Key Points
A novel TM mutation results in shedding of active TM into the blood.
Subsequent activation of the protein C anticoagulant system causes bleeding.
Abstract
In this study, we describe a novel thrombomodulin (TM) mutation (c.1611C>A) that codes for a change from cysteine 537 to a premature stop codon (p.Cys537Stop). Three members of a family with a history of posttraumatic bleeding were identified to be heterozygous for this TM mutation. All coagulation screening tests, coagulation factor assays, and platelet function test results were within normal limits. However, the endogenous thrombin potential was markedly reduced at low-tissue factor concentration, and failure to correct with normal plasma indicated the presence of a coagulation inhibitor. Plasma TM levels were highly elevated (433-845 ng/ml, normal range 2-8 ng/ml, equating to 5 to 10 nM), and the addition of exogenous protein C further decreased thrombin generation. The mutation, p.Cys537Stop, results in a truncation within the carboxyl-terminal transmembrane helix. We predict that as a consequence of the truncation, the variant TM is shed from the endothelial surface into the blood plasma. This would promote systemic protein C activation and early cessation of thrombin generation within a developing hemostatic clot, thereby explaining the phenotype of posttraumatic bleeding observed within this family.
Introduction
Thrombomodulin (TM) is a 557 amino acid type-1 transmembrane glycoprotein expressed on the surface of endothelial cells, certain epithelial cells, monocytes, and megakaryocytes. It is comprised of an amino-terminal C-type lectin domain, an epidermal growth factor (EGF)-like domain comprising 6 EGF modules, an O-glycosylation domain, a helical transmembrane domain, and a short cytoplasmic domain of 36 amino acid residues.1-3 TM has well-described roles in the regulation of hemostasis.2-4 Central to hemostasis is the role of thrombin, which when formed in trace amounts following tissue factor (TF) exposure, activates platelets by cleavage of protease activated receptors on the platelet surface.5-7 This is an important step for both platelet activation and for provision of the anionic phospholipid membrane surface required for coagulation complex assembly. Thrombin also activates factor VIII and factor V, which are critical for the formation of tenase and prothrombinase membrane complexes to create a burst of thrombin production of sufficient magnitude to cleave fibrinogen, factor XIII and form a stable fibrin clot.5,7 The ability of TM to bind thrombin with high affinity and transform thrombin from a procoagulant to a facilitator of anticoagulation was noted in 1982 by Esmon et al.8 The activation of protein C to activated protein C (APC) by the thrombin-TM complex, and the subsequent downregulation of coagulation by cleavage of factors Va and VIIIa, has been widely described.9,10 The thrombin-TM complex also activates thrombin activatable fibrinolysis inhibitor (TAFI) at a 1250-fold higher rate than thrombin alone.11 Activated TAFI inhibits the localization of plasminogen to partially degraded fibrin by cleavage of carboxyl-terminal (C-terminal) lysine and arginine residues from the fibrin surface, and thus, reduces plasmin-mediated fibrinolysis.12,13 TM, therefore, plays a pivotal role for regulating both the size and strength of the hemostatic clot. The location of TM on the surface of endothelial cells ensures that these effects normally occur at the periphery of a hemostatic clot, and are therefore, key to limiting clot expansion.
We report a heterozygous c.1611C>A mutation within the TM gene sequence (THBD) that codes for a change from cysteine 537 to a premature stop codon (p.Cys537Stop). The mutation was identified within a family where 15 members spanning 4 generations suffered from a bleeding disorder of unknown etiology. Affected individuals were reported to suffer from excessive bleeding following physical trauma or surgery, although two incidences of apparent spontaneous abdominal bleeding had been reported. Both males and females were affected and disease transmission was indicative of an autosomal dominant mode of inheritance. Investigations performed previously at other diagnostic centers had identified a low factor IX level by a 2-stage assay, however, the 1-stage factor IX assay result was normal. DNA sequencing was unable to identify any factor IX mutations. The only other abnormality detected was that of decreased coagulation factor consumption when a prothrombin consumption index (PCI) was performed.
A 31-year-old male from this family with end-stage kidney failure secondary to diabetic nephropathy was referred to the Regional Haemophilia Centre at Addenbrooke’s Hospital (Cambridge, United Kingdom [UK]) and was scheduled for combined renal and pancreas transplant surgery. Prior to previous surgical procedures, he had received prothrombin complex concentrate (PCC) and the only instance of excessive bleeding documented was following an appendectomy when he had prolonged bleeding and suffered a left rectus sheath hematoma. We describe the laboratory investigations performed on the propositus described above, as well as on his brother and mother. Based on laboratory data, all 3 were previously reported to have been affected, although only the propositus and mother had clinical histories of excessive bleeding. Other family members were not available for investigation. The initial aim of the investigation was to identify the underlying cause of the bleeding abnormality, so that treatment could be most appropriately targeted to cover the forthcoming surgery.
Methods
Materials
Biological materials and reagents used were as follows: TF was Innovin (Sysmex UK Ltd.); corn trypsin inhibitor (CTI) and protein C (Haematologic Technologies, Inc.); polyclonal protein C antibody (Dako); fibrinogen (CSL Behring); and recombinant C-terminal truncated human TM, lacking the transmembrane and cytoplasmic domains (REF 2374) was obtained from American Diagnostica/Sekisui Diagnostics, LLC. Phospholipid vesicles were prepared from 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (PS), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (PE), and 1,2-dioleoyl-sn-glycero-3-phosphocholine (PC) obtained from Avanti Polar Lipids, Inc. For routine use, a 20/20/60 mix of PS/PE/PC vesicles was prepared as described.14 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered saline albumin was used for dilution of TF and PS/PE/PC vesicles (20 mM HEPES, 140 mM NaCl, 5 mg/mL bovine serum albumin and pH 7.35). Thrombin fluorogenic substrate was Z-Gly-Gly-Arg-AMC (Bachem UK Ltd.), and was diluted to 2.5 mM in 20 mM HEPES, 100 mM CaCl2, 60 mg/mL bovine serum albumin and pH 7.35.
Blood samples
The patient and relatives gave informed consent for additional blood tests in accordance with study approval (Local Research Ethics Committee Study 03/41) and the Declaration of Helsinki. PCI testing was performed on blood collected into tubes without anticoagulant (Sarstedt) and allowed to clot for 1 hour at 37°C, as described by Parry et al.15 Blood for coagulation and platelet function studies was collected into 3.2% (0.106 mM) trisodium citrate (Sarstedt) or into 3.2% trisodium citrate supplemented with CTI to give a final concentration of 1.45 µM CTI in whole blood. Platelet-poor plasma was prepared by centrifugation at 2000 g for 10 minutes (min) at room temperature.
PCI
Prothrombin consumption index (PCI) was performed on blood as described by Parry et al.15 Essentially, patient serum obtained after whole blood clotting was mixed with thromboplastin at 37°C, and after 60 seconds an aliquot of human fibrinogen (3 g/l) was added and the time taken for a clot to form was recorded. The procedure was then repeated using patient citrated plasma, with the initial clot forming after the addition of thromboplastin being squashed and removed immediately prior to the addition of fibrinogen. The PCI was calculated from the clotting time of the plasma in seconds, divided by clotting time of the serum, and then multiplied by 100. The PCI was also performed on whole blood spiked with recombinant C-terminal truncated human TM to final concentrations of 3.75, 7.5, and 15 nM.
Coagulation studies
Unless otherwise stated, coagulation screening, factor assays, and thrombophilia testing were performed on platelet-poor citrated plasma on an ACL TOP 700 (Instrumentation Laboratory) according to the manufacturer’s instructions. SynthASil colloidal silica/synthetic phospholipid and calcium chloride reagents were used for activated partial thromboplastin time (APTT) testing and for 1-stage assay of factors, VIII, IX, XI, and XII with the appropriate factor deficient plasma (Instrumentation Laboratory). Recombiplastin 2G recombinant human TF/synthetic phospholipid/calcium chloride was used for the prothrombin time (PT), the PCI and for 1-stage assay of factor II, V, VII, and X with the appropriate factor deficient plasma (Instrumentation Laboratory). Thrombin times and fibrinogen levels (Clauss assay) were determined using bovine thrombin (Instrumentation Laboratory). Factor XIII, von Willebrand cofactor activity, and protein S levels were determined by immunoturbidimetric assays (Instrumentation Laboratory); and protein C levels and APC sensitivity ratios were determined by commercial kits (Instrumentation Laboratory). The antithrombin concentration was determined in the presence of unfractionated heparin using Stachrom AT III reagents (Diagnostica Stago, Inc.).
Platelet function testing
The whole blood platelet counts were determined by aperture impedance (Beckman Coulter, Inc.). Platelet function defects were screened for in citrated whole blood using a PFA-100 analyzer (Sysmex UK Ltd.) and collagen/adenosine 5′-diphosphate (ADP) cartridges according to the manufacturer’s instructions. Light transmission platelet aggregation studies were performed on platelet-rich plasma on a PAP-8E (Bio/Data Corporation) also according to the manufacturer’s instructions. Platelet aggregation reagents used were 20 µM ADP (Sigma-Aldrich), 500 µg/mL arachidonic acid (Helena Laboratories UK Ltd.), 300 µM epinephrine (Helena Laboratories UK Ltd.), 10 µg/mL collagen (Helena Laboratories UK Ltd.), and 1.5 and 0.5 mg/mL ristocetin (Helena Laboratories UK Ltd.). Agonist concentrations given were final concentrations.
CAT
Calibrated automated thrombography (CAT) was performed on platelet-poor citrated plasma using a TF trigger (final concentration = 5 pM) and on citrated plasma containing CTI (final concentration = 1 pM TF). Analysis was performed on a Fluoroskan Ascent Microplate Fluorometer (Thermo Scientific) using Thrombinoscope software (Diagnostica Stago, Inc.), as described by Dargaud et al.16 To screen for the presence of a coagulation inhibitor, CAT was performed on citrated-CTI plasma from all 3 family members using a 1 pM TF trigger after the addition of an equal volume of normal citrated CTI plasma (obtained in-house). To evaluate the protein C pathway, CAT was performed on citrated plasma using a 5 pM TF trigger after the addition of purified protein C to a final concentration of 350 nM, and on citrated CTI plasma with a 1 pM TF trigger after the addition of a polyclonal protein C antibody (final concentration = 350 nM). To evaluate the effect of TM on thrombin generation, CAT was performed on normal citrated-CTI plasma using a 1 pM TF trigger after the addition of recombinant C-terminal truncated human TM to final concentrations of 3.75, 7.5, and 15 nM (approximately 250, 500, and 1000 ng/mL).
Plasma TM assay
Plasma TM levels were quantified by a solid phase sandwich enzyme-linked immunosorbent assay (ELISA) to human TM according to the manufacturer’s instructions (Abcam Plc).
DNA analysis
Genomic DNA was extracted from peripheral blood leukocytes using standard procedures (Qiagen). Molecular screening for factor V Leiden was performed by melting curve analysis of polymerase chain reaction (PCR)-amplified DNA, using a factor V Leiden kit and a LightCycler 1.5 (Roche Diagnostics Ltd.). Genomic DNA was amplified between EGF6 and the cytoplasmic domain of human TM gene sequence on a DNA thermal cycler (MJ Research Inc.) using PCR oligonucleotide primers 5′-ACTGCCTGTCCAGCCGACTG and 5′-ACGGAGGCCGCTCAGAGTCT. Amplified DNA was separated by agarose gel electrophoresis, purified by gel extraction (Qiagen), and Sanger DNA sequencing was performed (Source BioScience LifeSciences). Sequence data were compared with the TM reference sequence, NM_000361.2.
Results
PCI testing
The PCI for the propositus, mother, and brother were 79%, 68%, and 60%, respectively (normal range, <30%). This indicated a significant decrease in coagulation factor consumption during whole blood clotting relative to simultaneously processed controls (all <20%). The PCI determined on normal whole blood spiked with recombinant C-terminal truncated human TM to final concentrations of 0, 3.75, 7.5, and 15 nM, and were 15%, 48%, 86%, and 100%, respectively.
Coagulation factor analysis
Coagulation screening testing using the PT, APTT, thrombin time, and fibrinogen assay detected no abnormality in any of the family cohorts (data not shown). Factors II, V, VII, VIII, IX, X, XI, XII, XIII, von Willebrand factor antigen, and von Willebrand cofactor activity performed on the propositus and his mother were all normal (data not shown). The APTTs on a pool of normal plasma following the addition of 0, 3.75, 7.5, and 15 nM recombinant C-terminal truncated human TM ± 2 standard deviations were 29.2 ± 0.8, 30.1 ± 1.3, 30.1 ± 1.5, and 31.1 ± 0.4 seconds.
Platelet function testing
Platelet counting and function analysis was performed on the family cohort. The whole blood platelet counts determined by aperture impedance were all within normal limits. Platelet function analysis performed on a PFA-100 using ADP/collagen cartridges were all within normal reference limits (data not shown), and no abnormalities were detected by platelet aggregation studies, including agglutination with ristocetin that was performed on platelet-rich plasma (data not shown).
Thrombophilia testing
The plasma antithrombin and protein C levels were within normal reference limits for all 3 family members (data not shown). Protein S levels were within normal reference limits in the propositus and brother, whereas in the mother it was 51% (normal reference limits, >63%). The APC sensitivity ratio was normal in the propositus and mother, whereas the brother had a low ratio of 1.8 (normal reference range, >2.2) indicating a resistance to APC. Fluorescent melting curve analysis of genomic DNA demonstrated that the brother was heterozygous for the factor V Leiden mutation (c.1601G>A, p.Arg534Gln), and that the propositus and mother had a normal genotype.
CAT measurement
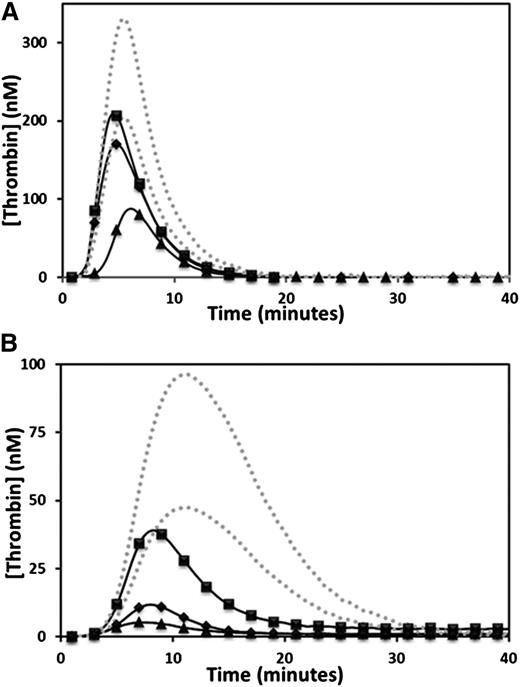
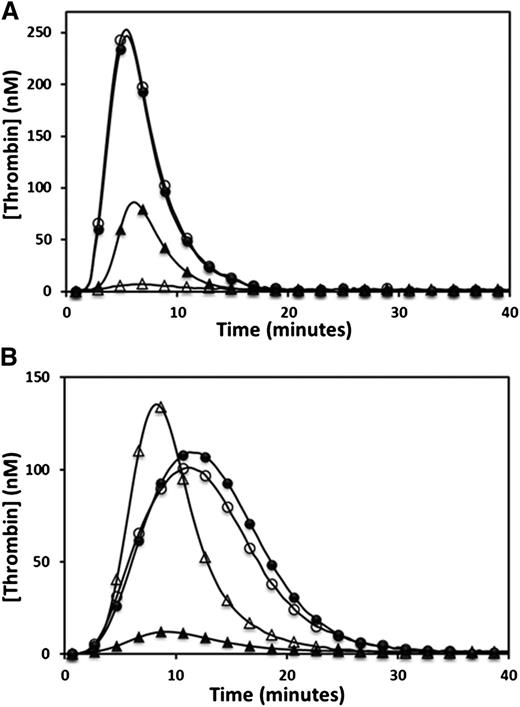
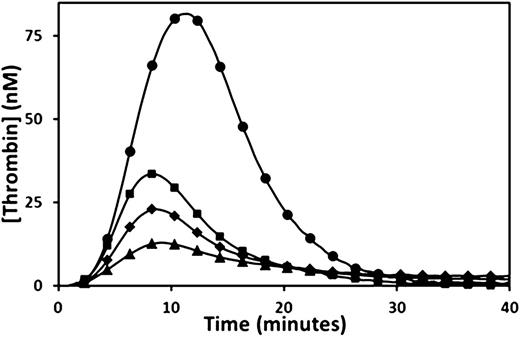
Thrombin generation was determined by CAT. The endogenous thrombin potential (ETP) was moderately reduced in the propositus and mother, and was at the low range of normal in the brother when 5 pM TF was the trigger (Figure 1A and Table 1). However, when 1 pM TF was used as the trigger, the propositus and his mother generated very low amounts of thrombin, whereas the brother showed only a moderate reduction in ETP (Figure 1B and Table 1). Normal plasma mixing studies increased the ETP from 28 to 140 nM/min in the propositus, 82 to 207 nM/min in the mother, and 392 to 426 nM/min in the brother, whereas the ETP of the normal plasma used was 745 nM/min (normal reference range, 609 to 1242 nM/min). The observation of only a partial correction of the ETP in all family members indicated the presence of a coagulation inhibitor. Further CAT studies were performed using citrated plasma supplemented with a fivefold physiological excess of protein C (350 nM) and triggered with 5 pM TF. Under these conditions, the ETP of all 3 family members was reduced (from 436 to 80 nM/min in the propositus, 872 to 246 nM/min in the mother, and 1214 to 458 nM/min in the brother), whereas the ETP of the normal control plasma was unaffected by the additional protein C (propositus and control shown in Figure 2A). A fivefold physiological excess of protein C polyclonal antibody relative to normal plasma protein C levels added to citrated CTI plasma restored a normal ETP in the propositus (1010 nM/min), while the ETP of a normal plasma control was unaffected (Figure 2B). An assessment of the effect of TM on thrombin generation performed by supplementation of normal plasma with recombinant C-terminal truncated human TM showed significant reductions in ETP at final TM concentrations of 3.75 to 15 nM, or approximately 250 to 1000 ng/mL (Figure 3).
Reduced thrombin generation in the propositus, mother, and brother. Thrombin generation was measured for the propositus (▲), mother (♦), and brother (▪), triggered by the addition of 5 pM TF (A) or 1 pM TF (B). The dotted gray lines indicate the upper and lower limits of the normal reference range.
Reduced thrombin generation in the propositus, mother, and brother. Thrombin generation was measured for the propositus (▲), mother (♦), and brother (▪), triggered by the addition of 5 pM TF (A) or 1 pM TF (B). The dotted gray lines indicate the upper and lower limits of the normal reference range.
ETP and plasma TM concentrations
| Analysis . | Normal . | Propositus . | Propositus* . | Mother . | Brother . |
|---|---|---|---|---|---|
| ETP (nM/min) | |||||
| 5 pM TF | 1121–1816 | 426 ± 54 | 700 ± 245 | 896 ± 58 | 1127 ± 204 |
| 1 pM TF | 609–1242 | 54 ± 63 | 206 ± 52 | 108 ± 62 | 446 ± 94 |
| Plasma TM (ng/ml) | 2–8 | 845 ± 44 | 553 ± 44 | 433 ± 55 | 498 ± 124 |
| Analysis . | Normal . | Propositus . | Propositus* . | Mother . | Brother . |
|---|---|---|---|---|---|
| ETP (nM/min) | |||||
| 5 pM TF | 1121–1816 | 426 ± 54 | 700 ± 245 | 896 ± 58 | 1127 ± 204 |
| 1 pM TF | 609–1242 | 54 ± 63 | 206 ± 52 | 108 ± 62 | 446 ± 94 |
| Plasma TM (ng/ml) | 2–8 | 845 ± 44 | 553 ± 44 | 433 ± 55 | 498 ± 124 |
1-year posttransplant.
Evaluation of the protein C pathway by CAT. The effect of the protein C pathway on thrombin generation was evaluated by adding excess protein C (A) or polyclonal antiprotein C antibody (B) to plasma of the propositus (▲) and a normal control (●). Open symbols represent the addition of the protein C or antiprotein C antibody.
Evaluation of the protein C pathway by CAT. The effect of the protein C pathway on thrombin generation was evaluated by adding excess protein C (A) or polyclonal antiprotein C antibody (B) to plasma of the propositus (▲) and a normal control (●). Open symbols represent the addition of the protein C or antiprotein C antibody.
Reduced thrombin generation in normal plasma after the addition of TM. Thrombin generation triggered by 1 pM TF was measured in normal plasma (●), and in normal plasma following the addition of recombinant human C-terminal truncated TM to a final concentration of 3.75 nM 250 ng/ml (▪), 7.5 nM (500 ng/ml) (♦), and 15 nM (1000 ng/ml) (▲).
Reduced thrombin generation in normal plasma after the addition of TM. Thrombin generation triggered by 1 pM TF was measured in normal plasma (●), and in normal plasma following the addition of recombinant human C-terminal truncated TM to a final concentration of 3.75 nM 250 ng/ml (▪), 7.5 nM (500 ng/ml) (♦), and 15 nM (1000 ng/ml) (▲).
Plasma TM
Plasma TM levels determined by ELISA in all 3 family members were 100-fold higher than in normal plasma. The plasma TM level of the propositus determined before the kidney and pancreas transplant was twice that of the mother and brother, but dropped to a comparable level 1 year after transplant (Table 1). Based on a molecular weight estimation of 68 000 Da for TM, a concentration of 510 ng/mL equates to 7.5 nM. The recombinant truncated TM reagent (American Diagnostica/Sekisui Diagnostics, LLC) that we used had a deletion of the cytoplasmic domain and transmembrane helix, and so was similar to the mutant TM in the affected family members.
Molecular analysis
Genomic DNA obtained from all 3 family members was amplified by PCR to yield 509 bp fragments of TM DNA (reference sequence THBD NM_000361.2). Sanger DNA sequencing showed that all 3 family members were heterozygous for a c.1611C>A mutation, Human Genome Variation Society nomenclature NM_000361.2:c.1611C>A. This mutation coded for a change from cysteine 537 to a premature stop codon (p.Cys537Stop). The propositus was also noted to be heterozygous for a c.1418C>T mutation coding for an alanine-to-valine amino acid change at residue 473 (p.Ala473Val).
Discussion
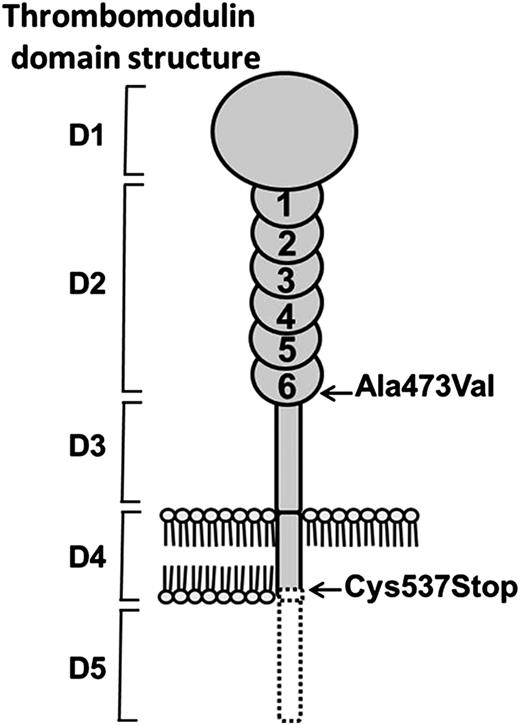
We investigated a family with a history of an autosomal dominant phenotype of posttraumatic bleeding. Routine coagulation tests, coagulation factor assays, and platelet function tests were all normal. However, the PCI showed decreased coagulation factor consumption during whole blood clotting, and CAT performed on platelet-poor plasma showed a decrease in thrombin generation as measured by the ETP (Table 1). To minimize the effect of contact activation on thrombin generation, further CAT studies were performed in the presence of CTI. Under these conditions, a larger reduction in ETP was observed, which was not corrected by the addition of an equal volume of normal plasma. The ETP results showed a milder laboratory phenotype in the brother of the propositus, who was found to be heterozygous for factor V Leiden. CAT performed after the addition of an excess of protein C showed that the effect of the coagulation inhibitor was potentiated by increased levels of protein C. Conversely, the addition of a polyclonal protein C antibody, previously shown in our laboratory to inhibit the function of APC, restored thrombin generation in the propositus to normal levels. Collectively, these results indicated that the low ETP values were caused by the action of APC. This is in keeping with the quantification of plasma TM by ELISA, which showed a 100-fold increase over the normal level. It also explains the higher thrombin generation in the brother who was heterozygous for the factor V Leiden mutation. Thus, the APC cofactor effect observed in the plasma from our family cohort appeared to be due to functional TM, which depending on its degree of glycosylation,17 was present at a concentration of approximately 5 to 10 nM. The 3 family members investigated were found to be heterozygous for a c.1611C>A mutation within the TM gene sequence (THBD). This is a new mutation within TM and codes for a change from cysteine 537 to a premature stop codon (p.Cys537Stop). Cysteine 537 lies within the transmembrane region of TM, and its mutation to a stop codon is predicted to result in a truncated TM molecule lacking the last three amino acids of the transmembrane helix and the entire C-terminal cytoplasmic domain (Figure 4). The introduction of a negatively charged C-terminus within the hydrophobic lipid bilayer could be predicted to have a destabilizing effect on anchoring TM. In addition, the cytoplasmic domain of TM has recently been shown to be linked via an adaptor protein to actin within the membrane cytoskeleton.18 Truncated TM resulting from the new premature stop codon would be expected to be shed from the endothelial cells into the blood, thus explaining the high level of plasma TM observed in our family members. The propositus differed from his mother and brother in that he was also noted to be heterozygous for an Ala473Val amino acid change (Figure 4). This is a previously described TM variant that has been investigated as a potential risk factor for thrombophilia,19 cardiac disease,20 and atypical hemolytic uremic syndrome.21
The predicted structural consequences of TM Cys537Stop mutation. The TM domains shown are: D1 = lectin; D2 = EGF-like; D3 = O-glycosylation; D4 = transmembrane; and D5 = cytoplasmic. The Ala473Val mutation in EGF6 was present only in the propositus. The Cys537Stop mutation was present in all 3 family members and resulted in loss of the C-terminal portion of the transmembrane helix and the entire cytoplasmic domain (dotted black line). The consequence of the introduction of a negatively charged C-terminus into the hydrophobic lipid bilayer, together with the loss of stabilizing protein interactions with cytoplasmic domain, resulted in the shedding of TM into the blood plasma.
The predicted structural consequences of TM Cys537Stop mutation. The TM domains shown are: D1 = lectin; D2 = EGF-like; D3 = O-glycosylation; D4 = transmembrane; and D5 = cytoplasmic. The Ala473Val mutation in EGF6 was present only in the propositus. The Cys537Stop mutation was present in all 3 family members and resulted in loss of the C-terminal portion of the transmembrane helix and the entire cytoplasmic domain (dotted black line). The consequence of the introduction of a negatively charged C-terminus into the hydrophobic lipid bilayer, together with the loss of stabilizing protein interactions with cytoplasmic domain, resulted in the shedding of TM into the blood plasma.
The propositus had previously been diagnosed with factor IX deficiency based on a low factor IX level in a 2-stage factor assay and had been treated with prothrombin complex concentrate with an apparent beneficial effect. The low factor IX level in the 2-stage assay was in retrospect explained by the inhibitory effect of the TM, which would be apparent in a 2-stage assay but not a 1-stage assay. We repeated the consumption index to confirm the abnormality. Notably, the addition of recombinant C-terminal truncated human TM produced a dose-dependent change in the consumption index in normal plasma comparable with the results in the family members, and this is in keeping with their plasma levels of TM as measured by ELISA. Despite the presence of the high level of TM, the phenotype of the affected family members was not characterized by a prolonged APTT. When recombinant C-terminal truncated human TM was added to normal plasma, the APTT was not prolonged, which is in keeping with the normal APTT results in the family members. When thrombin is generated in the PT and APTT, 2 µmol of thrombin are generated rapidly and so the ability of TM-bound thrombin to retard the thrombin explosion is likely overwhelmed. Therefore, the speed of thrombin generation during clotting in the PT or APTT possibly explains the apparent paradox of a normal APTT, despite a high TM level.
At present, we can only speculate on the effect of the mutation on fibrinolysis and clot stability and the influence of these factors on bleeding tendency. It has been demonstrated that in hemophila, the amount of thrombin generated may not be sufficient for optimal activation of factor XIII, and TM specifically has been shown to delay factor XIII activation.22,23 Therefore, the high plasma TM level may result in reduced factor-XIII–dependent fibrin crosslinking and clot strength. On the other hand, TM has been shown to increase the rate of TAFI activation with a reduction in fibrinolytic activity.24 The balance between these effects in vivo is likely to be influenced by local variable factors at sites of thrombin generation, but the low rate of spontaneous bleeding in the family suggests there may be a compensatory reduction in fibrinolytic activity generally.
In view of the apparent clinical benefit of PCC, the patient received PCC prior to kidney and pancreatic transplantation. Further PCC, fresh frozen plasma, platelets, and cryoprecipitate were also given by the anesthetist during the procedure. The surgery was essentially uneventful and bleeding was not excessive. However, despite daily administration of PCC postoperatively, bleeding occurred from the wound site necessitating blood transfusion. Laparotomies performed at day 1 and day 7 postsurgery identified extensive hematomas within the abdomen (which were removed), but no specific bleeding site was identified. After the second laparotomy, no further surgical intervention was necessary. Thus, severe postoperative bleeding occurred despite substantial blood product support, in keeping with an “inhibitor-type” bleeding phenotype.
Further investigation of the propositus 1-year after kidney and pancreas transplant showed that his plasma TM had fallen to a level equivalent to that of his mother and brother, and his ETP had risen to a level similar to that of his mother (Table 1). Therefore, the initial results for these determinations in the propositus were likely modified by kidney and pancreatic disease rather than as a consequence of the second TM mutation (c.1418C>T). The propositus and his mother now have a similar laboratory phenotype and also share the clinical phenotype of posttraumatic bleeding. The brother of the propositus demonstrated significantly higher ETP values and the abnormality could only be detected at low TF concentrations. His milder laboratory phenotype is most likely due to APC resistance as a consequence of being heterozygous for factor V Leiden. He has also experienced no significant bleeding episodes to date, therefore, it appears that co-inheritance of factor V Leiden moderates the clinical severity of this bleeding disorder.
In conclusion, we have identified a new TM mutation (c.1611C>A) that codes for premature termination of transcription (p.Cys537Stop), and hence, formation of truncated TM that is readily shed from endothelial cell surfaces into the plasma. In the laboratory, we identified decreased coagulation factor consumption during whole blood clotting, a decrease in thrombin generation at low TF concentration, elevated plasma TM levels, and an accompanying increase in protein C activation. The clinical phenotype of this mutation is moderate-to-severe bleeding.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Daniel Johnson and Ty Adams for their helpful advice.
Authorship
Contribution: All authors designed the study, analyzed and interpreted the data, and wrote the manuscript; and J.L. performed the majority of the laboratory investigations and was the primary author of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Trevor P. Baglin, Department of Haematology, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge CB20QQ, UK; e-mail: trevor.baglin@addenbrookes.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal