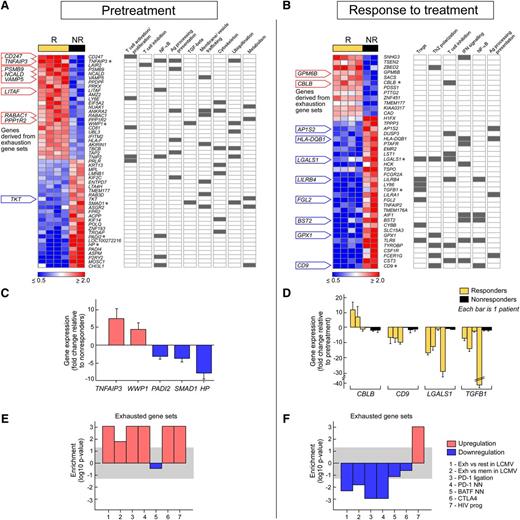
We performed 2 general analyses. First, in our “pretreatment” analysis, we compared gene expression patterns of pre-DLI time points between responders and nonresponders and selected all genes with unadjusted P values < .001. Global mRNA expression profiling of pre-DLI T cells identified 25 significantly upregulated and 26 significantly downregulated genes in responders relative to nonresponders (Figure 5A and supplemental Table 3). Strikingly, our analysis of the associations between these specific upregulated genes and biological processes revealed 8 of 25 (32%) to play key roles in T-cell exhaustion (Figure 5A). Based on a literature review, we further identified multiple biological processes and pathways with published and/or putative associations to these 51 genes. These pathways segregated themselves into those enriched in the responders vs those involving genes significantly altered in both responders and nonresponders. Although responders demonstrated upregulation of genes involved in T-cell exhaustion, T-cell activation and proliferation and antigen processing and presentation, they showed only minimal relative upregulation of genes involved in T-cell inhibition (including the processes of T-cell anergy or tolerance). Consistent with the T-cell source of mRNA for expression profiling, genes related to NF-κB signaling were affected in both cohorts. Several processes not unique to T-cell biology also emerged as differentially regulated in both patient groups: membrane vesicle/trafficking, cytoskeleton, ubiquitination, and metabolism (Figure 5A). In addition to individual gene analysis, we undertook GSEA using ∼880 curated gene sets from the canonical pathways collection of the Molecular Signatures Database, spiked with 7 specifically curated gene sets for T-cell exhaustion (supplemental Table 4). Consistent with our gene pathway observations, 3 of the top 12 sets positively enriched in responders relative to nonresponders before DLI directly implicated T-cell exhaustion. In contrast, 17 of the top 20 negatively enriched gene sets were related to cell cycle progression (DNA synthesis and replication, G1/S transition, E2F transcriptional targets) (supplemental Figure 2).
Expression profiling reveals T-cell exhaustion as a strongly associated signature of response to DLI therapy. Differential gene expression from CD3+ T cells isolated from the marrow of DLI responders (R) and nonresponders (NR) before DLI therapy (A) or in response to treatment (B). The annotation of genes within key biological processes is indicated. The asterisks refer to genes selected for qPCR validation (see C-D). (C-D) qPCR validation of changes in microarray gene expression in selected genes (C) differentially expressed between R and NR before therapy and (D) differentially affected by DLI over time in R compared with NR. Data are represented as mean ±SEM, with each sample performed in triplicate. (E-F) GSEAs from the pretreatment and response to treatment comparisons. The bar graphs show P value of enrichment (signed according to enrichment score) for distinct T-cell exhaustion gene sets in R vs NR before (E) or after (F) DLI therapy. Detailed descriptions of gene sets are provided in supplemental Table 4. The shaded region indicates P > .05.
Expression profiling reveals T-cell exhaustion as a strongly associated signature of response to DLI therapy. Differential gene expression from CD3+ T cells isolated from the marrow of DLI responders (R) and nonresponders (NR) before DLI therapy (A) or in response to treatment (B). The annotation of genes within key biological processes is indicated. The asterisks refer to genes selected for qPCR validation (see C-D). (C-D) qPCR validation of changes in microarray gene expression in selected genes (C) differentially expressed between R and NR before therapy and (D) differentially affected by DLI over time in R compared with NR. Data are represented as mean ±SEM, with each sample performed in triplicate. (E-F) GSEAs from the pretreatment and response to treatment comparisons. The bar graphs show P value of enrichment (signed according to enrichment score) for distinct T-cell exhaustion gene sets in R vs NR before (E) or after (F) DLI therapy. Detailed descriptions of gene sets are provided in supplemental Table 4. The shaded region indicates P > .05.
Second, in a “response to treatment” analysis, we examined whether DLI differentially affects gene expression of marrow-infiltrating T cells over time after therapy in responders compared with nonresponders. As before, all genes with unadjusted P values < .001 were selected. Twelve upregulated and 30 downregulated genes in responders after DLI treatment, relative to nonresponders, were identified (Figure 5B and supplemental Table 3). Consistent with our prior results, 8 of the 30 (∼27%) downregulated individual genes were now associated with T-cell exhaustion pathways (indicated in Figure 5B). Genes upregulated in nonresponders after treatment were not only enriched for phenotypes associated with regulatory T cells, Th2 polarization, and T-cell inhibition, but also for pathways involved in interferon and NF-κB signaling, as well as antigen processing and presentation, suggesting the wide-ranging effects on T-cell activity induced by DLI.
These microarray data were confirmed by quantitative (qPCR) of representative significantly up- or downregulated genes. From the pretreatment group, qPCR of TNFAIP3 and WWP1 revealed seven- and fourfold upregulation in responders, replicating the directionality of change in the microarray data. PADI2, SMAD1, and HP were similarly downregulated in microarray and qPCR assays (three-, four-, and eightfold, respectively) (Figure 5C). In the response to treatment analysis, fold change in expression values from the paired pre- and post-DLI samples are shown for each responder and nonresponder. CBLB was upregulated in 2 of 3 assessable responders and downregulated in 2 of 2 nonresponders, mirroring the microarray pattern. CD9, LGALS1, and TGFB1 (genes associated with T-cell exhaustion) were downregulated in the responders with minimal change in nonresponders after DLI, consistent with the observed changes from microarray data (Figure 5D).
To examine the involvement of T-cell exhaustion more specifically, we analyzed the behavior of 7 exhaustion gene sets after DLI. In accordance with our previous results, we observed upregulation of 6 of 7 exhaustion gene sets in the pretreatment group of responders vs nonresponders and repression of multiple, distinct T-cell exhaustion gene sets during response to DLI (Figure 5E-F). Together, these data implicate T-cell exhaustion in distinguishing responders from nonresponders and suggest this unique T-cell transcriptional state as a potential marker and mechanism of DLI responsiveness in relapsed CML.
Finally, the central role of the PD-1 molecule to T-cell exhaustion led us to examine its expression by immunohistochemistry (IHC).33 We found a significant increase in the number of responders with PD-1+ infiltrates after DLI, whereas no PD-1 positivity was detected in nonresponders before or after DLI (supplemental Figure 3). The upregulation of PD-1 strongly suggests increased T-cell receptor signaling and T-cell activation after DLI.34 Moreover, this phenomenon of PD-1 upregulation with reversal of T-cell exhaustion is consistent with the blockade of LAG-3 or PD-L1, or both, in a murine model of chronic viral infection.35 These studies illustrate the complexity of assigning a unique immunohistochemical profile for T-cell exhaustion and highlight the use of transcriptional profiling to interrogate exhaustion in human cancer.
