In this issue of Blood, Florek et al from Stanford describe an interesting new approach to the problem of graft-versus-host disease (GVHD).1
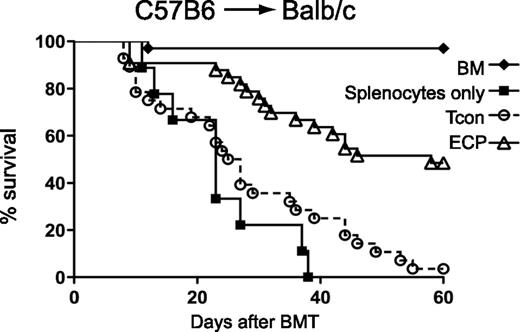
Exposure to apoptotic cells prior to BMT improves survival. BALB/c mice were injected with C57BL/6 BM plus conventional T cells (Tcon) only (circles) or with prior injection of ECP-treated BALB/c-treated cells (triangles) or with prior injection of BALB/c cells treated with 8MOP but no UV light (squares). See Figure 1A in the article by Florek et al that begins on page 1832.
Exposure to apoptotic cells prior to BMT improves survival. BALB/c mice were injected with C57BL/6 BM plus conventional T cells (Tcon) only (circles) or with prior injection of ECP-treated BALB/c-treated cells (triangles) or with prior injection of BALB/c cells treated with 8MOP but no UV light (squares). See Figure 1A in the article by Florek et al that begins on page 1832.
GVHD remains the scourge of allogeneic bone marrow transplantation (BMT) and limits the use of this important, curative therapy to patients with very-high-risk diseases. The mainstay of GVHD prevention remains broad-spectrum immunosuppression that succeeds in only half of patients, and innovative strategies are desperately needed. The central event of the graft-versus-host reaction is the activation of donor T cells by host antigen-presenting cells (APCs). High-dose chemoradiotherapy prior to BMT eliminates most, but not all, hematopoietic APCs; the APCs that remain, however, are activated and elicit inflammatory responses from the T cells in the donor graft, eventually resulting in GVHD. Phagocytosis of apoptotic cells by APCs can change their function and make them more regulatory or tolerogenic, thereby limiting the responses of donor T cells that drive GVHD. The authors of the current study reasoned that extracorporeal photopheresis (ECP), a modality that exposes cells to methoxypsoralen (8MOP) and UV radiation to induce apoptosis, might be used to prevent GVHD. Indeed, they show that this strategy reduced mortality from GVHD (see figure) and increased the number of regulatory T cells (Tregs), but importantly did not reduce beneficial graft-versus-leukemia effects. Injections of lipopolysaccharide abrogated the protective effect, confirming that modulation of the inflammatory milieu surrounding APCs is key to the success of the strategy.
Others have demonstrated that several infusions of donor ECP-treated cells following BMT can reverse ongoing acute GVHD in mouse models through the induction of donor Tregs.2 Clinically, successful treatment of acute GVHD by ECP has also been associated with an increase in Tregs.3,4 The principal innovation of this report is the use of host apoptotic cells to modulate the function of host APCs to prevent GVHD. This advance is important because no single APC subset appears to initiate GVHD. Dendritic cells (DCs), the APC subset par excellence, may even regulate GVHD rather than amplify it.5 Current data indicate that donor CD8+ T cells are activated by hematopoietic APCs6,7 but that donor CD4+ T cells can be activated by nonhematopoietic APCs in the gastrointestinal tract.5 Once activated, donor T cells may be further stimulated by donor APCs and contribute to GVHD.8 Given this complex variety of APCs that may activate donor T cells, an approach that modulates APC function is more attractive than a strategy that targets a specific cell type, potentially leaving other APC populations unaffected. It should also be noted that the use of donor apoptotic cells from HLA-identical siblings may effectively prevent GVHD. A small dose-escalation trial tested a single infusion on day −1 of BMT of apoptotic mononuclear cells from HLA-identical sibling donors when added to cyclosporine/methotrexate prophylaxis. The resulting incidence of grade II to IV GVHD was 23% (with 0% for the 2 highest cell doses) and nonrelapse mortality was 8% at day 100.9 Incubation of host DCs with these apoptotic cells also significantly decreased the cell surface expression of the activation markers HLA-DR and CD86. Future clinical trials of cellular therapy with apoptotic cells (potentially of either donor or host origin) to prevent GVHD will therefore be of great interest.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal