Key Points
Endothelial-restricted complement activation occurs in aHUS, and clinical remission relies on efficient endothelial complement inhibition.
Ex vivo serum-induced endothelial C5b-9 deposits are a sensitive tool to monitor complement activation and eculizumab effectiveness in aHUS.
Abstract
Atypical hemolytic-uremic syndrome (aHUS) is associated with genetic complement abnormalities/anti–complement factor H antibodies, which paved the way to treatment with eculizumab. We studied 44 aHUS patients and their relatives to (1) test new assays of complement activation, (2) verify whether such abnormality occurs also in unaffected mutation carriers, and (3) search for a tool for eculizumab titration. An abnormal circulating complement profile (low C3, high C5a, or SC5b-9) was found in 47% to 64% of patients, irrespective of disease phase. Acute aHUS serum, but not serum from remission, caused wider C3 and C5b-9 deposits than control serum on unstimulated human microvascular endothelial cells (HMEC-1). In adenosine 5′-diphosphate–activated HMEC-1, also sera from 84% and 100% of patients in remission, and from all unaffected mutation carriers, induced excessive C3 and C5b-9 deposits. At variance, in most patients with C3 glomerulopathies/immune complex-associated membranoproliferative glomerulonephritis, serum-induced endothelial C5b-9 deposits were normal. In 8 eculizumab-treated aHUS patients, C3/SC5b-9 circulating levels did not change posteculizumab, whereas serum-induced endothelial C5b-9 deposits normalized after treatment, paralleled or even preceded remission, and guided drug dosing and timing. These results point to efficient complement inhibition on endothelium for aHUS treatment. C5b-9 endothelial deposits might help monitor eculizumab effectiveness, avoid drug overexposure, and save money considering the extremely high cost of the drug.
Introduction
Atypical hemolytic-uremic syndrome (aHUS) consists of microangiopathic hemolytic anemia, thrombocytopenia, and renal failure.1
Mutations in genes encoding complement factor H (CFH), I (CFI), and B (CFB); membrane-cofactor protein (MCP); complement C3 and thrombomodulin (THBD); and anti-CFH autoantibodies (www.fh-hus.org)1-6 have been found in about 50% to 60% of patients with aHUS, underscoring the importance of uncontrolled complement activation in this devastating disease. Incomplete penetrance of aHUS has been reported in mutation carriers, indicating that complement gene mutations confer predisposition to develop aHUS, with additional hits necessary for disease manifestation.1,7
Specific and sensitive markers of complement activation in aHUS are lacking. C3 serum levels are of limited prognostic significance. Indeed, reduced C3 levels have been found in only 30% to 50% of patients with mutations in CFH, MCP, CFI, and THBD or with anti-CFH antibodies.2 An exception is represented by patients with C3 or CFB mutations who mostly (70% to 100%) present with hypocomplementemia. Mutant CFH, MCP, CFI, and THBD cannot fully regulate the alternative complement pathway (AP) on endothelial cells, as documented by in vitro tests.1,8,9 By contrast, aHUS-associated mutant proteins effectively regulate complement in fluid phase, which would explain the normal or near-normal circulating C3 levels in many mutation carriers. Gain-of-function mutations of CFB and C3 form a C3 convertase resistant to decay by endothelial cell regulators.3,4,9 These findings suggested that aHUS is a disease of unrestricted endothelial complement activation, which eventually causes renal microvascular thrombosis.1 In vivo evidence of the above pathogenetic hypothesis came from findings that transgenic mice expressing a mutant CFH lacking surface-recognition domains develop spontaneous aHUS.10 Interestingly, in this mouse model, complement C5 deficiency protected from thrombotic microangiopathy, suggesting a critical role of C5 in aHUS.11 This represented a strong rationale for use of the anti-C5 humanized monoclonal antibody eculizumab in aHUS.12,13 This drug, by blockade of C5 cleavage, protected from microvascular thrombosis and radically improved the outcome of aHUS.13-15 However, how to titrate anti-C5 treatment in clinical practice has not been addressed so far.
This study was designed in patients with aHUS and their relatives with the aims to (1) test new sensitive assays of complement activation at the endothelial cell level in aHUS patients, (2) clarify whether unaffected relatives carrying complement gene mutations show impaired complement regulation on endothelium, and (3) search for a tool for monitoring and/or titrating anti-C5 treatment in clinical practice, taking into account that complement activation occurs on endothelium and not in fluid phase.
By specific ex vivo assays, we demonstrate that aHUS patients with or without identified complement gene mutations or anti-CFH antibodies consistently and chronically activate complement on endothelium. Also, unaffected gene mutation carriers show dysregulated complement activation at the endothelial level. Finally, we document that the level of C5 blockade on endothelium found in our ex vivo test predicts clinical effectiveness of eculizumab in vivo and could guide drug dosing and timing. This topic has particular clinical relevance not only given the impressive therapeutic potential of eculizumab13 but also taking into consideration that the cost of this drug is so high that even in high-income countries, public health systems and private insurances tend to limit eculizumab use.16,17
Methods
Study participants
aHUS was diagnosed in cases reported to have one or more episodes of nonimmune hemolytic anemia, thrombocytopenia, and renal impairment (details about disease diagnosis and definitions of acute disease and remission are provided in supplemental “Methods” available at the Blood Web site).1 Patients in whom HUS was associated with Shiga toxin–producing bacteria were excluded.
Among patients of the International Registry of HUS/thrombotic thrombocytopenia purpura (TTP)2 with mutations in CFH, CFI, C3, and CFB (encoding circulating complement proteins) or anti-CFH antibodies, or without mutations in known genes, who consented to participate in this study (36 patients [supplemental Table 1], plus 8 treated with eculizumab) were enrolled. Seven consenting healthy relatives carrying complement gene mutations and 7 healthy relatives without mutations/anti-CFH antibodies were also studied. This study did not include patients with mutations in DGKE, recently identified in infantile recessive aHUS,18 or in MCP or THBD, all encoding intracellular or transmembrane proteins.
Fifteen patients with C3 glomerulopathies (C3G)19 or immune complex–associated membranoproliferative glomerulonephritis (IC-MPGN; supplemental “Methods” and supplemental Table 2), all diseases associated with complement activation in fluid phase,20,21 were also studied.
Controls were 30 healthy subjects age and sex matched with patients. The protocol was approved by the ethical committee of the Azienda Sanitaria Locale Bergamo, Italy. Participants or their legal guardians provided written informed consent in accordance with the Declaration of Helsinki.
Study assessments
Methods used for measuring serum and plasma complement profile; complement deposits in kidney biopsy specimens (available for 5 patients); the effect of incubation with serum from patients, healthy relatives, and controls on complement deposits on cultured human microvascular endothelial cells (HMEC-1); and statistical analyses are detailed in supplemental “Methods.”
Results
Circulating complement profile in patients with aHUS with or without mutations
During the acute phase, we found lower than normal serum C3 levels in 56% of patients (Table 1). Lower than normal serum C3 levels were found in 47% of patients in remission (Table 1).
Circulating complement profile in aHUS patients
| Complement parameters . | Disease phase . | Overall . | Mutations or anti-CFH Ab . | No mutations . |
|---|---|---|---|---|
| Reduced C3 serum levels (83-180 mg/dL)* | Acute† | 10 (18) | 5 (9) | 5 (9) |
| Remission‡ | 15 (32) | 11 (25) | 4 (7) | |
| Increased C5a plasma levels (1.9-13.1 ng/mL)* | Acute† | 9 (19) | 3 (10) | 6 (9) |
| Remission‡ | 21 (36) | 15 (27) | 6 (9) | |
| Increased SC5b-9 plasma levels (127-400 ng/mL)* | Acute† | 10 (19) | 4 (10) | 6 (9) |
| Remission‡ | 23 (36) | 20 (27) | 3 (9) |
| Complement parameters . | Disease phase . | Overall . | Mutations or anti-CFH Ab . | No mutations . |
|---|---|---|---|---|
| Reduced C3 serum levels (83-180 mg/dL)* | Acute† | 10 (18) | 5 (9) | 5 (9) |
| Remission‡ | 15 (32) | 11 (25) | 4 (7) | |
| Increased C5a plasma levels (1.9-13.1 ng/mL)* | Acute† | 9 (19) | 3 (10) | 6 (9) |
| Remission‡ | 21 (36) | 15 (27) | 6 (9) | |
| Increased SC5b-9 plasma levels (127-400 ng/mL)* | Acute† | 10 (19) | 4 (10) | 6 (9) |
| Remission‡ | 23 (36) | 20 (27) | 3 (9) |
Ab, antibody.
Limits of normal ranges (as defined in supplemental “Methods”). In parentheses are the numbers of patients for whom data were available, and outside the parentheses are the numbers of patients with reduced C3 or increased C5a or SC5b-9 levels.
One patient was receiving eculizumab at the time of the test.
Eight patients were receiving eculizumab at the time of the tests.
During the acute phase, plasma levels of the anaphylatoxin C5a and of the cytolytically inactive terminal-complement complex (SC5b-9) were higher than normal in 47% and 53% of patients, respectively. At remission, increased C5a and SC59b-9 plasma levels were found in 58% and 64% of patients, respectively (Table 1).
No significant difference was found in the prevalence of circulating complement abnormalities among patients with or without complement gene mutations/anti-CFH antibodies (Table 1) either during the acute phase or at remission.
Renal complement deposition profile in aHUS patients with or without mutations
As microangiopathic lesions of aHUS mainly localize in the kidney microcirculation, we looked at markers of complement activation in kidney biopsy specimens taken during the acute phase from 5 out of 44 patients (CFH mutations, n = 1; C3 mutation, n = 1; CFI mutation, n = 1; no mutation, n = 2). Strong immunostaining for C3 (Figure 1A) and C9 neoantigen (Figure 1B-D), which reflects C5b-9 formation, was found in glomeruli of all patients. In small arteries, strong endothelial C3 reactivity was observed (Figure 1E-F), whereas C9 staining mainly displayed a subendothelial localization (Figure 1G). C3 and C9 kidney deposits were found irrespective of whether the patients had increased (Figure 1C) or normal plasma SC5b-9 (Figure 1A).
Immunohistochemical analysis of C3 and C9 (C5b-9) staining in kidney biopsy specimens from aHUS patients. Representative results are shown. (A) C3 deposits with main endothelial localization in a glomerulus from a patient with a CFI mutation and normal SC5b-9 plasma levels. (B) C9 staining restricted to hilar area in a glomerulus from a patient with a C3 mutation. (C) Diffuse C9 deposits in 2 glomeruli with marked ischemic injury from a patient with CFH mutation and increased SC5b-9 levels. (D) C9 deposits in glomeruli from a patient without mutations/anti-CFH antibodies. (E-F) endothelial C3 staining in arterioles from patients with CFH (E) and CFI (F) mutations. (G) Subendothelial localization of C9 staining in an arteriole from a patient with a CFH mutation and normal SC5b-9 level. (H) Control section (healthy portion of nephrectomy for cancer, C9 staining). Original magnification ×400, counterstaining with hematoxylin.
Immunohistochemical analysis of C3 and C9 (C5b-9) staining in kidney biopsy specimens from aHUS patients. Representative results are shown. (A) C3 deposits with main endothelial localization in a glomerulus from a patient with a CFI mutation and normal SC5b-9 plasma levels. (B) C9 staining restricted to hilar area in a glomerulus from a patient with a C3 mutation. (C) Diffuse C9 deposits in 2 glomeruli with marked ischemic injury from a patient with CFH mutation and increased SC5b-9 levels. (D) C9 deposits in glomeruli from a patient without mutations/anti-CFH antibodies. (E-F) endothelial C3 staining in arterioles from patients with CFH (E) and CFI (F) mutations. (G) Subendothelial localization of C9 staining in an arteriole from a patient with a CFH mutation and normal SC5b-9 level. (H) Control section (healthy portion of nephrectomy for cancer, C9 staining). Original magnification ×400, counterstaining with hematoxylin.
Serum from aHUS patients with or without mutations or anti-CFH antibodies induced C3 and C5b-9 deposition on microvascular endothelial cells
To find a sensitive test of complement activation on endothelium, resting or adenosine 5′-diphosphate (ADP)-activated confluent HMEC-1 were incubated for 4 hours with serum (diluted 1:2 in the test medium Hanks balanced salt solution with bovine serum albumin; supplemental “Methods”) from the 36 aHUS patients not treated with eculizumab (eculizumab-treated patients were studied apart [see below]), with or without identified complement gene mutations/anti-CFH antibodies. Thereafter, HMEC-1 were stained with anti-human C3c or anti-human C5b-9 antibodies and complement deposits were analyzed by confocal microscopy (supplemental “Methods”). Supplemental Figure 1 shows a schematic representation of ex vivo studies of HMEC-1. Seven of the 36 patients were studied both during the acute phase and in remission, 7 only in the acute phase, and 22 only in remission (Table 2). Serum from patients with acute aHUS, but not serum from most patients at remission, caused more C3 and/or C5b-9 deposition on resting HMEC-1 than control serum (Figure 2A-B and Table 2). On HMEC-1 pre-exposed to ADP, all acute aHUS sera and most aHUS sera taken in remission (sensitivity 84%) induced more C3 deposition than control sera (Table 2, Figure 2A,C, and D, and supplemental Table 1). ADP was used to mimic an activated/perturbed endothelium resulting in exocytosis of P-selectin (supplemental Figure 2), an adhesive molecule that can bind and activate C3.22,23 The same results were obtained with thrombin- or lipopolysaccharide-activated HMEC-1 (supplemental Figure 3).
Complement activation markers in aHUS patients
| Patients . | Mutations or anti-CFH Ab . | Complement parameters . | Endothelial complement deposits . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum C3 (83-180 mg/dL)* . | Serum C4 (10-40 mg/dL)* . | Plasma C5a (1.9-13.1 ng/mL)* . | Plasma SC5b-9 (127-400 ng/mL)* . | C3 deposits (% of control) . | C5b-9 deposits (% of control) . | ||||
| Resting . | ADP-activated . | Resting . | ADP-activated . | ||||||
| Acute | |||||||||
| Patient 1 | CFH-R1210C | 122 | 38 | 16.1 | 375 | 450%† | 286%† | 340%† | 309%† |
| Patient 2 | CFH-R1210C | n.d. | n.d. | 7.3 | 1160 | n.d. | n.d. | 441%† | 609%† |
| Patient 3 | CFH-R78G | 55 | 23 | 7.8 | 381 | n.d. | n.d. | 285%† | 306%† |
| Patient 4 | Anti-CFH Ab | 58 | 10 | 10 | 653 | 489%† | 288%† | 371%† | 599%† |
| Patient 5 | No | 84 | 25 | 5 | 220 | 301%† | 297%† | 722%† | 857%† |
| Patient 6 | No | 108 | 28.8 | n.a. | n.a. | n.d. | n.d. | 787%† | 577%† |
| Patient 7 | No | 51 | 11 | 1.8 | 69 | n.d. | n.d. | n.d. | 1055%† |
| Patient 8 | No | 89 | 22 | 31.1 | 335 | n.d. | n.d. | 1044%† | 1087%† |
| Patient 9 | No | n.d. | n.d. | 49.8 | 933 | n.d. | n.d. | 1509%† | 1060%† |
| Patient 10 | No | 82 | 13 | 61.5 | 541 | n.d. | n.d. | 470%† | n.d. |
| Patient 11 | No | 58 | 18 | 61 | 1432 | n.d. | n.d. | 167% | n.d. |
| Patient 12 | No | n.d. | n.d. | n.a. | n.a. | n.d. | n.d. | 650%† | n.d. |
| Patient 13 | No | n.d. | n.d. | 21.2 | 713 | n.d. | n.d. | 476%† | n.d. |
| Patient 14 | No | 103 | 18.7 | n.a. | n.a. | n.d. | n.d. | 881%† | n.d. |
| Remission | |||||||||
| Patient 1 | CFH-R1210C | 108 | 30 | 31.2 | 233 | 213% | 240%† | 108% | 187%† |
| Patient 2 | CFH-R1210C | n.d. | n.d. | n.a. | n.a. | n.d. | n.d. | 94% | 644%† |
| Patient 3 | CFH-R78G | 51 | 17 | 12.2 | 725 | n.d. | 86% | 86% | 195%† |
| Patient 15 | CFH-S1191L | 109 | 36 | 14.9 | 656 | n.d. | 234%† | n.d. | 468%† |
| Patient 16 | CFH-S1191L | 115 | 21 | 8.3 | 447 | n.d. | 258%† | n.d. | 504%† |
| Patient 17 | CFH-S1191L | 51 | 33 | 14.5 | 178 | n.d. | 387%† | n.d. | 590%† |
| Patient 18 | CFH-E1172X | 51 | 26 | 3.6 | 476 | n.d. | 167%† | n.d. | 244%† |
| Patient 19 | CFH-R1215G | 73 | 24 | 16 | 655 | n.d. | 167%† | n.d. | 242%† |
| Patient 20 | CFH-1183-1194 dup | n.d. | n.d. | 10.7 | 342 | n.d. | 287%† | n.d. | 351%† |
| Patient 21 | CFH-N516K | 65 | 40 | 29.8 | 905 | n.d. | 112% | n.d. | 255%† |
| Patient 22 | CFH-G1011Vfs4X | 80 | 22 | 14.8 | 802 | n.d. | 149% | n.d. | 158%† |
| Patient 23 | CFHR1/CFH hybrid | n.d. | n.d. | 29.6 | 236 | n.d. | n.d. | n.d. | 605%† |
| Patient 24 | CFI-G261D | 136 | 38 | 11.3 | 1100 | n.d. | 342%† | n.d. | 209%† |
| Patient 25 | CFI-1340T/G424D | 136 | 72 | 21.2 | 719 | n.d. | 341%† | n.d. | 326%† |
| Patient 26 | CFI-P50A | 125 | 41 | 18.6 | 676 | n.d. | 482%† | n.d. | 250%† |
| Patient 27 | CFI-1357M | 94 | 21 | 8.8 | 515 | n.d. | 291%† | n.d. | 520%† |
| Patient 28 | C3-K1029M | 55 | 29 | 13.3 | 348 | n.d. | 294%† | n.d. | 253%† |
| Patient 29 | C3-T140R | 82 | 26 | 8.6 | 1052 | n.d. | 151%† | n.d. | 213%† |
| Patient 30 | C3-S1041R | 86 | 11 | 7.2 | 444 | n.d. | 311%† | n.d. | 171%† |
| Patient 31 | CFB-R138W | 65 | 15 | 9.5 | 455 | n.d. | 223%† | n.d. | 182%† |
| Patient 4 | Anti-CFH Ab | 149 | 38 | 8.1 | 591 | 93% | 344%† | 79% | 516%† |
| Patient 32 | Anti-CFH Ab | 75 | 14 | 24.9 | 1206 | n.d. | 99% | n.d. | 316%† |
| Patient 5 | No | n.d. | n.d. | 3 | 183 | 112% | 302%† | 157% | 504%† |
| Patient 6 | No | 120 | 20 | 14.7 | 280 | n.d. | n.d. | 104% | 431%† |
| Patient 7 | No | n.d. | n.d. | 2.5 | 117 | n.d. | n.d. | n.d. | 937%† |
| Patient 33 | No | 79 | 28 | 31.1 | 1780 | n.d. | 283%† | n.d. | 282%† |
| Patient 34 | No | 63 | 28 | 13.5 | 209 | n.d. | 343%† | n.d. | 567%† |
| Patient 35 | No | 204 | 58 | 15.6 | 850 | n.d. | 286%† | n.d. | 468%† |
| Patient 36 | No | 57 | 32 | 16.8 | 1490 | n.d. | 450%† | n.d. | 504%† |
| Patients . | Mutations or anti-CFH Ab . | Complement parameters . | Endothelial complement deposits . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum C3 (83-180 mg/dL)* . | Serum C4 (10-40 mg/dL)* . | Plasma C5a (1.9-13.1 ng/mL)* . | Plasma SC5b-9 (127-400 ng/mL)* . | C3 deposits (% of control) . | C5b-9 deposits (% of control) . | ||||
| Resting . | ADP-activated . | Resting . | ADP-activated . | ||||||
| Acute | |||||||||
| Patient 1 | CFH-R1210C | 122 | 38 | 16.1 | 375 | 450%† | 286%† | 340%† | 309%† |
| Patient 2 | CFH-R1210C | n.d. | n.d. | 7.3 | 1160 | n.d. | n.d. | 441%† | 609%† |
| Patient 3 | CFH-R78G | 55 | 23 | 7.8 | 381 | n.d. | n.d. | 285%† | 306%† |
| Patient 4 | Anti-CFH Ab | 58 | 10 | 10 | 653 | 489%† | 288%† | 371%† | 599%† |
| Patient 5 | No | 84 | 25 | 5 | 220 | 301%† | 297%† | 722%† | 857%† |
| Patient 6 | No | 108 | 28.8 | n.a. | n.a. | n.d. | n.d. | 787%† | 577%† |
| Patient 7 | No | 51 | 11 | 1.8 | 69 | n.d. | n.d. | n.d. | 1055%† |
| Patient 8 | No | 89 | 22 | 31.1 | 335 | n.d. | n.d. | 1044%† | 1087%† |
| Patient 9 | No | n.d. | n.d. | 49.8 | 933 | n.d. | n.d. | 1509%† | 1060%† |
| Patient 10 | No | 82 | 13 | 61.5 | 541 | n.d. | n.d. | 470%† | n.d. |
| Patient 11 | No | 58 | 18 | 61 | 1432 | n.d. | n.d. | 167% | n.d. |
| Patient 12 | No | n.d. | n.d. | n.a. | n.a. | n.d. | n.d. | 650%† | n.d. |
| Patient 13 | No | n.d. | n.d. | 21.2 | 713 | n.d. | n.d. | 476%† | n.d. |
| Patient 14 | No | 103 | 18.7 | n.a. | n.a. | n.d. | n.d. | 881%† | n.d. |
| Remission | |||||||||
| Patient 1 | CFH-R1210C | 108 | 30 | 31.2 | 233 | 213% | 240%† | 108% | 187%† |
| Patient 2 | CFH-R1210C | n.d. | n.d. | n.a. | n.a. | n.d. | n.d. | 94% | 644%† |
| Patient 3 | CFH-R78G | 51 | 17 | 12.2 | 725 | n.d. | 86% | 86% | 195%† |
| Patient 15 | CFH-S1191L | 109 | 36 | 14.9 | 656 | n.d. | 234%† | n.d. | 468%† |
| Patient 16 | CFH-S1191L | 115 | 21 | 8.3 | 447 | n.d. | 258%† | n.d. | 504%† |
| Patient 17 | CFH-S1191L | 51 | 33 | 14.5 | 178 | n.d. | 387%† | n.d. | 590%† |
| Patient 18 | CFH-E1172X | 51 | 26 | 3.6 | 476 | n.d. | 167%† | n.d. | 244%† |
| Patient 19 | CFH-R1215G | 73 | 24 | 16 | 655 | n.d. | 167%† | n.d. | 242%† |
| Patient 20 | CFH-1183-1194 dup | n.d. | n.d. | 10.7 | 342 | n.d. | 287%† | n.d. | 351%† |
| Patient 21 | CFH-N516K | 65 | 40 | 29.8 | 905 | n.d. | 112% | n.d. | 255%† |
| Patient 22 | CFH-G1011Vfs4X | 80 | 22 | 14.8 | 802 | n.d. | 149% | n.d. | 158%† |
| Patient 23 | CFHR1/CFH hybrid | n.d. | n.d. | 29.6 | 236 | n.d. | n.d. | n.d. | 605%† |
| Patient 24 | CFI-G261D | 136 | 38 | 11.3 | 1100 | n.d. | 342%† | n.d. | 209%† |
| Patient 25 | CFI-1340T/G424D | 136 | 72 | 21.2 | 719 | n.d. | 341%† | n.d. | 326%† |
| Patient 26 | CFI-P50A | 125 | 41 | 18.6 | 676 | n.d. | 482%† | n.d. | 250%† |
| Patient 27 | CFI-1357M | 94 | 21 | 8.8 | 515 | n.d. | 291%† | n.d. | 520%† |
| Patient 28 | C3-K1029M | 55 | 29 | 13.3 | 348 | n.d. | 294%† | n.d. | 253%† |
| Patient 29 | C3-T140R | 82 | 26 | 8.6 | 1052 | n.d. | 151%† | n.d. | 213%† |
| Patient 30 | C3-S1041R | 86 | 11 | 7.2 | 444 | n.d. | 311%† | n.d. | 171%† |
| Patient 31 | CFB-R138W | 65 | 15 | 9.5 | 455 | n.d. | 223%† | n.d. | 182%† |
| Patient 4 | Anti-CFH Ab | 149 | 38 | 8.1 | 591 | 93% | 344%† | 79% | 516%† |
| Patient 32 | Anti-CFH Ab | 75 | 14 | 24.9 | 1206 | n.d. | 99% | n.d. | 316%† |
| Patient 5 | No | n.d. | n.d. | 3 | 183 | 112% | 302%† | 157% | 504%† |
| Patient 6 | No | 120 | 20 | 14.7 | 280 | n.d. | n.d. | 104% | 431%† |
| Patient 7 | No | n.d. | n.d. | 2.5 | 117 | n.d. | n.d. | n.d. | 937%† |
| Patient 33 | No | 79 | 28 | 31.1 | 1780 | n.d. | 283%† | n.d. | 282%† |
| Patient 34 | No | 63 | 28 | 13.5 | 209 | n.d. | 343%† | n.d. | 567%† |
| Patient 35 | No | 204 | 58 | 15.6 | 850 | n.d. | 286%† | n.d. | 468%† |
| Patient 36 | No | 57 | 32 | 16.8 | 1490 | n.d. | 450%† | n.d. | 504%† |
Patients studied both during the acute phase and at remission are in bold.
Ab, antibody; n.a. sample not available; n.d. not done.
Limits of normal ranges (as defined in supplemental “Methods”).
P < .05 vs control (statistical comparisons were made for each patient by comparing deposits in pixel2 recorded in the 15 fields analyzed for the patient and for the corresponding control run in parallel, as detailed in supplemental “Methods” and in supplemental Table 1).
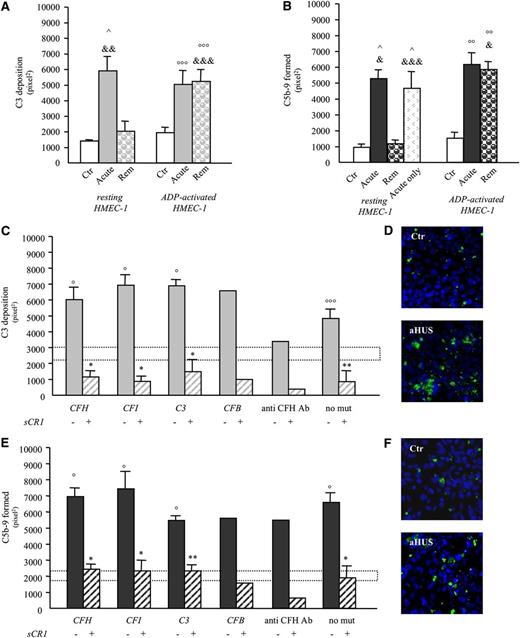
aHUS serum induces C3 and C5b-9 deposition on microvascular endothelial cells (HMEC-1). (A-B) Endothelial surface area covered by C3 (A) or C5b-9 (B) staining after incubation of unstimulated (resting) or ADP-activated HMEC-1 for 4 hr with serum (diluted 1:2 in test medium) from healthy subjects (Ctr; n = 4) or from aHUS patients (C3: n = 3, 1 with CFH mutation, 1 with anti-CFH antibodies, and 1 without identified mutations/anti-CFH antibodies; C5b-9: n = 7, 3 with CFH mutation, 1 with anti-CFH antibodies, and 3 without identified mutations/anti-CFH antibodies) studied both during the acute phase of the disease (Acute) and at remission (Rem) or from 7 aHUS patients studied in the acute phase only (panel B, C5b-9, acute only, all without identified mutations/anti-CFH antibodies). Data are mean ± standard error (SE). ^P < .01 vs control resting; °°P < .01, °°°P < .05 vs control ADP-activated; &P < .001, &&P < .01, &&&P < .05 vs remission resting. (C,E) Endothelial surface area covered by C3 (C) or C5b-9 (E) staining after incubation of ADP-activated HMEC-1 for 4 hr with serum from aHUS patients studied in remission (C3: n = 25, CFH mutations: n = 10; CFI mutations: n = 4; C3 mutations: n = 3; CFB mutation: n = 1; anti-CFH antibodies: n = 2; without identified mutations/anti-CFH antibodies: n = 5 ; C5b-9: n = 29, CFH mutations or CFHR1/CFH hybrid gene: n = 12; anti-CFH antibodies: n = 2; CFI mutations: n = 4; C3 mutations: n = 3; CFB mutation: n = 1; without identified mutations/anti-CFH antibodies: n = 7), in the presence or not of the complement inhibitor sCR1 (150 μg/mL). Range of deposits induced by control serum (mean ± SE): dotted horizontal areas. (D,F) Representative confocal microscopy images of C3 (D, in green) or C5b-9 (F, in green) staining of ADP-activated HMEC-1 exposed to serum from an healthy subject (Ctr) or an aHUS patient in remission (aHUS) (original magnification ×400). Additional images are shown in supplemental Figure 11. Data are mean ± SE. °P < .001, °°°P < .05 vs control serum; *P < .001, **P < .01 vs aHUS serum without sCR1. Both C3 and C5b-9 deposits were prevented by addition of sCR1 (an inhibitor of all the 3 complement pathways) to patient serum, indicating that the staining was specifically related to complement activation products. mut, mutation.
aHUS serum induces C3 and C5b-9 deposition on microvascular endothelial cells (HMEC-1). (A-B) Endothelial surface area covered by C3 (A) or C5b-9 (B) staining after incubation of unstimulated (resting) or ADP-activated HMEC-1 for 4 hr with serum (diluted 1:2 in test medium) from healthy subjects (Ctr; n = 4) or from aHUS patients (C3: n = 3, 1 with CFH mutation, 1 with anti-CFH antibodies, and 1 without identified mutations/anti-CFH antibodies; C5b-9: n = 7, 3 with CFH mutation, 1 with anti-CFH antibodies, and 3 without identified mutations/anti-CFH antibodies) studied both during the acute phase of the disease (Acute) and at remission (Rem) or from 7 aHUS patients studied in the acute phase only (panel B, C5b-9, acute only, all without identified mutations/anti-CFH antibodies). Data are mean ± standard error (SE). ^P < .01 vs control resting; °°P < .01, °°°P < .05 vs control ADP-activated; &P < .001, &&P < .01, &&&P < .05 vs remission resting. (C,E) Endothelial surface area covered by C3 (C) or C5b-9 (E) staining after incubation of ADP-activated HMEC-1 for 4 hr with serum from aHUS patients studied in remission (C3: n = 25, CFH mutations: n = 10; CFI mutations: n = 4; C3 mutations: n = 3; CFB mutation: n = 1; anti-CFH antibodies: n = 2; without identified mutations/anti-CFH antibodies: n = 5 ; C5b-9: n = 29, CFH mutations or CFHR1/CFH hybrid gene: n = 12; anti-CFH antibodies: n = 2; CFI mutations: n = 4; C3 mutations: n = 3; CFB mutation: n = 1; without identified mutations/anti-CFH antibodies: n = 7), in the presence or not of the complement inhibitor sCR1 (150 μg/mL). Range of deposits induced by control serum (mean ± SE): dotted horizontal areas. (D,F) Representative confocal microscopy images of C3 (D, in green) or C5b-9 (F, in green) staining of ADP-activated HMEC-1 exposed to serum from an healthy subject (Ctr) or an aHUS patient in remission (aHUS) (original magnification ×400). Additional images are shown in supplemental Figure 11. Data are mean ± SE. °P < .001, °°°P < .05 vs control serum; *P < .001, **P < .01 vs aHUS serum without sCR1. Both C3 and C5b-9 deposits were prevented by addition of sCR1 (an inhibitor of all the 3 complement pathways) to patient serum, indicating that the staining was specifically related to complement activation products. mut, mutation.
C3 deposits were blocked by adding AP inhibitors to serum (Figure 3A-B). This finding, together with lack of C4 or immunoglobulin G staining on ADP-activated HMEC-1 exposed to aHUS serum (supplemental Figure 4), indicates selective activation of the AP.
Effect of complement inhibitors on aHUS serum-induced C3 and C5b-9 deposition on ADP-activated HMEC-1. (A-B) Effect of selective inhibitors of the alternative pathway of complement, an anti-CFB antibody (anti-CFB, 150 μg/mL), the CR2-FH fusion protein (CR2-FH, 150 μg/mL and 300 μg/mL), and a CFH concentrate from human plasma (CFH conc, at levels comparable to those of normal human serum, 230 μg/mL) on C3 deposition induced on ADP-activated HMEC-1 by serum from 3 patients with aHUS and CFH mutations studied in remission in 3 independent experiments. (C) Effect of terminal complement pathway inhibitors, an anti-C5 minibody (anti-C5, 135 μg/mL), or an anti-C7 goat polyclonal antibody (anti-C7, 350 μg/mL) or eculizumab (Ecu, 150 μg/ml) on C5b-9 deposition induced on ADP-activated HMEC-1 by serum of patients with aHUS studied in remission. Data are from 3 different experiments in 3 patients (CFH mutations, n = 2; C3 mutation, n = 1) and 3 controls. (D) Effect of 3 doses of eculizumab (Ecu; 100, 50, or 25 μg/mL) on C5b-9 deposition on ADP-activated HMEC-1 induced by serum of patients with aHUS studied in the acute phase before any treatment. Data are from 3 different experiments in 3 patients (without mutations or anti-CFH antibodies) and 3 controls. Data are mean ± SE. °P < .001, °°P < .01, °°°P < .05 vs control serum; *P < .001, **P < .01, ***P < .05 vs aHUS serum untreated. Control range: dotted horizontal areas. ctr, control.
Effect of complement inhibitors on aHUS serum-induced C3 and C5b-9 deposition on ADP-activated HMEC-1. (A-B) Effect of selective inhibitors of the alternative pathway of complement, an anti-CFB antibody (anti-CFB, 150 μg/mL), the CR2-FH fusion protein (CR2-FH, 150 μg/mL and 300 μg/mL), and a CFH concentrate from human plasma (CFH conc, at levels comparable to those of normal human serum, 230 μg/mL) on C3 deposition induced on ADP-activated HMEC-1 by serum from 3 patients with aHUS and CFH mutations studied in remission in 3 independent experiments. (C) Effect of terminal complement pathway inhibitors, an anti-C5 minibody (anti-C5, 135 μg/mL), or an anti-C7 goat polyclonal antibody (anti-C7, 350 μg/mL) or eculizumab (Ecu, 150 μg/ml) on C5b-9 deposition induced on ADP-activated HMEC-1 by serum of patients with aHUS studied in remission. Data are from 3 different experiments in 3 patients (CFH mutations, n = 2; C3 mutation, n = 1) and 3 controls. (D) Effect of 3 doses of eculizumab (Ecu; 100, 50, or 25 μg/mL) on C5b-9 deposition on ADP-activated HMEC-1 induced by serum of patients with aHUS studied in the acute phase before any treatment. Data are from 3 different experiments in 3 patients (without mutations or anti-CFH antibodies) and 3 controls. Data are mean ± SE. °P < .001, °°P < .01, °°°P < .05 vs control serum; *P < .001, **P < .01, ***P < .05 vs aHUS serum untreated. Control range: dotted horizontal areas. ctr, control.
Sera from all patients either in acute phase or in remission, including those with normal SC5b-9 plasma levels, caused significantly higher C5b-9 deposits on ADP-activated HMEC-1 than control sera run in parallel (Table 2; Figure 2B,E,F; and supplemental Table 1), documenting the higher sensitivity (100%) of this ex vivo assay vs elevated plasma SC5b-9 levels (Table 2) in detecting complement dysregulation in aHUS. By testing different serum dilutions, we found that the effect of aHUS serum on C5b-9 deposits was dose dependent (supplemental Figure 5). C5b-9 deposits were prevented by an anti-C5 minibody, by eculizumab (Soliris, Alexion Pharmaceuticals), or by an anti-C7 antibody added to aHUS serum, supporting the specificity of the anti–C5b-9 antibody used for the staining (Figure 3C-D).
Concordance correlation test of C5b-9 deposits induced on ADP-activated HMEC-1 by serum from 8 patients tested twice in different experiments revealed a coefficient of 0.879, documenting the good reproducibility of the test (supplemental Figure 6).
aHUS serum-induced C5b-9 deposits on ADP-activated HMEC-1 (% of controls) did not correlate with SC5b-9 levels in the same sera or in plasma (supplemental “Results” and supplemental Figure 7), which would exclude that endothelial C5b-9 deposits derived from preformed serum SC5b-9.
To evaluate whether the test specifically picked out endothelial-restricted complement activation of aHUS, additional experiments were done with sera from 15 patients with C3G or IC-MPGN and AP complement activation in the fluid phase.20,21 In all but one patient with C3G/IC-MPGN, serum-induced C5b-9 deposits on ADP-activated HMEC-1 did not differ between patients and healthy controls (supplemental Table 2).
Serum from unaffected carriers of complement gene mutations induced C3 and C5b-9 deposition on microvascular endothelial cells
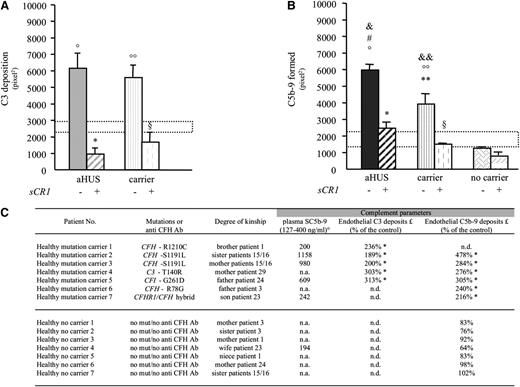
To evaluate whether C3 and C5b-9 deposition was the consequence of aHUS disease status or whether mutations per se predisposed to complement dysregulation on endothelium, serum from 7 unaffected relatives (supplemental Figure 1) that carried CFH (n = 4), CFI (n = 1), or C3 (n = 1) mutations or a CFHR1/CFH hybrid gene (n = 1)24 was tested. Serum from unaffected mutation carriers induced more C3 and C5b-9 deposition than control serum on ADP-activated HMEC-1 (Figure 4A-C). At variance, serum from 7 unaffected relatives without mutations induced C5b-9 deposition comparable to control serum (Figure 4B-C).
Serum from healthy carriers of complement gene mutations induces C3 and C5b-9 deposition on ADP-activated microvascular endothelial cells (HMEC-1). (A-B) Endothelial surface area covered by C3 (A) or C5b-9 (B) staining after incubation of ADP-activated HMEC-1 for 4 hr with serum (diluted 1:2 in test medium) from aHUS patients (C3 deposits: n = 6, 4 with CFH mutations, 1 with C3 mutation, and 1 with CFI mutation; C5b-9 deposits: n = 7, 4 with CFH mutations, 1 with C3 mutation, 1 with CFI mutation, and 1 with CFHR1/CFH hybrid gene) or from their healthy relatives carrying the same mutations (C3 deposits: n = 5, 3 with CFH mutation, 1 with C3 mutation, and 1 with CFI mutation; C5b-9 deposits: n = 6, 3 with CFH mutation, 1 with C3 mutation, 1 with CFI mutation, and 1 with CFHR1/CFH hybrid gene) in the presence or not of the complement inhibitor sCR1 (150 μg/mL) or from 7 healthy relatives without mutations (C5b-9 deposits). Control serum range: dotted horizontal areas. Data are mean ± SE. °P < .001, °°P < .01, vs control serum; *P < .001, **P < .01 vs aHUS patients without sCR1; §P < .001 vs mutation carriers without sCR1; #P < .01 vs carrier; &P < .001, &&P < .01 vs no carrier. (C) Data of plasma SC5b-9 levels and serum-induced C3 and C5b-9 deposition on ADP-activated HMEC-1 in unaffected relatives carrying complement gene mutations (healthy mutation carrier, n = 7) and unaffected relatives without mutations (only C5b-9, healthy no carrier, n = 7). °Limits of normal ranges (as defined in supplemental “Methods”). *P < .05 vs control serum (statistical comparisons were made for each relative by comparing deposits in pixel2 recorded in 15 fields analyzed for the relative and for the corresponding control run in parallel, as detailed in supplemental “Methods”). £: Serum-induced C3 or C5b-9 deposits on ADP-activated HMEC-1. mut, mutation; n.a., not available; n.d., not done.
Serum from healthy carriers of complement gene mutations induces C3 and C5b-9 deposition on ADP-activated microvascular endothelial cells (HMEC-1). (A-B) Endothelial surface area covered by C3 (A) or C5b-9 (B) staining after incubation of ADP-activated HMEC-1 for 4 hr with serum (diluted 1:2 in test medium) from aHUS patients (C3 deposits: n = 6, 4 with CFH mutations, 1 with C3 mutation, and 1 with CFI mutation; C5b-9 deposits: n = 7, 4 with CFH mutations, 1 with C3 mutation, 1 with CFI mutation, and 1 with CFHR1/CFH hybrid gene) or from their healthy relatives carrying the same mutations (C3 deposits: n = 5, 3 with CFH mutation, 1 with C3 mutation, and 1 with CFI mutation; C5b-9 deposits: n = 6, 3 with CFH mutation, 1 with C3 mutation, 1 with CFI mutation, and 1 with CFHR1/CFH hybrid gene) in the presence or not of the complement inhibitor sCR1 (150 μg/mL) or from 7 healthy relatives without mutations (C5b-9 deposits). Control serum range: dotted horizontal areas. Data are mean ± SE. °P < .001, °°P < .01, vs control serum; *P < .001, **P < .01 vs aHUS patients without sCR1; §P < .001 vs mutation carriers without sCR1; #P < .01 vs carrier; &P < .001, &&P < .01 vs no carrier. (C) Data of plasma SC5b-9 levels and serum-induced C3 and C5b-9 deposition on ADP-activated HMEC-1 in unaffected relatives carrying complement gene mutations (healthy mutation carrier, n = 7) and unaffected relatives without mutations (only C5b-9, healthy no carrier, n = 7). °Limits of normal ranges (as defined in supplemental “Methods”). *P < .05 vs control serum (statistical comparisons were made for each relative by comparing deposits in pixel2 recorded in 15 fields analyzed for the relative and for the corresponding control run in parallel, as detailed in supplemental “Methods”). £: Serum-induced C3 or C5b-9 deposits on ADP-activated HMEC-1. mut, mutation; n.a., not available; n.d., not done.
In aHUS patients, eculizumab normalized ex vivo complement deposition on ADP-activated endothelial cells and induced stable remission
We then retrospectively evaluated the effect of eculizumab treatment on serum-induced C5b-9 deposits in ADP-activated HMEC-1 in 4 patients (cases 1-4; Table 3) studied both during the acute phase before eculizumab and in stable remission under chronic eculizumab (supplemental Figure 1).
Complement profile in eculizumab-treated aHUS patients
| Case . | Mutation or anti-CFH Ab . | Disease phase treatment . | Clinical parameters* . | Complement parameters . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Platelets (150-400 × 103/μL) . | LDH (266-500 IU/L) . | Hemoglobin (14-18 g/dL) . | Serum creatinine (0.55-1.25 mg/dL) . | Serum C3 (83-180 mg/dL)† . | Plasma SC5b-9 (127-400 Eq/mL)† . | Serum CH50 (79-187 U Eq/mL)† . | C5b-9 deposits‡ (% of control) . | |||
| 1 | CFHR1/CFH | Acute (pre-Ecu) | 111 000 | 2000 | 5.1 | 6 | 79 | 329 | 227 | 309§ |
| Hybrid gene | 9 d post-Ecu 1200 mg | 318 000 | 741 | 12.6 | 1.7 | 91 | 533 | 5 | 71 | |
| 2 | CFB -L433S | Acute (pre-Ecu) | 121 000 | 2011 | 5.5 | 27 | 77 | 156 | 100 | 1019§ |
| 14 d post-Ecu 1200 mg | 280 000 | 357 | 12.9 | 1.22 | 65 | 967 | 11 | 106 | ||
| 3 | No mutations | Acute (pre-Ecu) | 19 000 | 2373 | 9.2 | 1.9 | 50 | 411 | 142 | 478§ |
| No anti-CFH Ab | 21 d post-Ecu 300 mg | 564 000 | n.d. | 12.5 | 0.6 | 76 | 116 | 46 | 105 | |
| 4 | No mutations | Acute (pre-Ecu) | 107 000 | 768 | 9.6 | 15.2 | 78 | 556 | 45 | 439§ |
| No anti-CFH Ab | 14 d post-Ecu 1200 mg | 161 000 | 342 | 11.7 | 4.77 | 88 | 260 | 4 | 59 | |
| 5 | CFH-S1191L | Remission (pre-Ecu) | 237 000 | 576 | 10.9 | 8.91 | 93 | 121 | 72 | 298§ |
| 8 d post-Ecu 300 mg | 67 000 | 560 | 13.5 | 0.52 | 103 | 195 | 3 | 152§ | ||
| 12 d post-Ecu 600 mg | 119 000 | 588 | 13.6 | 0.59 | 95 | 214 | 3 | 43 | ||
| 11 d post-Ecu 600 mg | 225 000 | 493 | 12.6 | 0.5 | n.d. | 167 | 6 | 76 | ||
| 6 | C3-K633R | Acute (pre-Ecu) | 39 000 | 5000 | 7.7 | 1.48 | 123 | 392 | 93 | 342§ |
| 6 d post-Ecu 600 mg | 159 000 | 854 | 8.5 | 4.29 | 126 | 231 | 1 | 119 | ||
| 6 d post-Ecu 900 mg | 232 000 | 473 | 11.5 | 1.5 | n.d. | 566 | 11 | 82 | ||
| 7 | CFH-R1210C | Acute (pre-Ecu) | 46 000 | 1962 | 7 | 5.7 | 79 | 421 | 118 | 474§ |
| 14 d post-Ecu 1200 mg | 277 000 | 336 | 11.9 | 1.4 | 89 | 505 | 10 | 102 | ||
| 21 d post-Ecu 1200 mg | 307 000 | 264 | 12.2 | 1.2 | 83 | 231 | 32 | 75 | ||
| 8 | CFI-R187Q | Acute (pre-Ecu) | 94 000 | 869 | 7.2 | 4.5 | 99 | 534 | n.d. | 177§ |
| 14 d post-Ecu 1200 mg | 212 000 | 401 | 10.1 | 2 | 89 | 435 | 6 | 24 | ||
| 1 mo post-Ecu 1200 mg | 185 000 | 434 | 11.6 | 1.93 | 85 | 298 | 22 | 92 | ||
| Case . | Mutation or anti-CFH Ab . | Disease phase treatment . | Clinical parameters* . | Complement parameters . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Platelets (150-400 × 103/μL) . | LDH (266-500 IU/L) . | Hemoglobin (14-18 g/dL) . | Serum creatinine (0.55-1.25 mg/dL) . | Serum C3 (83-180 mg/dL)† . | Plasma SC5b-9 (127-400 Eq/mL)† . | Serum CH50 (79-187 U Eq/mL)† . | C5b-9 deposits‡ (% of control) . | |||
| 1 | CFHR1/CFH | Acute (pre-Ecu) | 111 000 | 2000 | 5.1 | 6 | 79 | 329 | 227 | 309§ |
| Hybrid gene | 9 d post-Ecu 1200 mg | 318 000 | 741 | 12.6 | 1.7 | 91 | 533 | 5 | 71 | |
| 2 | CFB -L433S | Acute (pre-Ecu) | 121 000 | 2011 | 5.5 | 27 | 77 | 156 | 100 | 1019§ |
| 14 d post-Ecu 1200 mg | 280 000 | 357 | 12.9 | 1.22 | 65 | 967 | 11 | 106 | ||
| 3 | No mutations | Acute (pre-Ecu) | 19 000 | 2373 | 9.2 | 1.9 | 50 | 411 | 142 | 478§ |
| No anti-CFH Ab | 21 d post-Ecu 300 mg | 564 000 | n.d. | 12.5 | 0.6 | 76 | 116 | 46 | 105 | |
| 4 | No mutations | Acute (pre-Ecu) | 107 000 | 768 | 9.6 | 15.2 | 78 | 556 | 45 | 439§ |
| No anti-CFH Ab | 14 d post-Ecu 1200 mg | 161 000 | 342 | 11.7 | 4.77 | 88 | 260 | 4 | 59 | |
| 5 | CFH-S1191L | Remission (pre-Ecu) | 237 000 | 576 | 10.9 | 8.91 | 93 | 121 | 72 | 298§ |
| 8 d post-Ecu 300 mg | 67 000 | 560 | 13.5 | 0.52 | 103 | 195 | 3 | 152§ | ||
| 12 d post-Ecu 600 mg | 119 000 | 588 | 13.6 | 0.59 | 95 | 214 | 3 | 43 | ||
| 11 d post-Ecu 600 mg | 225 000 | 493 | 12.6 | 0.5 | n.d. | 167 | 6 | 76 | ||
| 6 | C3-K633R | Acute (pre-Ecu) | 39 000 | 5000 | 7.7 | 1.48 | 123 | 392 | 93 | 342§ |
| 6 d post-Ecu 600 mg | 159 000 | 854 | 8.5 | 4.29 | 126 | 231 | 1 | 119 | ||
| 6 d post-Ecu 900 mg | 232 000 | 473 | 11.5 | 1.5 | n.d. | 566 | 11 | 82 | ||
| 7 | CFH-R1210C | Acute (pre-Ecu) | 46 000 | 1962 | 7 | 5.7 | 79 | 421 | 118 | 474§ |
| 14 d post-Ecu 1200 mg | 277 000 | 336 | 11.9 | 1.4 | 89 | 505 | 10 | 102 | ||
| 21 d post-Ecu 1200 mg | 307 000 | 264 | 12.2 | 1.2 | 83 | 231 | 32 | 75 | ||
| 8 | CFI-R187Q | Acute (pre-Ecu) | 94 000 | 869 | 7.2 | 4.5 | 99 | 534 | n.d. | 177§ |
| 14 d post-Ecu 1200 mg | 212 000 | 401 | 10.1 | 2 | 89 | 435 | 6 | 24 | ||
| 1 mo post-Ecu 1200 mg | 185 000 | 434 | 11.6 | 1.93 | 85 | 298 | 22 | 92 | ||
Ab, antibody; Ecu, eculizumab; n.d. not done.
Clinical data in the table are those recorded the same days when complement parameters were evaluated.
Limits of normal ranges (as defined in supplemental “Methods”).
Serum-induced C5b-9 deposits on ADP-activated endothelial cells (HMEC-1).
P < .01 vs control (serum from healthy subjects run in parallel, with 15 fields analyzed, as detailed in supplemental “Methods”).
The 4 patients are a 20-year-old woman with familial aHUS and a hybrid CFHR1/CFH gene24 (case 1, the daughter of patient 23), a 20-year-old woman with sporadic aHUS and a heterozygous p.L433S CFB mutation (case 2), a 1-year-old infant (case 3), and a 45-year-old man (case 4), both without identified mutations/anti-CFH antibodies. Cases 1 to 4 started eculizumab treatment (cases 1, 2, and 4 received 4 infusions 900 mg weekly, then 1200 mg fortnightly; case 3 received 2 infusions 300 mg weekly, then 300 mg every 2-3 weeks) at 13, 8, 8, and 30 days after aHUS onset, respectively. Details of familial and clinical history, genetic analysis, and eculizumab treatment are provided in supplemental “Results.”
In the 4 cases, serum taken in the acute phase before eculizumab caused intense C5b-9 deposition on ADP-activated HMEC-1 (Table 3), consistent with complement dysregulation at endothelial level, while plasma SC5b-9 was increased in 2 out of 4 cases (Table 3). Eculizumab-induced disease remission was accompanied by normalization of ex vivo C5b-9 deposits (Table 3). At that time, plasma SC5b-9 levels were higher than normal in 2 out of 4 cases and complement hemolytic activity (CH50) was 5, 11, 46, and 4 U Eq/mL in cases 1, 2, 3, and 4, respectively.
In aHUS patients, prospective ex vivo endothelial complement deposition evaluation was instrumental to titrate eculizumab dosage
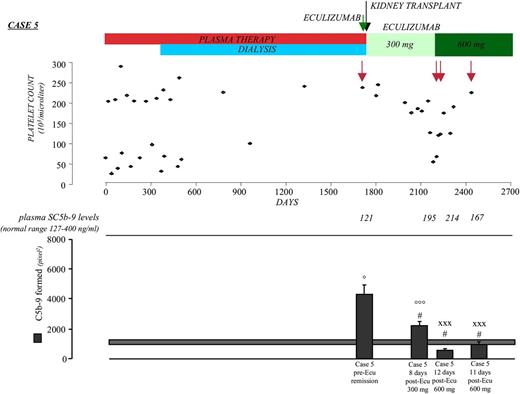
Case 5.
A 7-year-old child with familial aHUS (supplemental Figure 8) and a heterozygous CFH mutation (p.S1191L)25 manifested aHUS at 6 months. Despite chronic plasma therapy, the child had 12 relapses and progressed to end-stage renal disease (Figure 5). At 5 years, the child received a kidney transplant under eculizumab prophylaxis to prevent posttransplant recurrence.26 Before transplant and eculizumab treatment, he had 237 000 platelets/μL. Serum taken at this point caused intense C5b-9 deposition on ADP-activated HMEC-1, whereas plasma SC5b-9 was normal (Figure 5 and Table 3).
Effect of eculizumab on clinical and complement parameters in case 5. Treatments, platelet count, plasma SC5b-9 levels, and serum-induced complement deposition on ADP-activated HMEC-1 (by calculating HMEC-1 area covered by C5b-9 staining in pixel2) after incubation (4 hr) with serum (diluted 1:2 with test medium) from case 5 taken immediately pretransplant before eculizumab treatment (pre-Ecu), at 15 months posttransplant after eculizumab treatment (post-Ecu, 300 or 600 mg), and 21 months posttransplant after eculizumab treatment (post-Ecu, 600 mg). Green arrow indicates eculizumab prophylaxis for kidney transplant. Black arrow indicates the time of kidney transplant. Red arrows indicate times of sampling for plasma SC5b-9 and serum-induced ex vivo complement deposits. Data are mean ± SE of 15 fields examined for each sample. The horizontal rectangle shows range of endothelial C5b-9 deposits with control sera (mean ± SE). °P < .001, °°°P < .05 vs control serum; #P < .01 vs case 5 pre-Ecu; xxxP < .05 vs case 5, 8 days post-Ecu 300 mg.
Effect of eculizumab on clinical and complement parameters in case 5. Treatments, platelet count, plasma SC5b-9 levels, and serum-induced complement deposition on ADP-activated HMEC-1 (by calculating HMEC-1 area covered by C5b-9 staining in pixel2) after incubation (4 hr) with serum (diluted 1:2 with test medium) from case 5 taken immediately pretransplant before eculizumab treatment (pre-Ecu), at 15 months posttransplant after eculizumab treatment (post-Ecu, 300 or 600 mg), and 21 months posttransplant after eculizumab treatment (post-Ecu, 600 mg). Green arrow indicates eculizumab prophylaxis for kidney transplant. Black arrow indicates the time of kidney transplant. Red arrows indicate times of sampling for plasma SC5b-9 and serum-induced ex vivo complement deposits. Data are mean ± SE of 15 fields examined for each sample. The horizontal rectangle shows range of endothelial C5b-9 deposits with control sera (mean ± SE). °P < .001, °°°P < .05 vs control serum; #P < .01 vs case 5 pre-Ecu; xxxP < .05 vs case 5, 8 days post-Ecu 300 mg.
The posttransplant course was uneventful until months 14 to 15, when he developed a drop in platelets with slightly increased LDH despite the low CH50 (3 U Eq/mL) measured 8 days after the former eculizumab infusion indicated almost complete circulating C5 inhibition (Table 3). Serum taken at this point caused higher C5b-9 deposits on ADP-activated HMEC-1 than control serum (Figure 5 and Table 3), whereas SC5b-9 plasma levels were normal. Higher than normal endothelial C5b-9 deposits suggested that the eculizumab dose was not enough to completely block complement activation on endothelium. The eculizumab dose was doubled (from 300 to 600 mg every other week), which resulted in prompt normalization of endothelial C5b-9 deposits. Platelet count increased, without fully normalizing (Table 3). After 6 months of 600 mg eculizumab, endothelial C5b-9 deposits were still normal, and platelets and LDH normalized (Figure 5 and Table 3). SC5b-9 and CH50 levels measured in parallel did not differ from values obtained under 300 mg eculizumab (Figure 5 and Table 3).
These findings can be interpreted as to suggest that although circulating C5 activation was well controlled by both eculizumab doses, only the highest dose efficiently prevented C5 endothelial activation. Additional clinical and familial information are provided in supplemental “Results.”
Case 6.
Sporadic acute aHUS was diagnosed in a 8 year-old boy with a heterozygous rare variant in C3 gene (p.K633R). He had a medical history of hemolytic crisis and epistaxis diagnosed as glucose-6-phosphate dehydrogenase deficiency. Serum taken at this point caused intense C5b-9 deposition on ADP-activated HMEC-1, whereas plasma SC5b-9 was normal (Table 3). The patient was treated with erythrocyte transfusions and plasma exchange. Because of worsening of renal function and oliguria, eculizumab was started (600 mg weekly for 2 infusions and then every 12/14 days), with progressive regression of neurologic impairment and respiratory failure. During the initial course of the therapy, the boy suffered from 2 hypertensive crises associated with pulmonary edema, an increase in serum creatinine levels, and persistent hemolytic anemia (Table 3). SC5b-9 levels were normal and CH50 very low (1 U Eq/mL; Table 3). Serum-induced C5b-9 deposits on ADP-activated HMEC-1 did not completely normalize (Table 3). Antihypertensive polytherapy was given, and the eculizumab dose was increased to 900 mg, obtaining blood pressure stabilization, progressive normalization of renal function, and complete normalization of platelet counts, LDH, and serum-induced C5b-9 deposits on ADP-activated HMEC-1 (Table 3), whereas SC5b-9 plasma levels were higher than normal and CH50 was detectable at 11 U Eq/mL (Table 3). Additional clinical information is provided in supplemental “Results.”
In aHUS patients, a prospective ex vivo endothelial complement deposition assay supported eculizumab dose spacing
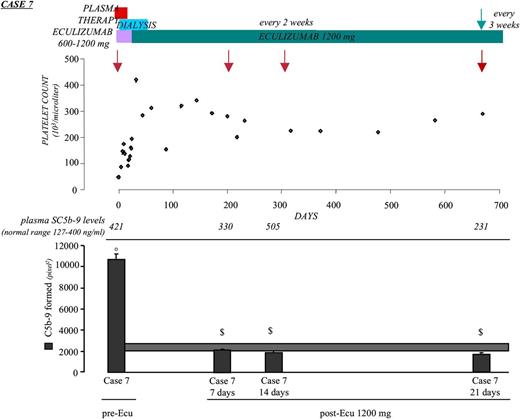
Case 7.
A male with a heterozygous CFH mutation (p.R1210C)27 manifested aHUS at 35 years. Intense serum-induced endothelial C5b-9 deposits were observed during the acute phase (Figure 6 and Table 3). Plasma SC5b-9 levels were slightly higher than normal (Table 3). He received 9 eculizumab infusions (600-1200 mg) during the first month and then 1200 mg fortnightly (Figure 6). Two months later, renal function improved and hemodialysis was stopped. Clinical remission was accompanied by normalization of ex vivo C5b-9 deposits on ADP-activated HMEC-1, whereas plasma SC5b-9 remained higher than normal and CH50 was 10 U Eq/mL (Figure 6 and Table 3). The following 18 months were uneventful, and eculizumab treatment was spaced every 3 weeks. At that time, a check of complement activation parameters 21 days after eculizumab (before the subsequent dose) revealed normal serum-induced C5b-9 deposits on ADP-activated HMEC-1 and SC5b-9 levels, despite a CH50 increase to 32 U Eq/mL (Figure 6 and Table 3). The treatment regimen was maintained. At the last follow-up, 1 year after eculizumab spacing, the patient was in stable condition. Additional clinical information is in supplemental “Results.”
Effect of eculizumab on clinical and complement parameters in case 7. Treatments, platelet count, plasma SC5b-9 levels, and complement deposition on ADP-activated HMEC-1 (by calculating HMEC-1 area covered by C5b-9 staining in pixel2) after 4-hr incubation with serum (diluted 1:2 in test medium) from case 7 taken during the acute phase before start of eculizumab treatment (pre-Ecu) and in full remission (normal renal and hematologic parameters) after eculizumab (at the adult dose of 1200 mg every 2 and 3 weeks; post-Ecu). Red arrows indicate times of sampling for plasma SC5b-9 and serum-induced ex vivo complement deposits. Green arrow: from this time, the patient was treated with eculizumab every 3 weeks. Data are mean ± SE of 15 fields examined for each sample. The horizontal rectangle shows range of endothelial C5b-9 deposits with control sera (mean ± SE). °P < .001 vs control serum; $P < .001 vs case 7 pre-Ecu.
Effect of eculizumab on clinical and complement parameters in case 7. Treatments, platelet count, plasma SC5b-9 levels, and complement deposition on ADP-activated HMEC-1 (by calculating HMEC-1 area covered by C5b-9 staining in pixel2) after 4-hr incubation with serum (diluted 1:2 in test medium) from case 7 taken during the acute phase before start of eculizumab treatment (pre-Ecu) and in full remission (normal renal and hematologic parameters) after eculizumab (at the adult dose of 1200 mg every 2 and 3 weeks; post-Ecu). Red arrows indicate times of sampling for plasma SC5b-9 and serum-induced ex vivo complement deposits. Green arrow: from this time, the patient was treated with eculizumab every 3 weeks. Data are mean ± SE of 15 fields examined for each sample. The horizontal rectangle shows range of endothelial C5b-9 deposits with control sera (mean ± SE). °P < .001 vs control serum; $P < .001 vs case 7 pre-Ecu.
Case 8.
A woman with a heterozygous CFI mutation (p.R187Q) manifested recurrent aHUS since 34 years of age. At admission, she showed worsening renal function, thrombocytopenia and hemolysis, and higher than normal serum-induced C5b-9 deposits on ADP-activated HMEC-1 and SC5b-9 plasma levels (Table 3). The woman was treated with 17 plasma exchanges and immunoglobulins without benefit. Eculizumab was given (900 mg weekly, 5 infusions), obtaining hematologic normalization and improvement of renal function. The patient was discharged under 1200 mg eculizumab fortnightly, with normal serum-induced C5b-9 deposits, almost normal plasma SC5b-9, and low CH50 (Table 3). Eighteen months later, the patient was in stable condition and eculizumab was spaced to 1200 mg once a month. At this time point, induced C5b-9 deposits on ADP-activated HMEC-1 by serum taken 1 month after eculizumab (before the subsequent dose) and SC5b-9 plasma levels were completely normal, whereas CH50 increased to 22 U Eq/mL (Table 3). The monthly eculizumab regimen was maintained, without sign of disease reactivation in the following 12 months. Additional clinical information is provided in supplemental “Results.”
In the 8 cases, serum-induced C5b-9 deposits on ADP-activated HMEC-1 during eculizumab treatment were lower (P < .001) than pretreatment values, whereas no significant change was observed among pre- and posteculizumab levels of either serum C3 or plasma SC5b-9 (supplemental Figure 9). Serum-induced endothelial C5b-9 deposits (% of controls) under eculizumab treatment did not correlate with platelet counts (r = 0.009; supplemental Figure 10), indicating that the ex vivo test is not just a surrogate of platelet count measurement.
Discussion
Here, we document that circulating levels of C3, SC5b-9, and C5a are normal in a substantial fraction of aHUS patients even during the acute phase, which indicates that serum C3 and plasma SC5b-9 and C5a are not suitable markers of complement activation in this disease. At variance, all sera from aHUS patients induced abnormal C3 and/or C5b-9 deposits on ADP-activated endothelial cells ex vivo. The in vivo counterparts of these findings are provided by intense glomerular and arteriolar C3 and C9 staining in patient biopsy specimens. The above findings confirm previous in vitro studies with complement mutant proteins, indicating that local complement activation on endothelial cells rather than in fluid phase plays a pathogenetic role in aHUS.1,3,8,9,25
The pathogenetic role of complement in aHUS was eventually confirmed by clinical trials showing that eculizumab, by blockade of C5 cleavage, protected from microvascular thrombosis and radically improved the outcome of aHUS patients.13 However, a letter to The New England Journal of Medicine raised a critique to the above article,13 because “yet 24 to 30% of the study patients had no proven genetic disease, and proof of ongoing complement activation was lacking.”28 The finding here that sera from patients without identified mutations or anti-CFH antibodies induced more intense C3 and C5b-9 deposits on ADP-activated HMEC-1 than control sera supports the concept that there are still unrecognized genetic or acquired complement abnormalities leading to aHUS. Previous studies have described a simple hemolytic assay with sheep erythrocytes and patient serum for detecting defects in the control of complement activation on cellular surfaces.29,30 However, the sensitivity of the hemolytic test appeared to be restricted to CFH-related HUS.29,30
Of relevance, serum from aHUS patients studied in the acute phase deposited complement both on resting and activated endothelial cells, which would reflect a massive in vivo complement activation as a consequence of both genetic defects and environmental triggers. At variance, complement deposition by aHUS serum collected in remission occurred only on activated endothelial cells. This finding fits with the “2-hit model” of aHUS: gene mutations predispose to aHUS, but the disease develops only in concomitance with an environmental trigger that perturbs microvascular endothelium.31 As multiple membrane-bound complement regulators bind to or are expressed at endothelial surface, in the absence of inciting events, the endothelium can control complement even when activity of one regulator is reduced by gene mutations.32 However, due to defective complement regulation, upon endothelial activation via diverse triggers (infections, drugs, pregnancy, or ischemia/reperfusion), complement products settle on endothelial cells and likely initiate the thrombotic microangiopathy process.2 Thus, remission state is plausibly metastable in patients with complement gene abnormalities, and any of the above triggers may precipitate a relapse. In this regard, the ex vivo test with resting endothelial cells shown here could represent a biomarker of disease burst in patients at risk of relapse either in the native kidneys or after kidney transplantation.
It is well known that the penetrance of aHUS in carriers of complement gene mutations is incomplete,1,2,4,5,7,27,33,34 and some subjects never develop aHUS or manifest the disease very late in adulthood. An epidemiologic study in familial cases reported that penetrance of aHUS by age 45 years was 50% among carriers of gene mutations.35 Our results showing that serum from unaffected mutation carriers also induced excessive C3 and C5b-9 deposits on ADP-activated endothelial cells suggest that these subjects have impaired complement regulation at the endothelial level and are at disease risk, though prospective studies are needed to prove our hypothesis.
Tanimoto et al,36 in another letter to The New England Journal of Medicine on the report of eculizumab trials in aHUS,13 underlined the need “to determine whether the terminal complement pathway is adequately blocked.” The authors’ reply that “the lack of standardization and validation of available complement assays currently makes them unsuitable for clinical use”37 is consistent with finding here that both serum C3 and plasma SC5b-9 levels did not appreciably change or even increased after eculizumab. Because eculizumab recognizes the C5b portion of C5,15 it could form with SC5b-9 clinically inactive complexes with slow plasma clearance. If this is the case, such complexes would be detected in the SC5b-9 enzyme-linked immunosorbent assay, rendering SC5b-9 measurement not suitable to monitor eculizumab’s therapeutic effect in aHUS. Neither CH50 was of help to monitor eculizumab effectiveness, because values measured during treatment did not parallel signs of disease activity.
On the other hand, we found that normalization of serum-induced C5b-9 deposits on ADP-activated endothelium paralleled or even preceded (as in case 5) eculizumab-induced clinical remission. Thus, the ex vivo test presented here is a tool to monitor eculizumab efficiency and personalize eculizumab therapy. In support of the above possibility are data showing that measurement of serum-induced endothelial C5b-9 deposits allowed us to document that the eculizumab dose initially given to 2 pediatric patients, to prevent posttransplant recurrence in one (case 5) and during the acute episode in the other (case 6), was not enough to fully control complement activation at endothelial level, despite normal plasma SC5b-9 suggesting an efficient complement control in fluid phase. Enhancing the eculizumab dose from 300 to 600 mg in the first case and from 600 mg to 900 mg in the second one normalized both ex vivo endothelial C5b-9 deposits and clinical parameters.
The effectiveness of eculizumab to inhibit complement-mediated thrombotic microangiopathy13,15 led regulatory agencies in the United States and Europe to approve this drug for the treatment of aHUS. However, the extremely high cost of eculizumab (about US $350 000 per patient per year) and the need of lifelong treatment may be important limitations to its widespread use in aHUS, even in relatively high-resource settings. The case of the UK health minister, who on May 2013 rejected a recommendation from an expert committee that the drug be “routinely provided nationally” and refused to fund eculizumab to a woman with aHUS, is paradigmatic.16,17 After that episode, the National Health System published an interim commissioning policy (NHS England, September 2013 E03/PS[HSS]/a) defining patients to whom eculizumab treatment will be provided, pending evaluation and final decision by the National Institute for Health and Care Excellence.
Thus, as highlighted by Tanimoto et al in their letter, “it is crucial to explore the most appropriate dose, dosage intervals and duration of treatment to reduce the enormous financial burden of eculizumab therapy.”36 In this regard, our ex vivo test, when performed in suitably equipped laboratories, could be a helpful tool to adjust the eculizumab dose and the interval between doses to the minimum necessary to block complement at the endothelial level, thus avoiding drug overexposure and waste of money. That this might be the case is supported here by the 2 adult patients (cases 7 and 8) in whom treatment could be spaced every 3 and 4 weeks, respectively, with the support of prospective evaluation of ex vivo C5b-9 deposits on ADP-activated endothelium, without changes in clinical parameters. Prospective studies in a larger number of patients are needed to prove the sensitivity of the ex vivo test proposed here to guide eculizumab dosage and spacing in aHUS patients, particularly in the presence of inciting events like infections or pregnancies.
Presented in part at the 13th European Meeting on Complement in Human Disease, Leiden, The Netherlands, August 21-24, 2011, and at the 24th International Complement Workshop, Crete, Greece, October 10-15, 2012.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Edwin Ades and Fransisco J. Candal of the Centers for Disease Control and Prevention and Dr Thomas Lawley of Emory University, Atlanta, who developed HMEC-1 used in this study; Adienne Pharma & Biotech (Bergamo, Italy) for providing the anti-C5 minibody; Prof R. Wurzner (Innsbruck, Austria) for providing the anti-C9 antibody; CellDex (Needham, MA) for providing sCR1; Drs L. Besso, D. Donati, A. Guarnieri, L. Peruzzi, V. Portalupi, and S. Rota for providing biological samples and clinical information on cases 1, 2, 3, 4, 5, 6, 7, and 8; and clinicians and patients for their membership of and support to the International Registry of Recurrent and Familial HUS/TTP. The authors also thank Anna Pezzotta, Paola Rizzo, and Fabio Sangalli for technical assistance in HMEC-1 cell culture and acquisition of microscope images. Drs Annalisa Perna and Antonietta Chianca performed statistical analysis.
This work was supported by a fellowship from Fondazione ART (Milan, Italy) (F.B.) and Fondazione ARMR (Bergamo, Italy) (S.B.). This research was funded by Fondazione ARMR, Fondazione ART, Taligen Therapeutics (Cambridge, MA), LFB Biotechnologies (Courtaboeuf, France), and European Community grant 2012-305608 EURenOmics.
Authorship
Contribution: M.N., M.G., and G.R. designed research, interpreted data, and wrote the paper; S.G. performed research and analyzed data; P.M., F.B., S.B., C.T., and E.V. performed research; E.B. coordinated clinical data collection and patient recruitment; R.D. analyzed data; A.A., R.C., P.R., and E.G. did clinical monitoring of eculizumab-treated patients; and F.T. provided complement reagents and inhibitors and contributed to interpretation of data.
Conflict-of-interest disclosure: P.M. received a personal grant from Adienne Pharma & Biotech, and M.N. received consultant fees from Alexion. The remaining authors declare no competing financial interests.
Correspondence: Miriam Galbusera, IRCCS - Istituto di Ricerche Farmacologiche Mario Negri, Centro Anna Maria Astori, Science and Technology Park Kilometro Rosso, Via Stezzano, 87, 24126 Bergamo, Italy; e-mail: miriam.galbusera@marionegri.it.
References
Author notes
M.N., M.G., and S.G. contributed equally to this study.