Discussion
Although clinical predictors of DLI response have been identified, biological predictors have not. We used a well-defined clinical setting to discover several new insights into DLI efficacy. First, our study highlights the site of disease (marrow) rather than peripheral blood as a more accurate indicator of T-cell immunity. Second, the pretreatment immunologic state of the marrow emerged as a strong novel predictor of DLI response. Finally, transcriptional profiling of marrow-infiltrating T cells uncovered T-cell exhaustion as a key pathway that distinguished clinical outcomes and indicated reversal of T-cell exhaustion as an underlying mechanism of DLI response in relapsed CML after HSCT.
Our studies confirm the unique immunologic role of the marrow microenvironment in shaping antileukemic responses. These results paralleled older studies demonstrating that lower marrow cellularity (associated with lower tumor burden) was predictive of better response rates and overall survival29,36 ; moreover, we analyzed the previously unexplored associations between the “burden” of infiltrating T cells and DLI response. Cytotoxic T cells play a crucial role in GvL responses, and our data suggest that CD8+ T-cell infiltrates are critical even in the setting of higher leukemic burden. Remarkably, even a small infiltrate of CD8+ T cells (∼5% marrow cellularity) at the time of DLI was sufficient to tip the balance in favor of subsequent antitumor response. Mechanistically, the observed robust expansion of marrow-infiltrating CD8+ T cells after DLI in responders strikingly supports the idea that CD4+ DLI provides immunologic help for marrow-residing, tumor-specific CD8+ T cells, thus awakening dormant antitumor immunity.18,37,38
Unexpectedly, preexisting responder T-cell infiltrates exhibited transcriptional profiles consistent with T-cell exhaustion, a reversible state of progressive T-cell dysfunction characterized by loss of effector and proliferative functions, as well as a distinct transcriptional profile.26,33 First described in chronic HIV, hepatitis C virus, and hepatitis B virus infection,27,33 T-cell exhaustion has been increasingly recognized as an important immunoevasive strategy in both solid and hematologic malignancies.33,39-41 The unique transcriptional profiles of T-cell exhaustion, distinct from those of T-cell anergy or senescence, allowed us to confirm their presence in our cohort. Indeed, the robust reversibility of T-cell exhaustion signatures after DLI therapy highlights this coherent gene module as a likely therapeutic target of DLI.
These data lead us to propose a model whereby DLI responders harbor reservoirs of antitumor CD8+ T cells at the tumor site that presumably have already encountered CML antigens (and hence are exhausted). Because these T cells already have specificity for leukemia cells, reinvigoration and reversal of their exhausted state by DLI allow for their subsequent expansion and effective elimination of leukemia cells (Figure 6). Because CD8+ T cells were effectively depleted from the DLI products given to these patients, our results implicate the remarkable ability of CD4+ T cells to reverse T-cell exhaustion in vivo and furthermore ascribe a positive therapeutic outcome to the reversal of T-cell exhaustion in patients. Undoubtedly, though, CD4+ DLI has other potent antileukemic effects, such as induction of coordinated innate and humoral immunity with cytotoxic CD8+ T-cell effectors.17,18,42 Conversely, in this schema, nonresponders harbor very few preexisting CD8+ T cells that lack phenotypic evidence of prior strong antigenic activation. Hence, these T cells are perhaps incapable of mounting a specific and potent antitumor response.
The proposed role of T-cell exhaustion in predicting clinical response to DLI. Our data support the idea that (A) response to DLI is associated with a preexisting reservoir of antitumor CD8+ T cells residing at the tumor site, to which CD4+ DLI provides immunologic help, not only to expand this reservoir but also to reverse T-cell exhaustion. The presence of T-cell exhaustion may signal that this reservoir exists. In contrast, in the absence of such a reservoir (B), a lack of DLI response is associated with both insufficient quantities of infiltrating T cells and the absence of phenotypic evidence of past T-cell activation.
