In this issue of Blood, Noris et al describe a functional complement assay that can be used to diagnose atypical hemolytic-uremic syndrome (aHUS).1 This assay might also be useful in identifying aHUS patients in remission and in detecting asymptomatic carriers of complement gene mutations. Besides being used for diagnostic purposes, this assay potentially can be used to monitor anticomplement therapy.
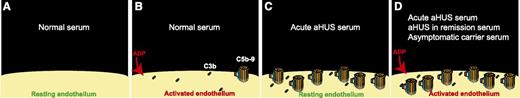
(A) Resting endothelium is protected against complement activation by complement regulators. (B) Normal serum deposited small amounts of C3b (and C3b degradation products) and C5b-9 on ADP-activated endothelial cells, but complement regulatory proteins presented in normal serum inhibited propagation of complement activation on the surface of endothelium. (C) In the presence of serum from a patient with acute aHUS, complement regulation on endothelial cells is ineffective, resulting in a larger number of C5b-9 complexes deposited on the surface of resting endothelium. (D) Serum samples from patients with acute aHUS, aHUS in remission, and healthy carriers of complement mutations deposited C5b-9 on the surface of ADP-activated endothelial cells. In this assay, HMEC-1 were used as the source of endothelial cells.
(A) Resting endothelium is protected against complement activation by complement regulators. (B) Normal serum deposited small amounts of C3b (and C3b degradation products) and C5b-9 on ADP-activated endothelial cells, but complement regulatory proteins presented in normal serum inhibited propagation of complement activation on the surface of endothelium. (C) In the presence of serum from a patient with acute aHUS, complement regulation on endothelial cells is ineffective, resulting in a larger number of C5b-9 complexes deposited on the surface of resting endothelium. (D) Serum samples from patients with acute aHUS, aHUS in remission, and healthy carriers of complement mutations deposited C5b-9 on the surface of ADP-activated endothelial cells. In this assay, HMEC-1 were used as the source of endothelial cells.
aHUS is a thrombotic microangiopathy that results in hemolysis, thrombocytopenia, and kidney failure. In contrast to typical hemolytic-uremic syndrome, aHUS is not a complication of infection with Shiga-toxin–producing enteropathogens and does not have a diarrheal prodrome. aHUS usually afflicts young patients and different members of the same family. It often has a recurrent and relapsing clinical course, resulting in end-stage renal disease, and can recur in the transplanted kidneys. In 1981, Thompson and Winterborn reported low serum levels of complement proteins in a patient with aHUS and his family members,2 and in 1998, Warwicker et al identified mutations in the factor H gene in aHUS patients.3 Since then, several complement mutations have been reported (www.fh-hus.org), including loss-of-function mutations in factor H, factor I, membrane cofactor protein (MCP), and thrombomodulin and gain-of-function mutations in C3 and factor B. In a small percentage of aHUS patients (5% to 7%), antifactor H antibodies, in association with deletions in genes encoding complement factor H–related proteins CFHR1 and CFHR3, were detected.4 Mutations in complement genes and antifactor H antibodies are present in about half of patients with a clinical diagnosis of aHUS. In the other half, despite the presence of complement dysregulation, no mutation in complement genes is detectable. Currently, there are no diagnostic tests available that can reliably confirm or refute a diagnosis of aHUS. This is an important shortcoming considering the fact that an effective treatment of aHUS is available. Eculizumab (Soliris; Alexion), which is an antibody against complement component 5 (C5) originally introduced to treat patients with paroxysmal nocturnal hemoglobinuria, was approved by the US Food and Drug Administration for the treatment of aHUS. The correct dosing of eculizumab in aHUS is unknown, and anecdotal reports on different dosing schedules guide physicians in treating aHUS patients, mostly in a trial-and-error manner. This is also an important shortcoming considering the high cost of eculizumab and the high morbidity associated with inadequately treated aHUS.
Currently, to diagnose aHUS, besides genetic studies on complement genes that may take several weeks to be completed, few diagnostic tests such as measuring concentration of complement proteins in the serum and sheep erythrocyte lysis assay are used. These serum assays have a low sensitivity and specificity. Serum C3 and soluble C5b-9 (terminal attack complex) levels or sheep erythrocyte lysis assay can be normal in a large percentage of aHUS patients or can be low in conditions other than aHUS. Additionally, measuring serum concentration of complement proteins is helpful to evaluate complement regulation in the fluid-phase, but not on cell surfaces, and the pathogenesis of aHUS is mainly related to complement dysregulation on the surface of endothelial cells.
Previously, it was shown that aHUS is associated with deposition of complement products on endothelial cells.5 In this issue of Blood, Noris et al1 provide data on an in vitro assay that is able to detect complement dysregulation on endothelial cells. In this assay, the patient’s serum sample was incubated with human microvascular endothelial cells (HMEC-1) for 4 hours. Prior to adding serum, HMEC-1 were either incubated with adenosine 5′-diphosphate (ADP) (activated) or not (resting). Subsequently, the amount of deposited C3 and C5b-9 on HMEC-1 was quantified by confocal microscopy. The authors used this assay to evaluate complement regulation in 36 aHUS patients: 7 during the acute phase of aHUS, 22 in remission, and 7 both during the acute phase and in remission. They also used 14 subjects as controls: 7 healthy relatives of the cohort who were also carriers of complement mutations and 7 healthy relatives who did not have complement mutations or antifactor H antibodies. Another important control group in this study was 15 patients with C3 glomerulonephritis or immune complex membranoproliferative glomerulonephritis who developed kidney disorders due to fluid-phase complement activation. In the reported results, the authors found that serum of patients with either acute aHUS or aHUS in remission deposited more C5b-9 on ADP-activated HMEC-1 than serum of control subjects (see figure). It is worth mentioning that 38% of aHUS patients in this study (14 out of 36) did not have any detectable complement mutations or antifactor H antibodies. Only serum of patients with acute aHUS deposited more C5b-9 on resting HMEC-1 than control sera. Interestingly, serum of healthy mutation carriers, and not serum of healthy noncarriers, deposited more C3 and C5b-9 on ADP-activated HMEC-1 compared with control sera. The amount of C5b-9 deposited on ADP-activated HMEC-1 by almost all of the serum samples from patients with glomerulonephritis due to fluid-phase complement activation (14 out of 15) was similar to that by control sera. From these results, one might conclude that an increase in C5b-9 deposition on ADP-activated HMEC-1 can be helpful in the diagnosis of aHUS (acute phase or in remission).
The authors found that treatment with eculizumab resulted in normalization of serum-induced C5b-9 deposition on ADP-activated HMEC-1 in patients with aHUS. Interestingly, the dosage of eculizumab was titrated according to the results of this assay in 4 patients. Normalization of the serum-induced C5b-9 deposition with higher doses of eculizumab coincided with improvement in the clinical parameters in 2 patients, and conversely, reducing the dosage or increasing the interval between infusions of eculizumab in 2 patients based on the results of this assay was not associated with any worsening of their clinical outcome.
In summary, the in vitro functional complement assay based on the serum-induced C5b-9 deposition on endothelial cells is potentially useful in the diagnosis of aHUS and in monitoring its treatment. The problems with this assay are that it is technically complex and cumbersome, hindering its widespread clinical use in its current form, and it cannot detect complement dysregulation due to the deficiency of cell-surface complement regulators (thrombomodulin and MCP) and aHUS due to mutations in cytoplasmic diacylglycerol kinase ε.6
Conflict-of-interest disclosure: The author participated on an advisory board and served as a consultant for Alexion Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal