Key Points
vWbp mediates adhesion of S aureus under flow to activated endothelial cells and the subendothelium via VWF.
vWbp activates prothrombin and triggers the formation of bacteria–fibrin–platelet aggregates, which enhance adhesion to vessels under flow.
Abstract
Adhesion of Staphylococcus aureus to blood vessels under shear stress requires von Willebrand factor (VWF). Several bacterial factors have been proposed to interact with VWF, including VWF-binding protein (vWbp), a secreted coagulase that activates the host’s prothrombin to generate fibrin. We measured the adhesion of S aureus Newman and a vWbp-deficient mutant (vwb) to VWF, collagen, and activated endothelial cells in a microparallel flow chamber. In vivo adhesion of S aureus was evaluated in the mesenteric circulation of wild-type (WT) and VWF-deficient mice. We found a shear-dependent increase in adhesion of S aureus to the (sub)endothelium that was dependent on interactions between vWbp and the A1-domain of VWF. Adhesion was further enhanced by coagulase-mediated fibrin formation that clustered bacteria and recruited platelets into bacterial microthrombi. In vivo, deficiency of vWbp or VWF as well as inhibition of coagulase activity reduced S aureus adhesion. We conclude that vWbp contributes to vascular adhesion of S aureus through 2 independent mechanisms: shear-mediated binding to VWF and activation of prothrombin to form S aureus–fibrin–platelet aggregates.
Introduction
Staphylococcus aureus is the most frequent cause of life-threatening bloodstream infections.1 S aureus sepsis carries a poor prognosis, with a fatal outcome in approximately 1 of 5 cases.2,3 A major determinant of this adverse outcome is its propensity to generate secondary sites of infection upon spreading through the bloodstream.3 To establish these metastatic infections and infective endocarditis in particular, circulating pathogens require a mechanism to adhere to the blood vessel wall and to overcome the shear stress of flowing blood.
Activation of endothelial cells (ECs) triggers the release of ultralarge von Willebrand factor (VWF) multimers that are temporarily retained on the EC surface. Furthermore, upon EC damage, circulating VWF binds to collagen fibers from the exposed subendothelial matrix.4 Vessel wall–bound VWF recruits platelets to sites of EC damage or activation through the shear-dependent interaction between the platelet glycoprotein GPΙbα and the VWF A1-domain that is exposed after unfolding of VWF multimers by flowing blood.5 A recent study revealed that S aureus may employ a similar VWF-dependent mechanism to adhere to ECs under shear stress.6 However, the bacterial factors involved in the S aureus–VWF interactions remained to be elucidated.
Several S aureus proteins were shown to bind to VWF, including staphylococcal protein A (SpA) and the VWF-binding protein (vWbp).7 However, the relevance of VWF as a ligand for vWbp was questioned in subsequent studies,8,9 and the role of vWbp–VWF interaction has not yet been studied in relevant disease models.
Nevertheless, vWbp has been shown to contribute to S aureus pathophysiology by its ability to induce blood clotting. This coagulase activity, which differentiates S aureus from less virulent coagulase-negative staphylococci, is the result of the conformational activation of prothrombin by 2 secreted proteins: staphylocoagulase (Coa)10,11 and vWbp.4 Staphylothrombin, the resulting complex of a bacterial coagulase and host prothrombin, converts fibrinogen into insoluble fibrin.11 Because of the direct activation of prothrombin, S aureus–mediated activity is insensitive to most anticoagulant drugs, which has hampered the study of coagulase activity as a virulence factor. However, the generation of mutant strains that lack vWbp, Coa, or both, and the recent finding that the direct thrombin inhibitor dabigatran effectively inhibits S aureus–mediated coagulation has allowed to demonstrate the contribution of this pathogen-induced clot formation to the virulence of S aureus in local infections as well as in sepsis.12-16 Furthermore, staphylothrombin-generated fibrin was shown to promote interaction between S aureus and platelets via the platelet fibrinogen receptor αΙΙbβ3, and contributes to the initiation and propagation of experimental infective endocarditis lesions in flowing blood.14
In this study, we have investigated the role of both vWbp functions—VWF binding and prothrombin activation—for the adhesion of S aureus to activated ECs and to subendothelial matrix under shear stress. We further evaluated the role of platelets in S aureus–vessel wall adhesion focusing on the role of the platelet fibrinogen receptor αΙΙbβ3, and present a model for the endovascular dissemination of S aureus based on VWF–vWbp–S aureus interactions, reinforced by coagulase activity and bacteria–fibrin–platelet interactions.
Materials and methods
Bacterial strains
The reference strain used in this study is S aureus Newman, originally isolated from a case of osteomyelitis, with a well-described profile of coagulase activity.12,16 The isogenic single and double coagulase–deficient mutants S aureus Newman vwb, coa, and coa/vwb with complementing plasmid have been described.16 The double coagulase–deficient mutant has no detectable coagulase activity (ie, is not able to activate host’s prothrombin and generate fibrin). Bacteria were fluorescently labeled with 5(6)-carboxy-fluorescein-N-hydroxysuccinimidyl ester (Sigma-Aldrich, Germany). Recombinant His6-vWbp without the signal sequence and lacking coagulase activity was cloned using plasmid pET15 and purified from Escherichia coli BL21(DE3) using Ni-trilotriacetic acid chromatography as previously described.16
In vitro perfusion experiments
Glass coverslips (24 × 50 mm; VWR International, Belgium) were coated with 50 μg/mL VWF (Haemate P; CSL Behring, Belgium) or 200 μg/mL Horm collagen (Takeda, Austria) in a humidified container at room temperature for 4 hours. The coverslips were mounted in a microparallel flow chamber17 and perfused for 10 minutes using a high-accuracy Harvard pump (PHD 2000 Infusion; Harvard Apparatus, Holliston, MA) generating flow rates of 250 seconds−1 to 2000 seconds−1. Coverslips were perfused with labeled bacteria (OD600 0.65 or 1.2, corresponding to approximately 3 × 108 and 6 × 108 colony-forming units/mL suspended in Dulbecco's modified Eagle medium (DMEM) (Invitrogen, Merelbeke, Belgium), platelet-poor plasma (PPP ), VWF-deficient plasma (Hyphen-BioMed, Neuville-sur-Oise, France) with or without added VWF (60 μg/mL), and platelet-rich plasma (PRP), with or without dabigatran (500 nM; a gift of Joanne van Ryn, Boehringer Ingelheim, Germany), a reversible specific thrombin inhibitor and equally potent inhibitor of staphylothrombin,16 and/or eptifibatide (7.5 μg/mL; GlaxoSmithKline, United Kingdom), antagonizing fibrin(ogen) and VWF binding to the platelet αΙΙbβ3-receptor. Where indicated, PPP and PRP were spiked with fluorescently labeled fibrinogen (F-13191, Alexa Fluor 488 labeled, Invitrogen) to a final concentration of 37.5 µg/mL to assess fibrin formation during perfusion. Bacterial supernatant was prepared by incubating washed bacteria for 4 hours at 37°C in phosphate-buffered saline. To investigate platelet adhesion, PRP was labeled with rhodamine-G (1 μg/mL). The neutralizing anti-VWF A1-domain murine monoclonal antibody 6D1 was added to DMEM, PPP, or PRP at a final concentration of 10 μg/mL. An anti-tPA monoclonal immunoglobulin G1 antibody was used as isotype control (10 μg/mL).
Live images were obtained using an inverted fluorescence microscope (Axio-observer DI; Carl-Zeiss NV, Belgium) and recorded using a black and white camera (Carl-Zeiss Axio-Cam MRm). Images were digitally stored and processed with the ImageJ analysis software (National Institutes of Health, Bethesda, MD) for fluorescent area quantification. Values are reported as Arbitrary Fluorescent Units (AFU) (1 AFU corresponds to the fluorescent area for 1 selected pixel).
Bacterial adhesion to ECs
Human umbilical vein ECs (HUVECs) were seeded on coverslips and mounted in a microparallel flow chamber. The ECs were activated via perfusion with 0.1 mM Ca2+-ionophore A23187 (Sigma-Aldrich, Germany) for 10 minutes, followed by a perfusion for 10 minutes with fluorescently labeled bacteria (OD600 1.2). Bacterial rolling and adhesion were monitored via videomicroscopic imaging, as described previously.
In vivo mesenteric perfusion model
The mouse mesenteric perfusion model was used to study real-time bacterial adhesion to the vascular bed in WT Vwf+/+ and homozygous Vwf−/− C57Bl/6 mice. Six- to 8-week old mice were anesthetized using ketamine/xylazine intraperitoneally and the right jugular vein was catheterized (Portex intravenous cannula, 2F). The peritoneal cavity was opened via midline abdominal incision to visualize the mesenteric arteriolar and venular circulation under an inverted microscope (Axio-observer D1). After topical application of 5 µL of the Ca2+-ionophore A23187 (10 mM) to trigger EC activation and VWF release, 300 µL of a suspension of labeled bacteria (OD600 1.8, corresponding to approximately 1 × 109 colony-forming units/mL) was injected through the jugular catheter. Where indicated, bacterial inoculation was preceded by a bolus of 50 µL of dabigatran (10 µM). Time-lapse images were acquired via online videomicroscopy for analysis. The fluorescent signal in the blood vessel was quantified manually for each frame and reported as AFU. Animal experiments were approved by the Ethical Committee of the University of Leuven.
Statistical analysis
All calculations were done using GraphPad Prism 5.0d (GraphPad Software, San Diego, CA). Groups were compared using a 1-way analysis of variance or a 2-tailed Student t test. All values are reported as mean ± standard error of the mean (SEM). A P value of < .05 was considered significant (*P < .05; **P < .01; ***P < .001).
Results
vWbp–VWF interactions mediate S aureus adhesion under flow
First, we assessed the adhesion of S aureus Newman (WT) to surface-bound VWF under static and flow conditions. Adhesion of S aureus to VWF increased with increasing shear rates (Figure 1A and supplemental Figure 1A-B on the Blood Web site). In contrast, adhesion of the vWbp-deficient mutant strain (vwb) remained low at all shear rates, and further decreased with increasing shear (Figure 1A).
vWbp mediates shear-dependent adhesion of S aureus to VWF. (A) Microparallel flow chamber perfusion over coated VWF (50 μg/mL) with fluorescently labeled wild-type (WT) and vwb strains at shear rates of 500 seconds−1 and 1000 seconds−1 in medium (n ≥ 6). (B) Perfusion over coated collagen with WT and vwb strains at 500 seconds−1 or 1000 seconds−1. VWF (60 μg/mL) was present in the medium where indicated (n ≥ 4). (C) Perfusion over coated collagen with WT or vwb strains in the presence of VWF-deficient plasma at 1000 seconds−1. VWF (60 μg/mL) was added where indicated (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001.
vWbp mediates shear-dependent adhesion of S aureus to VWF. (A) Microparallel flow chamber perfusion over coated VWF (50 μg/mL) with fluorescently labeled wild-type (WT) and vwb strains at shear rates of 500 seconds−1 and 1000 seconds−1 in medium (n ≥ 6). (B) Perfusion over coated collagen with WT and vwb strains at 500 seconds−1 or 1000 seconds−1. VWF (60 μg/mL) was present in the medium where indicated (n ≥ 4). (C) Perfusion over coated collagen with WT or vwb strains in the presence of VWF-deficient plasma at 1000 seconds−1. VWF (60 μg/mL) was added where indicated (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001.
Reconstitution of vWbp expression restored the adhesive phenotype, confirming that vWbp is required for the shear-dependent increase in adhesion of S aureus to VWF (supplemental Figure 2A). Furthermore, the addition of exogenous vWbp increased the adhesion of vwb to VWF under flow (supplemental Figure 3A-C). Preperfusion of the coated VWF with recombinant His6–vWbp decreased the subsequent adhesion of the WT strain, suggesting competition between bound His6–vWbp and subsequently perfused bacterial vWbp. In contrast, the vwb strain showed a mildly increased adhesion when VWF was preperfused with the His6–vWbp (supplemental Figure 3D). Both WT and vwb were found to bind under flow to coated His6–vWbp with comparable efficacies (data not shown), showing that deletion of vWbp in vwb had no effect on the binding of exogenous vWbp to S aureus.
Second, we studied whether VWF–vWbp interaction contributes to the adhesion of S aureus to collagen, the main subendothelial component. The addition of VWF significantly increased the adhesion of WT (Figure 1B; supplemental Figure 1C), but had only a limited effect on the binding of vwb. At 1000 seconds−1, a large difference in adhesion was observed between the WT and the vwb strains (35 195 ± 6959 AFU vs 5847 ± 1644 AFU, n = 12, P < .001) (Figure 1B). Again, addition of exogenous vWbp from WT supernatant or in recombinant form increased the adhesion of vwb (supplemental Figure 3A) and WT (supplemental Figure 3B), and the decreased adhesion of the coa/vwb strain was rescued by a plasmid carrying the coa and vwb gene (supplemental Figure 2B).
We further compared the adhesion of the WT and vwb strains to collagen in VWF-deficient plasma, before and after spiking with VWF. When suspended in VWF-deficient plasma, the WT and vwb strains bound comparably to collagen, but VWF repletion only increased the adhesion of the WT strain significantly (Figure 1C).
S aureus coagulase activity enhances bacterial adhesion to collagen
Activation of coagulation through coagulase activity is a hallmark of S aureus. Indeed, in the presence of plasma, we noted a time-dependent formation of microscopic aggregates that facilitated bacterial adhesion to collagen when perfused under flow. To study the interaction between coagulase activity and vWbp–VWF interactions on flow-dependent adhesion, we compared adhesion of S aureus WT, which possesses 2 coagulases: vwb, which is deficient in vWbp but retains Coa-mediated coagulase activity; and the coa/vwb strain, which has no detectable coagulase activity.
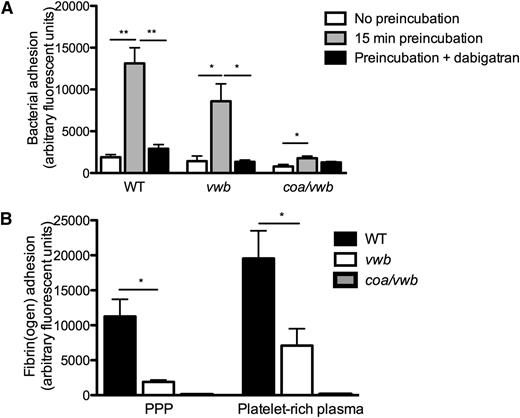
Preincubation of the WT strain in plasma led to a ∼sevenfold increase in adhesion for the WT strain (1891 ± 314 AFU vs 13 130 ± 1867 AFU, n ≥ 5, P < .01) (Figure 2A and supplemental Videos 1 and 2), which could be prevented by the (staphylo)thrombin inhibitor dabigatran. This increased adhesion of the WT strain was paralleled by a deposition of fibrin fibers (Figure 2B). In contrast, preincubation of the coagulase-deficient coa/vwb strain had only a minor effect on bacterial adhesion (Figure 2A), and no fibrin formation could be detected (Figure 2B).
Coagulase activity increases S aureus adhesion to collagen under shear stress. (A) Microparallel flow chamber perfusion over coated collagen with fluorescently labeled WT, vwb, and coa/vwb strains in plasma with or without preincubation (37°C for 15 minutes) at 1000 seconds−1. The ability of bacteria to generate coagulase-mediated fibrin during the preincubation phase in plasma increases subsequent bacterial adhesion. Where indicated, dabigatran (500 nm) was added to the plasma (preincubation 37°C for 15 minutes) (n ≥ 5). (B) Perfusion over coated collagen with fluorescently labeled fibrinogen and WT, vwb, and coa/vwb strains in the presence of plasma and platelet-rich plasma (preincubation 37°C for 15 minutes) (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001.
Coagulase activity increases S aureus adhesion to collagen under shear stress. (A) Microparallel flow chamber perfusion over coated collagen with fluorescently labeled WT, vwb, and coa/vwb strains in plasma with or without preincubation (37°C for 15 minutes) at 1000 seconds−1. The ability of bacteria to generate coagulase-mediated fibrin during the preincubation phase in plasma increases subsequent bacterial adhesion. Where indicated, dabigatran (500 nm) was added to the plasma (preincubation 37°C for 15 minutes) (n ≥ 5). (B) Perfusion over coated collagen with fluorescently labeled fibrinogen and WT, vwb, and coa/vwb strains in the presence of plasma and platelet-rich plasma (preincubation 37°C for 15 minutes) (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001.
Interestingly, absence of vWbp (vwb strain), which abolished shear-mediated adhesion in the absence of plasma (Figure 1C), had only a limited effect on adhesion if coagulase activity was present (Figure 2A). Although fibrin deposition of vwb was reduced by approximately 80% compared with WT, fibrin fibers could still be detected (Figure 2B). When absence of vWbp was combined with pharmacological inhibition of coagulase (dabigatran), bacterial adhesion to collagen was completely abrogated (Figure 2A).
The finding that coagulase activity could promote flow-dependent adhesion of S aureus independent from vWbp was confirmed by studying adhesion of the coa strain in which the vWbp-prothrombin complex is the only source of staphylothrombin (supplemental Figure 4).
S aureus–platelet interactions contribute to vessel wall adhesion
Platelets interact with S aureus via fibrin(ogen) bridges14 and they also display typical shear-dependent adhesion to VWF.18 To study the influence of platelet–VWF, platelet–fibrin, and platelet–S aureus19 interactions on the VWF-dependent adhesion of S aureus to collagen, perfusion experiments were carried out using both PPP and PRP, spiked with fluorescently labeled bacteria or platelets.
The presence of platelets increased the adhesion of the WT strain to collagen (13 130 ± 1867 AFU vs 39 504 ± 6431 AFU, n ≥ 11, P < .01) (Figure 3A) in control conditions. The increased adhesion of S aureus in the presence of platelets was no longer found when coagulase activity was prevented by dabigatran (Figure 3A). Interestingly, when the platelet fibrin(ogen) receptor αΙΙbβ3 was inhibited by eptifibatide, addition of platelets decreased S aureus WT adhesion to collagen (Figure 3A). Finally, the combined inhibition of the coagulase activity of S aureus and the αΙΙbβ3 platelet integrin almost completely abolished bacterial adhesion (39 504 ± 6431 AFU vs 739 ± 258 AFU, n ≥ 4, P < .01) (Figure 3A).
Platelets increase S aureus adhesion under shear stress. (A) Microparallel flow chamber perfusions over coated collagen with fluorescently labeled WT, vwb, and coa/vwb strains in the presence of plasma and platelet-rich plasma (preincubation 37°C for 15 minutes) at 1000 seconds−1. Addition of dabigatran (500 nM) or eptifibatide (7.5 μg/mL) where indicated (n ≥ 5). (B) Perfusion over coated collagen with WT, vwb, and coa/vwb strains in platelet-rich plasma at 1000 seconds−1. Platelets were labeled with rhodamine-G (preincubation 37°C for 15 minutes). Adhesion of platelets was lower when perfused with a strain lacking vWbp compared with WT and was further reduced when perfused together with a mutant strain lacking both coagulases (vwb/coa) (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01. (C) SEM image (×20 000) of WT perfusion in PRP over collagen at 1000 seconds−1, illustrating the interactions among bacteria, fibrin, and platelets. Bar represents 1 μm. (D) SEM image (×5000) of WT perfusion in PRP over collagen at 1000 seconds−1. Bar represents 2 μm. Full white arrows indicate bacteria; dotted white arrows indicate platelets.
