In this issue of Blood, Thon et al present the first microfluidic bioreactor design that recapitulates central features of the bone marrow in vitro and enables high-yield platelet production and high-resolution real-time visualization of (pro)platelet formation by megakaryocytes ex vivo.1
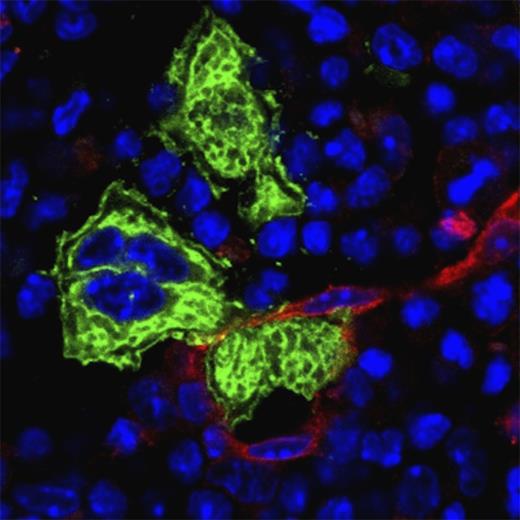
Proplatelet extension across the endothelial barrier (red) and subsequent release of (pre)platelets by megakaryocytes (green), as occurring in bone marrow sinusoids in vivo, has been very difficult to reproduce in vitro. The microfluidic bioreactor design by Thon and colleagues now recapitulates many critical aspects of this process, enabling platelet production and its high-resolution real-time visualization in vitro.1 Bone marrow cryosection was probed with anti-CD105 (Alexa Fluor 647; red), anti-GPIb (Alexa Fluor 488; green) antibodies and counterstained with 4′,6 diamidino-2-phenylindole (blue).
Proplatelet extension across the endothelial barrier (red) and subsequent release of (pre)platelets by megakaryocytes (green), as occurring in bone marrow sinusoids in vivo, has been very difficult to reproduce in vitro. The microfluidic bioreactor design by Thon and colleagues now recapitulates many critical aspects of this process, enabling platelet production and its high-resolution real-time visualization in vitro.1 Bone marrow cryosection was probed with anti-CD105 (Alexa Fluor 647; red), anti-GPIb (Alexa Fluor 488; green) antibodies and counterstained with 4′,6 diamidino-2-phenylindole (blue).
Platelets are small anuclear cell fragments generated from bone marrow megakaryocytes (MKs) by a cytoskeleton-driven process. Thereby, mature MKs form long cytoplasmic protrusions (proplatelets) that extend into bone marrow sinusoids where (pre)platelets are finally released (see figure) and circulate in the blood stream for about 10 days, where their principal function is to maintain vascular integrity and seal vessel injuries. Consequently, impaired platelet function or low platelet counts are associated with an increased bleeding risk, which may under certain conditions become life threatening. Thrombocytopenia is relatively frequently observed in humans and can be caused by different mechanisms in the context of a large variety of pathologies or medical treatments. Accordingly, there is a growing demand for platelet transfusions that faces limited availability of platelet units due to a short shelf life and a lack of artificial platelet substitutes or in vitro–generated platelets.2 The latter can be explained by the fact that even though MKs have been identified as the wellspring of platelets more than a century ago, the exact process of platelet production by these cells is still poorly understood and has therefore not been reproduced in vitro.3 This lack of knowledge is to a great extent attributed to the localization of MKs within the bone marrow, which makes them hardly accessible and difficult to visualize or manipulate in living animals.
In vitro cultivation systems have provided the first insights into the enigmatic process of platelet formation and allowed basic studies on MK development and function. However, under static conditions in vitro, MKs derived from the bone marrow or fetal liver cells of mice, as well as from different human precursor cells, randomly form multiple proplatelet protrusions to different directions reflecting imperfect polarization and indicating that critical determinants of platelet production in vivo are missing in this setting.4-6
Only recently, studies using intravital 2-photon microscopy of the bone marrow in mice revealed for the first time directly that in vivo MKs polarize and unidirectionally extend large proplatelets into the bone marrow sinusoids, where (pre)platelets are released, presumably by shear forces generated by the circulating blood.7-9 The molecular cues guiding proplatelets toward the sinusoids are only partially understood. A study by Zhang et al on sphingosine-1-phosphate and its receptor S1pr1 in MKs provided first insights into the mechanisms regulating transendothelial proplatelet guiding.8 Importantly, proplatelet extension within the microfluidic bioreactor design by Thon et al strongly resembles proplatelet formation in vivo as visualized by intravital microscopy in mice.1,7-9 Random (or premature) proplatelet formation by MKs as seen in static cultures could in vivo only be observed under pathological conditions, for example, in mice lacking S1pr1, Wiskott-Aldrich syndrome protein, or profilin1 in MKs.4,8,9
What makes the difference between the situation in vitro and in vivo? Previous studies revealed the importance of cytokines, cell-cell and cell-matrix contacts, and vascular shear stress as key regulators of platelet production.3 However, due to their experimental setup, these studies had limitations in combining the different parameters into one model or could not sufficiently control them to allow definitive conclusions. In a series of elegant experiments, Thon and colleagues now show that their bioreactor design allows one to tightly control these parameters and assess their impact on platelet formation, for example, by the supplementation or removal of different extracellular matrix components (collagens, fibronectins, or laminins) or variation of MK distance to the “sinusoids” (lower channel).1
Another important advantage of the here-described bioreactor is the striking increase in platelet yields by a magnitude of twofold compared with static conditions. The authors speculate that this setup, upon upscaling, could help to satisfy the demand for platelet transfusions. In support of this, Thon and colleagues assessed the quality of their instant platelet product, generated from murine fetal liver– and human-induced pluripotent stem cell–derived MKs, by performing morphologic and functional studies. With this, they show that their platelet product reflects the cytoskeletal organization and functionality of human and mouse platelets.1 The next logical step will be to test the quality of the product under in vivo conditions, for example, by substituting platelet-depleted mice with in vitro–generated platelets and assessment of platelet lifetime and function in models of not only hemostasis but also thrombosis and thromboinflammation.
Although the “bioreactor-on-a-chip” is not the first attempt to mimic key features of the MK bone marrow microenvironment,10 it represents a major advance in this field of research as it not only provides high yields of (apparently) functional platelets but also allows high-resolution real-time visualization of the dynamic process of proplatelet formation in vitro. We are convinced that this design will serve as a basis for further optimization and will stimulate new research in the field of MK biology. These studies will certainly advance our understanding of platelet formation and ultimately the molecular causes of platelet formation defects in patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.