The proposed role of T-cell exhaustion in predicting clinical response to DLI. Our data support the idea that (A) response to DLI is associated with a preexisting reservoir of antitumor CD8+ T cells residing at the tumor site, to which CD4+ DLI provides immunologic help, not only to expand this reservoir but also to reverse T-cell exhaustion. The presence of T-cell exhaustion may signal that this reservoir exists. In contrast, in the absence of such a reservoir (B), a lack of DLI response is associated with both insufficient quantities of infiltrating T cells and the absence of phenotypic evidence of past T-cell activation.
The identification of marrow immune features as a prognostic marker of DLI response has powerful clinical potential. Patients with relapsed CML after HSCT whose marrow biopsies reveal low numbers of infiltrating CD8+ T cells or “unfavorable” transcriptional signatures may likely not benefit from CD4+ DLI and could be spared the toxicity and cost of a potentially futile treatment. Instead, a more effective approach may be the administration of cytotoxic or targeted therapeutics to reduce tumor burden and change the tumor milieu or perhaps even allow the consideration of a DLI product containing CD8+ T cells. In addition, our results could suggest that additional immunologic priming of the recipient (ie, by antigen-specific or tumor cell vaccination) to generate an adequate antitumor T-cell infiltrate with antileukemic antigen specificity would be warranted. We acknowledge that the strength of the conclusions from our retrospective study is limited by the relatively small size of this discovery cohort. Thus larger studies will be needed to definitively establish whether this paradigm is generalizable across hematologic malignancies or is restricted to CML. We have preliminarily examined a small cohort of 9 patients with relapsed CLL after HSCT treated with DLI and have observed a nonsignificant increase in pre-DLI CD8+ T-cell marrow infiltrates in responders (data not shown). Nevertheless, these findings hint that pre-DLI CD8+ T-cell infiltrates may hold predictive value for treatment efficacy in other hematologic malignancies.
By linking the mechanism of action for a clinically established immunotherapy to reversal of an exhausted state, we propose a compelling new therapeutic direction to target T-cell exhaustion to address leukemic relapse. The clinical debut of agents that may reverse T-cell exhaustion, such as anti-PD1/anti-PDL1 antibodies,43-47 suggests their use in lieu of DLI to promote GvL responses after allogeneic HSCT. These agents can be dosed and titrated in a far more standardized fashion than cellular products, providing an avenue for treatment optimization that has been elusive for DLI.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the expert clinical care provided by the DFCI clinical transplant team and assistance from the DFCI Stem Cell Processing laboratory and Pasquarello Tissue Bank. We also thank Gordon Freeman (DFCI) for insightful discussions. C.J.W. is a Damon-Runyon Clinical Investigator.
This study was supported by the National Institutes of Health National Cancer Institute (5R21CA115043-2) and National Heart, Lung and Blood Institute (5R01HL103532-03), the Claudia Adams Barr Program in Cancer Research, the Leukemia and Lymphoma Translational Research Program, the Early Career Physician-Scientist Award of the Howard Hughes Medical Institute, and the Damon-Runyon Cancer Research Foundation (CI-38-07) (C.J.W.). Statistical analysis was supported by the National Cancer Institute PO1 (CA142106-06A1).
Authorship
Contribution: P.B. and U.H. designed, performed, and analyzed the experiments; collected the data; and wrote and edited the manuscript; M.R. and K.O. analyzed and interpreted data; O.P. analyzed data; W.Z. and X.L. performed experiments; J.A. and H.T.K. performed statistical analyses; W.N.H. and F.S.H. contributed vital new reagents; N.R.G. and C.M.C. collected data and cared for patients; R.J.S., J.R., N.H., and E.P.A. contributed valuable reagents and edited the manuscript; and C.J.W. designed and analyzed the experiments, wrote and edited the manuscript, and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine J. Wu, M.D., Associate Professor in Medicine, Harvard Medical School, Dana-Farber Cancer Institute, 450 Brookline Ave, Dana 540B, Boston, MA 02215; e-mail: cwu@partners.org.