Platelets increase S aureus adhesion under shear stress. (A) Microparallel flow chamber perfusions over coated collagen with fluorescently labeled WT, vwb, and coa/vwb strains in the presence of plasma and platelet-rich plasma (preincubation 37°C for 15 minutes) at 1000 seconds−1. Addition of dabigatran (500 nM) or eptifibatide (7.5 μg/mL) where indicated (n ≥ 5). (B) Perfusion over coated collagen with WT, vwb, and coa/vwb strains in platelet-rich plasma at 1000 seconds−1. Platelets were labeled with rhodamine-G (preincubation 37°C for 15 minutes). Adhesion of platelets was lower when perfused with a strain lacking vWbp compared with WT and was further reduced when perfused together with a mutant strain lacking both coagulases (vwb/coa) (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01. (C) SEM image (×20 000) of WT perfusion in PRP over collagen at 1000 seconds−1, illustrating the interactions among bacteria, fibrin, and platelets. Bar represents 1 μm. (D) SEM image (×5000) of WT perfusion in PRP over collagen at 1000 seconds−1. Bar represents 2 μm. Full white arrows indicate bacteria; dotted white arrows indicate platelets.
To explain this, we hypothesized that S aureus and platelets individually compete for VWF-mediated binding to collagen. However, clustering of bacteria to platelets, which requires coagulase-mediated fibrin formation and subsequent αΙΙbβ3-mediated fibrin bridging,14 increases the binding capacity. To test this hypothesis, we performed scanning electron microscopy imaging of the coverslips after perfusion, which showed fibrin bridging of bacteria and platelets (Figure 3C-D). Hence, inhibition or absence of one of these players mitigated the increased bacterial adhesion.
Platelets had less effect on the adhesion of the vwb strain compared with the WT strain, and both eptifibatide and dabigatran further reduced the adhesion of the vwb strain in the presence of platelets (Figure 3A). The elimination of both vWbp–VWF interactions and staphylothrombin-mediated fibrin formation in the coa/vwb strain resulted in a limited adhesion, irrespective of the presence of platelets (Figure 3A).
The changes in bacterial adhesion were paralleled by changes in platelet deposition on the collagen surface. Absent vWbp reduced platelet adhesion to collagen twofold. Adhesion of platelets to collagen was further decreased upon perfusion of coa/vwb (Figure 3B), underpinning the role of both fibrin generation and vWbp–VWF interactions in the generation of S aureus–platelet microthrombi.
vWbp binds to VWF via the VWF A1-domain
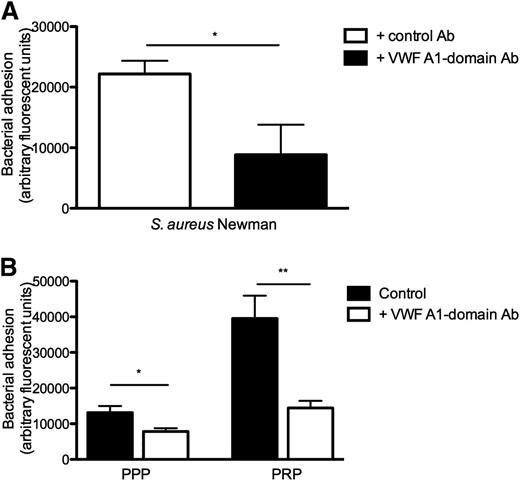
Neutralization of the VWF A1-domain by a neutralizing anti-A1 monoclonal antibody reduced bacterial adhesion of S aureus to collagen (Figure 4A-B), demonstrating the importance of the A1-domain of VWF for the shear-dependent adhesion of S aureus to VWF. The impact of the anti–A1-domain was larger in the presence of platelets, which also bind to the VWF A1-domain via the GP1bα receptor.
Blocking A1-domain of VWF inhibits S aureus binding to collagen under shear stress. (A) Perfusion over coated collagen with fluorescently labeled WT strain and 60 μg/mL VWF in medium at a shear rate of 1000 seconds−1. Anti-A1 VWF domain antibody (Ab) 6D1 (final concentration 10 μg/mL) or anti-tPa monoclonal immunoglobulin G1 Ab (10 μg/mL) were added where indicated (n ≥ 5). (B) Perfusion over coated collagen at 1000 seconds−1 with WT strain in PPP and PRP (PPP or PRP preincubation of 15 minutes at 37°C). 6D1 (final concentration 10 μg/mL) was added where indicated (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01.
Blocking A1-domain of VWF inhibits S aureus binding to collagen under shear stress. (A) Perfusion over coated collagen with fluorescently labeled WT strain and 60 μg/mL VWF in medium at a shear rate of 1000 seconds−1. Anti-A1 VWF domain antibody (Ab) 6D1 (final concentration 10 μg/mL) or anti-tPa monoclonal immunoglobulin G1 Ab (10 μg/mL) were added where indicated (n ≥ 5). (B) Perfusion over coated collagen at 1000 seconds−1 with WT strain in PPP and PRP (PPP or PRP preincubation of 15 minutes at 37°C). 6D1 (final concentration 10 μg/mL) was added where indicated (n ≥ 4). All results are expressed as mean ± SEM. *P < .05, **P < .01.
vWbp enhanced bacterial adhesion to ECs
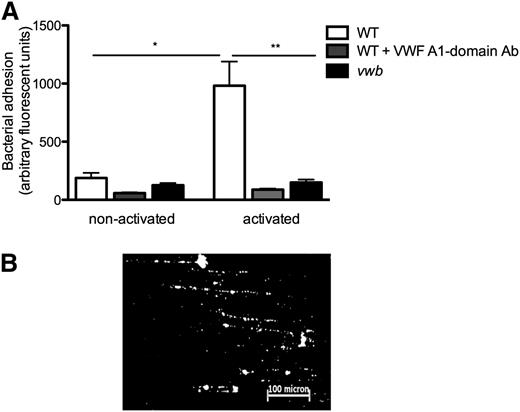
To investigate the adhesion of S aureus to ECs under flow, HUVECs were perfused with fluorescently labeled bacteria at 1000 seconds−1 with or without EC activation, which led to the release of VWF (Figure 5A). Specific VWF staining on activated ECs confirmed release of multimeric VWF (not shown). EC activation and VWF release increased adhesion of the WT strain, which formed typical “string” patterns of fluorescently labeled bacterial clusters aligned in the direction of the shear force (Figure 5B), suggesting the binding of bacteria along a linear-stretched VWF molecule. Compared with WT, vwb adhered threefold less (982 ± 207 AFU vs 305 ± 150 AFU, n ≥ 5, P < .05) (Figure 5A). The VWF A1-domain antibody, but not an isotype control antibody, significantly decreased the adhesion of the WT strain to activated ECs (Figure 5A).
vWbp mediates bacterial adhesion to activated HUVECs under flow conditions. (A) Microparallel flow chamber perfusions. ECs were activated with the Ca2+-ionophore A23187 (0.1 mM) followed by a 10-minute perfusion of fluorescently labeled WT and vwb strains at a shear rate of 1000 seconds−1 in DMEM. Where indicated, the anti-A1 VWF domain antibody 6D1 (final concentration 10 μg/mL) was present. No difference was observed in the presence or absence of the anti-tPa monoclonal immunoglobulin G1 antibody. All results are expressed as mean ± SEM. *P < .05, **P < .01, n ≥ 5. (B) Image of microparallel flow chamber perfusion over activated HUVECs with WT at a shear rate of 1000 seconds−1. S aureus forms strings on VWF over a distance of >200 microns.
vWbp mediates bacterial adhesion to activated HUVECs under flow conditions. (A) Microparallel flow chamber perfusions. ECs were activated with the Ca2+-ionophore A23187 (0.1 mM) followed by a 10-minute perfusion of fluorescently labeled WT and vwb strains at a shear rate of 1000 seconds−1 in DMEM. Where indicated, the anti-A1 VWF domain antibody 6D1 (final concentration 10 μg/mL) was present. No difference was observed in the presence or absence of the anti-tPa monoclonal immunoglobulin G1 antibody. All results are expressed as mean ± SEM. *P < .05, **P < .01, n ≥ 5. (B) Image of microparallel flow chamber perfusion over activated HUVECs with WT at a shear rate of 1000 seconds−1. S aureus forms strings on VWF over a distance of >200 microns.
In vivo bacterial adhesion in splanchnic veins is mediated by vWbp–VWF interactions
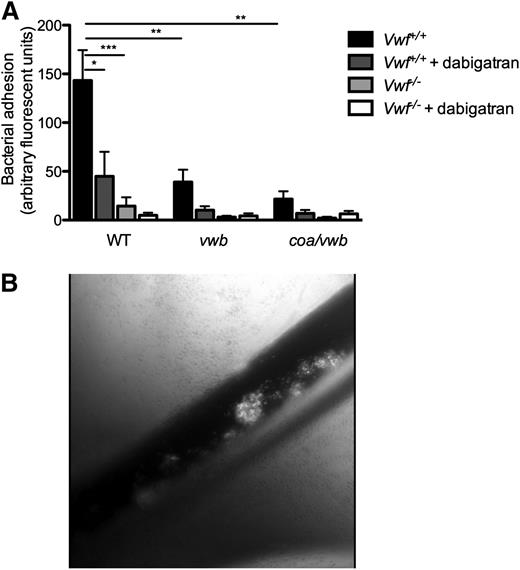
Finally, we validated our findings in an in vivo model. Real-time videomicroscopy of the murine splanchnic veins allowed the in vivo visualization of circulating fluorescently labeled S aureus, injected via a catheter in the jugular vein. After pharmacological activation of the endothelium by the Ca2+-ionophore, we observed rapid local accumulation of individual bacteria and aggregates of bacteria to the vessel wall of WT mice (Figure 6B) (supplemental Videos 3 and 4). Inoculation of the vwb strain resulted in reduced vessel wall adhesion of bacteria (64.5 ± 28.1 AFU vs 143 ± 31.2 AFU, n ≥ 10, P < .01) (Figure 6A) (supplemental Video 5). Inhibition of coagulase activity by dabigatran reduced the formation and adhesion of aggregates of the WT strain, leading to a large reduction in total bacterial adhesion, but did not prevent the adhesion of individual bacteria (Figure 6A). When the vwb strain was inoculated along with dabigatran treatment, the combined genetic deletion of vWbp and inhibition of coagulase activity resulted in the strongest reduction in adhesion (WT vs vwb + dabigatran: 143 ± 31.2 AFU vs 10.2 ± 4.01 AFU, n ≥ 7, P < .01 and WT vs coa/vwb: 143 ± 31.2 AFU vs 21.6 ± 7.89 AFU, n ≥ 10, P < .01).
Bacterial adhesion to activated endothelium in vivo is VWF and vWbp -mediated. (A) In vivo venous mesenteric perfusion model with C57Bl/6-Vwf+/+ and C57Bl/6-Vwf−/− mice. A total of 5 µL of the Ca2+-ionophore A23187 (10 mM) was applied to the region of the visualized vascular bed to trigger EC activation and VWF release. A suspension of carboxy-fluorescein–labeled WT, vwb, or coa/vwb strains was injected through the jugular catheter. Where indicated, bacterial inoculation was preceded by a bolus of 50 µL of dabigatran (10 µM). All results are expressed as mean ± SEM. **P < .01, ***P < .001, n ≥ 7. (B) Image of in vivo venous mesenteric perfusion model with C57Bl/6-Vwf+/+ mice.
Bacterial adhesion to activated endothelium in vivo is VWF and vWbp -mediated. (A) In vivo venous mesenteric perfusion model with C57Bl/6-Vwf+/+ and C57Bl/6-Vwf−/− mice. A total of 5 µL of the Ca2+-ionophore A23187 (10 mM) was applied to the region of the visualized vascular bed to trigger EC activation and VWF release. A suspension of carboxy-fluorescein–labeled WT, vwb, or coa/vwb strains was injected through the jugular catheter. Where indicated, bacterial inoculation was preceded by a bolus of 50 µL of dabigatran (10 µM). All results are expressed as mean ± SEM. **P < .01, ***P < .001, n ≥ 7. (B) Image of in vivo venous mesenteric perfusion model with C57Bl/6-Vwf+/+ mice.
Almost no adhesion of bacteria was observed on the activated vessel wall of VWF-deficient mice as compared with adhesion in WT mice (Vwf+/+ mice vs Vwf−/− mice: 143 ± 31.2 AFU vs 14.4 ± 9.01 AFU, n ≥ 13, P < .001) (Figure 6A) (supplemental Video 6). No further reduction was found when we compared the adhesion of the WT strain with the vwb or the coa/vwb strains to the activated vessel wall of VWF-deficient mice, with or without the inhibition of staphylothrombin.
In conclusion, the absence of both Coa and vWbp or the absence of VWF completely abolished the ability of S aureus to adhere to the activated vessel wall.
Discussion
In this study, we identify vWbp as a key protein involved in the early steps of vascular infections by S aureus through a unique synergism between its shear-dependent interaction with VWF and its coagulase activity. vWbp mediates bacterial binding to collagen and to ECs under shear via vWbp–VWF interactions. Bacterial adhesion is further enhanced by vWbp- and Coa-mediated fibrin formation and by S aureus–fibrin–platelet interactions. S aureus adhesion to activated mesenteric endothelium required both VWF and vWbp, whereas the coagulase activity of Coa and vWbp increased the local infective burden through the formation of S aureus–containing microthrombi.
The ability for bacteria to adhere to the vessel wall is a crucial first step to initiate metastatic infections and infective endocarditis.20,21 Activation or inflammation of ECs will induce rapid release of highly reactive VWF multimers on the vessel wall. Temporarily retained VWF multimers are a ligand for circulating platelets but also for S aureus, as recently reported.6,22 However, the mechanisms by which S aureus adheres to VWF under flow conditions remained uncertain.
The ability of S aureus to bind to VWF had previously been reported to be mediated by SpA.23 SpA binding to VWF has been documented in static conditions and at low shear stress24,25 ; however, in high flow, the recruitment of S aureus to ECs appeared to be SpA-independent.6 In 2002, vWbp was described as a novel secreted VWF-binding molecule with coagulase activity, present in all tested strains of S aureus.7 However, other studies could not confirm VWF as an important ligand for vWbp when assessed in plasma in static conditions.9
Our results indicate that vWbp interactions with VWF are enhanced in a flow field. VWF circulates in a compact globular form, but is progressively unfolded in a shear field26 or when bound to collagen,27,28 thereby exposing the VWF A1-, A2-, and A3-domains. The A1-domain is the ligand-binding site for platelet GPIbα, the major VWF receptor on platelets,29 whereas the A3-domain is required for VWF binding to collagen fibers. We determined that not only binding of circulating platelets, but also of circulating S aureus, could largely be blocked by an A1 neutralizing antibody. Hence, S aureus has developed an adhesion mechanism very similar to that used by platelets.
vWbp also interacts with prothrombin to mediate the conversion of soluble fibrinogen into fibrin. S aureus staphylothrombin activity constitutes an important determinant of bacterial virulence in local abscess formation, sepsis mortality, and device-related infections.12,13,15 Comparing adhesion of the single and double mutants coa, vwb, and coa/vwb, respectively, revealed a unique synergistic action in vessel wall adhesion between coagulation activation and adhesion to VWF. Fibrin clusters bacteria, and these aggregates interact with immobilized VWF more effectively than single bacterium. Cluster formation was abolished upon staphylothrombin inhibition by dabigatran or when using the double coa/vwb mutant, in line with our earlier findings.14
Platelets rapidly bind to collagen under high shear stress, with VWF acting as a bridging molecule. Our results show that, rather than reducing the adhesion of bacteria by competing for VWF binding, the presence of platelets increased the adhesion of S aureus to collagen under flow conditions. This increased adhesion in the presence of platelets required both coagulase activity and platelet–fibrin binding through αΙIbβ3. Fibrin bridges between bacteria and platelets, may facilitate bacterial recruitment and the formation of bacteria–platelet aggregates.14 We have previously shown that even when staphylothrombin, in contrast to thrombin, does not directly activate the platelet thrombin receptor, S aureus–mediated fibrin generation indirectly facilitates bacteria–platelet interactions via common interactions with fibrin.14
The presence of platelets also increased the adhesion of the vwb mutant. Because this effect was lost in the presence of dabigatran or in the double coa/vwb mutant strain, these findings confirm that the role of platelets in adhesion requires coagulase activity and fibrin formation, even when direct bacterial receptors on platelets have been described.19 The bacteria–platelet interactions via fibrin may allow bacteria to dock more firmly onto VWF. Platelets will likewise interact with VWF multimers and use GPIbα-VWF interaction to dock to collagen, further stabilizing adhesion of the conglomerate between bacteria and platelets. When αΙIbβ3 fibrinogen–mediated platelet–platelet and αΙIbβ3 fibrin–mediated platelet–bacteria interactions are prevented by eptifibatide, single platelets compete for VWF binding with vWbp and reduce S aureus adhesion to collagen.
Interactions of bacteria with VWF represent an efficient strategy to focus circulating pathogens to breaches in the protective endothelial cell lining of the vasculature. Inflammation-triggered exposure of VWF allowed the recruitment of S aureus to the mesenteric vasculature. Adhesion was abrogated in Vwf−/− mice. The role of staphylothrombin in vivo was also apparent from the formation of large vegetations, a process abrogated by dabigatran. The supplemental videos further illustrate how large bacterial conglomerates embolize in the face of increasing shear forces, potentially giving rise to metastatic infectious foci.
Previous studies using S aureus coa mutants failed to provide insight into the role of Coa in the initial infection of heart valves.30 Because vWbp had not been discovered at the time of these investigations, the molecular contribution of this secreted factor could not have been appreciated. Here, we show that vWbp is a key molecule for bacterial binding to the vasculature and that it represents an interesting therapeutic target to contain intravascular S aureus infections.
In conclusion, our work identifies vWbp as a key protein in S aureus–vessel wall interactions, through unique bimodal adhesive properties and by combining adhesive and procoagulant activities. Combined inhibition of coagulase activity and VWF binding completely abolished S aureus adhesion to the vessel wall, despite the redundancy of S aureus mechanisms including 2 coagulases and potentially other vWbps. To explain the propensity of S aureus to adhere to the endothelium under shear stress, we have shown synergistic interactions between S aureus, platelets, fibrin(ogen), and adhesive bacteria–vessel wall interactions. Disruption of such interactions may lead to novel therapeutic strategies, reducing the high mortality of S aureus bloodstream infections, including infective endocarditis.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen G0466.10; “Eddy Merckx Research Grant” and the “Sporta Research Grant” for Pediatric Cardiology, UZ Leuven, Belgium (J.C.); the Center for Molecular and Vascular Biology is supported by the Programmafinanciering KU Leuven (PF/10/014), by the “Geconcerteerde Onderzoeksacties” (GOA 2009/13) from the University of Leuven and a research grant from Boehringer-Ingelheim. C.V., M.P., and L.L. are fellows and P.V. is a senior clinical investigator of the FWO.
The skillful assistance of Katrien Cludts, Soetkin Van kerckhoven, and Marleen Lox is acknowledged.
Authorship
Contribution: P.V., M.F.H., and R.H. designed the research, analyzed the data, and wrote the manuscript; J.C. and T.V. designed and performed the research, analyzed the data, and wrote the manuscript; K.V., M.P., L.L. and C.V. performed experiments and helped interpret data; and D.M. and O.S designed the research, contributed vital new reagents, and contributed to writing the manuscript.
Conflict-of-interest disclosure: P.V. has received honoraria from Boehringer-Ingelheim for lectures and advisory committees. The remaining authors declare no competing financial interests.
Correspondence: Peter Verhamme, Center for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven – University of Leuven, Herestraat 49, Box 901, 3000 Leuven, Belgium; e-mail: peter.verhamme@uzleuven.be.
References
Author notes
J.C. and T.V. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal