Key Points
CYR61/CCN1 is a bone marrow microenvironmental biomarker for myeloma progression and for transformation of MGUS and asymptomatic disease to overt myeloma.
CCN1 reduces myeloma bone disease and tumor growth and is a potential therapeutic target for myeloma.
Abstract
Secreted protein CCN1, encoded by CYR61, is involved in wound healing, angiogenesis, and osteoblast differentiation. We identified CCN1 as a microenvironmental factor produced by mesenchymal cells and overexpressed in bones of a subset of patients with monoclonal gammopathy of undetermined significance (MGUS), asymptomatic myeloma (AMM), and multiple myeloma (MM). Our analysis showed that overexpression of CYR61 was independently associated with superior overall survival of MM patients enrolled in our Total Therapy 3 protocol. Moreover, elevated CCN1 was associated with a longer time for MGUS/AMM to progress to overt MM. During remission from MM, high levels of CCN1 were associated with superior progression-free and overall survival and stratified patients with molecularly defined high-risk MM. Recombinant CCN1 directly inhibited in vitro growth of MM cells, and overexpression of CYR61 in MM cells reduced tumor growth and prevented bone destruction in vivo in severe combined immunodeficiency-hu mice. Signaling through αvβ3 was required for CCN1 prevention of bone disease. CYR61 expression may signify early perturbation of the microenvironment before conversion to overt MM and may be a compensatory mechanism to control MM progression. Therapeutics that upregulate CYR61 should be investigated for treating MM bone disease.
Introduction
Multiple myeloma (MM) is a malignancy of terminally differentiated plasma cells that typically grow in the bone marrow (BM) and produce osteolytic lesions and bone disease in 80% of patients.1,2 Experimental data3 and clinical observations1 suggest that bone disease drives MM progression and that early changes in bone remodeling precede transformation of MM from monoclonal gammopathy of undetermined significance (MGUS),4 a benign stage of MM.5 Compared with control subjects, patients with MGUS have decreased bone mineral density (BMD) and cortical and trabecular thickness6 and increased risk of fractures.7 A population-based study showed that patients with MGUS (N = 5326) had a 1.6-fold increased risk of any fracture at 10 years after diagnosis.1
Because recent reports implicated CYR61 upregulation by osteoblast-activating agents, we sought to investigate its role in MM. CYR61 is a growth factor–inducible gene8,9 that encodes a cysteine-rich, extracellular matrix–associated heparin-binding protein, CCN1.10,11 CYR61 is upregulated by endothelin-1,12 WNT3A,13 and parathyroid hormone (PTH)14 during induction of osteoblastogenesis15 and bone formation, and we previously showed that administration of exogenous WNT3A13 or intermittent administration of PTH14 prevent MM-induced bone disease, promote bone formation, and delay tumor progression. Further, CCN1 has been detected in BM from patients with MM.16
As a matrix-associated protein, CCN1 supports adhesion of fibroblasts, endothelial cells, epithelial cells, blood platelets, and other cell types17 and stimulates chemotaxis of fibroblasts and vascular endothelial cells. CCN1 synergizes with mitogenic growth factors to enhance growth factor–induced DNA synthesis.18 It also directly stimulates osteoblastogenesis19 and angiogenesis20,21 in an αvβ3-dependent manner and inhibits formation of osteoclasts independently of αvβ3 and αvβ5.22 Thus, CCN1 may be involved in bone remodeling and its activity can vary according to integrin combinations and cell context.
Based on these findings, we investigated CYR61 expression and levels of circulating CCN1 in patients with MGUS and various stages of MM, analyzed associations with patient survival and disease progression, and examined direct effects of CCN1 on MM cell growth and bone disease.
Materials and methods
Patient population
BM aspirates or whole bone biopsies were obtained from healthy donors, patients with MGUS, asymptomatic multiple myeloma (AMM), and MM. The majority of MM patients analyzed at diagnosis were enrolled in National Institutes of Health–sponsored clinical trial UARK 03-033 Total Therapy TT3A and TT3B protocols; therefore, outcome analyses were limited to this cohort. Patients’ baseline characteristics were described previously.23 Diagnostic criteria for patients at our institute with MGUS and AMM were based on the International Myeloma Working Group convention.24 Of the 89 MGUS/AMM patients analyzed, 6/37 MGUS patients and 35/52 AMM patients progressed to overt MM. Of the progression events, 24/41 were based on Calcium, Renal, Anemia, and Bone lesion criteria and 17/41 were based on other criteria; however, all required therapy. Risk assessments were based on parameters defined by Mayo25 and gene expression profiling 70 (GEP70)26 risk of transformation to overt MM. The Institutional Review Board of the University of Arkansas for Medical Sciences approved the research studies, and all subjects provided written informed consent in accordance with the Declaration of Helsinki.
Statistical analysis
Hazard ratios were calculated by the Cox proportional hazards model. Multivariate survival analysis was applied to select independent features that were most significantly associated with patient outcomes. All statistical analyses were performed with statistical software R, available from The R Project for Statistical Computing (http://www.r-project.org), and with R developed by the BioConductor project (http://www.bioconductor.org). Kaplan-Meier statistical methods were used to plot overall survival, progression-free survival, time to progression, complete response (CR) duration, and time to CR, and the log-rank test was used for comparisons. X-tile software (Yale University, New Haven, CT) was used to determine optimal cut points. Comparisons of CYR61/CCN1 levels between 2 groups were examined by Student t test or 1-way analysis of variance for multiple groups. Differences in BMD, MM burden, and osteoblast and osteoclast numbers were analyzed with Student t test.
Methods and information on cells, growth assays, gene expression profiling, quantitative reverse-transcription polymerase chain reaction, cell transduction, small interfering RNA (siRNA) transfection, CCN1 enzyme-linked immunosorbent assay (ELISA) and western blots, and myelomatous severe combined immunodeficiency (SCID)-hu mice studies are described in the supplemental data on the Blood Web site.
Results
CYR61 is variably expressed in the BM microenvironment of patients with MGUS, AMM, and MM
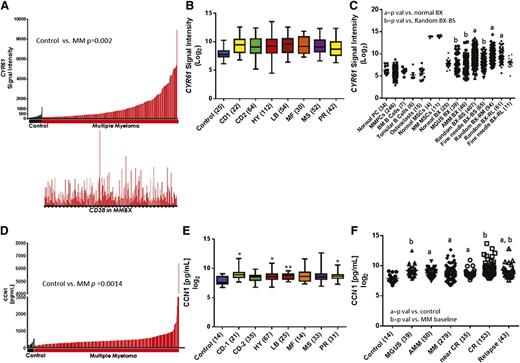
To determine the prevalence of CYR61 expression in the MM microenvironment, we examined the GEP data of plasma cells and whole bone biopsies from patients with MM and from healthy donors. CYR61 expression was significantly higher in bone biopsies from MM patients (n = 246) than in those from healthy donors (n = 24; P = .0019) (Figure 1A, top). Quantitative reverse-transcription polymerase chain reaction analysis confirmed CYR61 expression in MM bone biopsies (supplemental Figure 1A). Expression of CYR61 in biopsies did not correlate with expression of CD38 (Figure 1A, bottom), suggesting that CYR61 in the biopsies did not reflect the level of plasmacytosis. Baseline CYR61 expression did not vary among molecular subtypes of MM27 (Figure 1B) or GEP70-defined high- and low-risk MM,28 although there was a trend toward higher CYR61 in MS, MF, and PR subgroups in patients with high-risk MM (supplemental Figure 2). To identify the cellular source of CYR61, we investigated its expression in cultured mesenchymal stem cells (MSCs), osteoclasts, and other cell types in BM. CYR61 was not expressed in normal plasma cells, MM plasma cells, osteoclasts, or B-lymphocytes, but it was detected at high levels in cultured MSCs from healthy donors and MM patients, indicating that CYR61 in myelomatous bone is produced mainly by cells of mesenchymal lineage (Figure 1C). Additional plasma cell and microenvironment-specific genes were analyzed to determine if they correlated with CYR61 expression in whole bone biopsies. Positive, significant correlation was observed with the microenvironmental genes FN1 (fibronectin), COL4A1, and MCAM (CD146), whereas plasma cell genes CD38, IRF4, and SDC1 (CD138) were not correlated with CYR61 expression, confirming that CYR61 is produced in the MM microenvironment (supplemental Figure 1C).
CYR61 is expressed at low levels in normal biopsies and normal or malignant plasma cells. (A) A bar view of CYR61 messenger RNA (mRNA) levels (y-axis) determined by GEP in whole biopsies from healthy donors (control; black, n = 24) and patients with MM (red, n = 246) (x-axis). Each bar represents an individual sample, and the height of the bar indicates the level of CYR61 mRNA. Samples are ordered based on increasing levels of CYR61 mRNA from left to right. CD38 mRNA levels in the corresponding biopsies are presented immediately below (lower panel). (B) CYR61 expression in whole biopsies from healthy donors (control) and patients with different molecular subtypes of MM; number of samples for each subtype is indicated in parentheses. (C) GEP analysis of CYR61 (presented as the Affymetrix signal; y-axis) in indicated cell types and bone biopsies (BX) (x-axis); number of samples for each cell type is indicated in parentheses. BS, baseline; RM, remission; RL, relapse. P < .05 vs normal BX = a or random BX-BS = b. (D) CCN1 levels were quantitated in BM serum from healthy donors (control, n = 14; black) and from patients with MM (n = 279; red) at diagnosis and (E) were grouped by molecular subtype of MM; number of samples for each subtype is indicated in parentheses. (P value vs control; *P <.05, **P < .001, ***P < .0001. (F) CCN1 levels in BM serum samples from indicated MM disease stages. The median and range of CCN1 levels among the subjects were as follows: healthy donors 198, 107-553 pg/mL; MGUS 572, 225-5 856 pg/mL; AMM 455, 107-6 415 pg/mL; active MM 394, 107-6 415 pg/mL; near complete remission MM 398, 199-1 952 pg/mL, complete remission MM 437, 199-13 156 pg/mL; relapse MM 471 195-7 235 pg/mL. (P < .05 vs control = a or MM baseline = b).
CYR61 is expressed at low levels in normal biopsies and normal or malignant plasma cells. (A) A bar view of CYR61 messenger RNA (mRNA) levels (y-axis) determined by GEP in whole biopsies from healthy donors (control; black, n = 24) and patients with MM (red, n = 246) (x-axis). Each bar represents an individual sample, and the height of the bar indicates the level of CYR61 mRNA. Samples are ordered based on increasing levels of CYR61 mRNA from left to right. CD38 mRNA levels in the corresponding biopsies are presented immediately below (lower panel). (B) CYR61 expression in whole biopsies from healthy donors (control) and patients with different molecular subtypes of MM; number of samples for each subtype is indicated in parentheses. (C) GEP analysis of CYR61 (presented as the Affymetrix signal; y-axis) in indicated cell types and bone biopsies (BX) (x-axis); number of samples for each cell type is indicated in parentheses. BS, baseline; RM, remission; RL, relapse. P < .05 vs normal BX = a or random BX-BS = b. (D) CCN1 levels were quantitated in BM serum from healthy donors (control, n = 14; black) and from patients with MM (n = 279; red) at diagnosis and (E) were grouped by molecular subtype of MM; number of samples for each subtype is indicated in parentheses. (P value vs control; *P <.05, **P < .001, ***P < .0001. (F) CCN1 levels in BM serum samples from indicated MM disease stages. The median and range of CCN1 levels among the subjects were as follows: healthy donors 198, 107-553 pg/mL; MGUS 572, 225-5 856 pg/mL; AMM 455, 107-6 415 pg/mL; active MM 394, 107-6 415 pg/mL; near complete remission MM 398, 199-1 952 pg/mL, complete remission MM 437, 199-13 156 pg/mL; relapse MM 471 195-7 235 pg/mL. (P < .05 vs control = a or MM baseline = b).
GEP analysis revealed lower CYR61 expression in biopsies from healthy donors and those with MGUS (P = .03) or AMM (P = .01) (Figure 1C). However, biopsies from MM patients had higher CYR61 expression at baseline (P = .003), during remission (P = .02), or at relapse (P = .002) than did those from healthy donors. Interestingly, compared with random biopsies, CYR61 expression was lower in computed tomography–guided fine-needle biopsies of magnetic resonance imaging–identified focal lesions, which are often associated with osteolytic lesions (GEP log2 mean 9.29 vs 8.21, respectively, P = .0002, Figure 1C). As further evidence, CYR61 expression was investigated in whole human bone of SCID-hu mice injected with primary MM cells and found to be suppressed compared with whole bone in the absence of MM cells. However, upon exposure of MM-bearing SCID-hu mice to intermittent PTH,14 CYR61 is upregulated suggesting that, although focal growth of MM (eg, patient’s focal lesion; SCID-hu model) may suppress CYR61 expression in MM, CYR61 can be upregulated by bone anabolic agents (supplemental Figure 1B).Taken together, these results indicate that CYR61 is upregulated in the BM microenvironment of a significant number of MM patients.
CYR61-encoded CCN1 is elevated in BM serum from a subset of MGUS and MM patients
Recently, CCN1 protein was detected in BM from MM patients.16 Because CCN1 is a secreted factor,29 we used ELISA to quantitate its levels in BM sera. CCN1 was present at higher levels in baseline samples from a subset of MM patients (n = 279) than in those from healthy donors (n = 14; P = .001) (Figure 1D), which corresponds with GEP findings (Figure 1A). Among patients for whom corresponding GEP signatures were available (n = 260), elevated CCN1 levels did not vary according to molecular subtype (Figure 1E). Similar to GEP, CCN1 levels were higher in patients with the MF subtype and GEP70-defined high-risk disease, and also were higher in high-risk than in low-risk MS subtype MM (P = .005, supplemental Figure 3).
CCN1 levels in BM sera from patients with MGUS and AMM and various stages of MM were compared with those from healthy donors (Figure 1F). CCN1 levels were higher in samples from patients with MGUS (P = .0028), AMM (P = .0002), active MM (P = .0001), near-CR from MM (P = .0054), CR (P < .0001), and relapsed MM (P = .0123) than in those from healthy donors. MM patients in CR had higher CCN1 levels than those with active MM (P = .0329). These results indicate that CYR61 gene expression is heterogeneously induced in myelomatous bone at different stages of the disease, resulting in variable secretion levels of CCN1.
CYR61 gene expression is an independent predictor of survival
Because levels of CYR61 expression and CCN1 are highly variable among each of the examined patient populations, we investigated their associations with patient outcome and disease progression. Univariate analysis of baseline variables27 linked superior overall survival to elevated CYR61 expression (hazard ratio [HR] 0.58, P = .048); inferior survival was linked to albumin <3.5 g/dL; beta-2 microglobulin (B2M) ≥3.5 mg/L; B2M >5.5 mg/L; hemoglobin <10 g/dL; lactate dehydrogenase (LDH) ≥190 U/L; presence of cytogenetic abnormalities; and GEP-defined high-risk disease, MF and PR molecular subgroups, proliferation index >10, and TP53 deletion (Table 1). In multivariate stepwise Cox regression analysis, elevated expression of CYR61 retained independent prognostic significance of overall survival in TT3 patients (HR 0.78, P = .006), as did albumin <3.5 g/dL, B2M >5.5 mg/L, LDH ≥190 U/L, MF molecular subgroup; age ≥65 years, presence of cytogenetic abnormalities, and GEP-defined proliferation index >10 represented highly adverse features for overall survival in TT3 patients (Table 1).
Univariate and multivariate stepwise Cox regression for baseline CYR61 in TT3ab (OS)
| . | Variable . | n/N (%) . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| Univariate | GEP MY subgroup | 28/264 (11%) | 1.04 (0.50-2.18) | .912 |
| Focal lesions at BS by MRI >0 | 184/264 (70%) | 1.04 (0.63-1.74) | .868 | |
| GEP LB subgroup | 34/264 (13%) | 0.93 (0.46-1.87) | .829 | |
| GEP CD-2 subgroup | 38/264 (14%) | 0.75 (0.36-1.57) | .446 | |
| GEP HY subgroup | 70/264 (27%) | 0.80 (0.46-1.41) | .446 | |
| CYR61 210764_s_at expression at BS | N = 264 | 0.92 (0.76-1.12) | .429 | |
| GEP CD-1 subgroup | 15/264 (6%) | 0.47 (0.12-1.92) | .294 | |
| CRP ≥8 mg/L | 77/264 (29%) | 1.43 (0.87-2.33) | .155 | |
| CYR61 201289_at expression at BS | N = 264 | 0.88 (0.75-1.03) | .101 | |
| GEP MS subgroup | 40/264 (15%) | 0.48 (0.21-1.11) | .087 | |
| Focal lesions at BS by PET >0 | 177/264 (67%) | 1.63 (0.94-2.82) | .079 | |
| Age ≥65 y | 71/264 (27%) | 1.56 (0.95-2.56) | .077 | |
| CYR61 210764_s_at at BS high in tt3ab (OS) | 35/264 (13%) | 0.37 (0.13-1.01) | .052 | |
| CYR61 201289_at at BS high in tt3ab (OS) | 90/264 (34%) | 0.58 (0.33-1.00) | .048 | |
| GEP MF subgroup | 21/264 (8%) | 2.35 (1.23-4.48) | .009 | |
| Hemoglobin <10 g/dL | 88/264 (33%) | 1.94 (1.21-3.10) | .006 | |
| GEP TP53 deletion | 32/264 (12%) | 2.27 (1.26-4.09) | .006 | |
| Creatinine ≥2.0 mg/dL | 17/264 (6%) | 2.65 (1.35-5.17) | .004 | |
| Albuminin <3.5 g/dL | 77/264 (29%) | 2.53 (1.57-4.07) | <.001 | |
| B2M ≥3.5 mg/L | 134/264 (51%) | 2.60 (1.56-4.31) | <.001 | |
| B2M >5.5 mg/L | 66/264 (25%) | 3.29 (2.05-5.29) | <.001 | |
| LDH ≥190 U/L | 64/264 (24%) | 2.76 (1.71-4.44) | <.001 | |
| Baseline 70-gene high risk | 40/264 (15%) | 5.07 (3.09-8.32) | <.001 | |
| Cytogenetic abnormalities | 90/264 (34%) | 3.31 (2.06-5.32) | <.001 | |
| GEP PR subgroup | 26/264 (10%) | 3.29 (1.83-5.93) | <.001 | |
| Proliferation index >10 | 27/264 (10%) | 4.72 (2.77-8.03) | <.001 | |
| Multivariate | GEP MF subgroup | 21/264 (8%) | 2.20 (1.11-4.38) | .024 |
| B2M > 5.5 mg/L | 66/264 (25%) | 1.92 (1.14-3.24) | .015 | |
| LDH ≥ 190 U/L | 64/264 (24%) | 1.94 (1.15-3.25) | .012 | |
| Albuminin < 3.5 g/dL | 77/264 (29%) | 1.93 (1.17-3.19) | .011 | |
| Age ≥65 y | 71/264 (27%) | 2.01 (1.19-3.38) | .009 | |
| CYR61 201289_at expression at BS | N = 264 | 0.78 (0.66-0.93) | .006 | |
| Cytogenetic abnormalities | 90/264 (34%) | 2.39 (1.47-3.89) | <.001 | |
| Proliferation index >10 | 27/264 (10%) | 3.83 (2.17-6.77) | <.001 |
| . | Variable . | n/N (%) . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| Univariate | GEP MY subgroup | 28/264 (11%) | 1.04 (0.50-2.18) | .912 |
| Focal lesions at BS by MRI >0 | 184/264 (70%) | 1.04 (0.63-1.74) | .868 | |
| GEP LB subgroup | 34/264 (13%) | 0.93 (0.46-1.87) | .829 | |
| GEP CD-2 subgroup | 38/264 (14%) | 0.75 (0.36-1.57) | .446 | |
| GEP HY subgroup | 70/264 (27%) | 0.80 (0.46-1.41) | .446 | |
| CYR61 210764_s_at expression at BS | N = 264 | 0.92 (0.76-1.12) | .429 | |
| GEP CD-1 subgroup | 15/264 (6%) | 0.47 (0.12-1.92) | .294 | |
| CRP ≥8 mg/L | 77/264 (29%) | 1.43 (0.87-2.33) | .155 | |
| CYR61 201289_at expression at BS | N = 264 | 0.88 (0.75-1.03) | .101 | |
| GEP MS subgroup | 40/264 (15%) | 0.48 (0.21-1.11) | .087 | |
| Focal lesions at BS by PET >0 | 177/264 (67%) | 1.63 (0.94-2.82) | .079 | |
| Age ≥65 y | 71/264 (27%) | 1.56 (0.95-2.56) | .077 | |
| CYR61 210764_s_at at BS high in tt3ab (OS) | 35/264 (13%) | 0.37 (0.13-1.01) | .052 | |
| CYR61 201289_at at BS high in tt3ab (OS) | 90/264 (34%) | 0.58 (0.33-1.00) | .048 | |
| GEP MF subgroup | 21/264 (8%) | 2.35 (1.23-4.48) | .009 | |
| Hemoglobin <10 g/dL | 88/264 (33%) | 1.94 (1.21-3.10) | .006 | |
| GEP TP53 deletion | 32/264 (12%) | 2.27 (1.26-4.09) | .006 | |
| Creatinine ≥2.0 mg/dL | 17/264 (6%) | 2.65 (1.35-5.17) | .004 | |
| Albuminin <3.5 g/dL | 77/264 (29%) | 2.53 (1.57-4.07) | <.001 | |
| B2M ≥3.5 mg/L | 134/264 (51%) | 2.60 (1.56-4.31) | <.001 | |
| B2M >5.5 mg/L | 66/264 (25%) | 3.29 (2.05-5.29) | <.001 | |
| LDH ≥190 U/L | 64/264 (24%) | 2.76 (1.71-4.44) | <.001 | |
| Baseline 70-gene high risk | 40/264 (15%) | 5.07 (3.09-8.32) | <.001 | |
| Cytogenetic abnormalities | 90/264 (34%) | 3.31 (2.06-5.32) | <.001 | |
| GEP PR subgroup | 26/264 (10%) | 3.29 (1.83-5.93) | <.001 | |
| Proliferation index >10 | 27/264 (10%) | 4.72 (2.77-8.03) | <.001 | |
| Multivariate | GEP MF subgroup | 21/264 (8%) | 2.20 (1.11-4.38) | .024 |
| B2M > 5.5 mg/L | 66/264 (25%) | 1.92 (1.14-3.24) | .015 | |
| LDH ≥ 190 U/L | 64/264 (24%) | 1.94 (1.15-3.25) | .012 | |
| Albuminin < 3.5 g/dL | 77/264 (29%) | 1.93 (1.17-3.19) | .011 | |
| Age ≥65 y | 71/264 (27%) | 2.01 (1.19-3.38) | .009 | |
| CYR61 201289_at expression at BS | N = 264 | 0.78 (0.66-0.93) | .006 | |
| Cytogenetic abnormalities | 90/264 (34%) | 2.39 (1.47-3.89) | <.001 | |
| Proliferation index >10 | 27/264 (10%) | 3.83 (2.17-6.77) | <.001 |
CI, confidence interval; P value from Wald χ-square test in Cox regression; MRI, magnetic resonance imaging; OS, overall survival; PET, positron emission tomography.
All univariate P values reported regardless of significance.
Multivariate model uses stepwise selection with entry level 0.1 and variable remains if meets the 0.05 level.
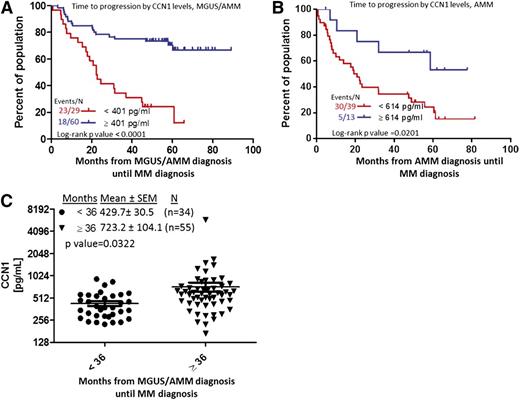
High levels of circulating CCN1 in MGUS/AMM patients are associated with longer time to progression
Because risk of disease progression is substantially lower for MGUS patients than for AMM patients,26 we sought to determine whether CCN1 may be an informative biomarker of MGUS/AMM progression. We initially combined the 2 patient populations and found that CCN1 ≥401 pg/mL was associated with longer time to progression (P < .0001; Figure 2A). However, for the group of patients with only AMM, CCN1 ≥614 pg/mL conferred longer time to progression (P = .02; Figure 2B). Patients who progress to MM within less than 3 years after MGUS/AMM diagnosis have significantly lower levels of CCN1 than those who progress 3 years or more after MGUS/AMM diagnosis (431 vs 723 pg/mL, P = .03; Figure 2C). Assessment of the relationship of risk of progression to overt MM, as defined by the Mayo clinic risk parameters25 or using our GEP-defined risk score,26 revealed no significant association with baseline CCN1 levels (supplemental Tables 1 and 2). However, 25 of 34 MGUS/AMM patients with >1 risk parameter who progressed had CCN1 levels of 406.7 ± 35.80, whereas 15 of 42 patients with >1 risk parameter26 who did not progress had CCN1 level of 958.5 ± 312.8 (P = .0593). Assessment of a similar relationship using the GEP-defined risk score,26 revealed that of the 32 patients defined as GEP70 high risk (GEP70 score > −0.26), 21 patients that progressed had a CCN1 level of 498.5 ± 39.32, whereas 11 patients defined as GEP70 high risk that did not progress had a CCN1 level of 748.1 ± 138.2 (P = .0345). Taken together, these data do not show a clear correlation between CCN1 levels and the risk factors examined and that CCN1 levels may serve as an independent risk marker for MGUS/AMM progression.
Elevated CCN1 at MGUS/AMM diagnosis is associated with longer time until progression to MM. (A) Time for overt MM to progress from MGUS/AMM, according to high (≥401 pg/mL, n = 60; blue) or low (<401 pg/mL, n = 29; red) levels of CCN1. (B) Time for overt MM to progress from AMM alone, according to high (≥614 pg/mL, n = 13; blue) or low (<614 pg/mL, n = 39; red) levels of CCN1. (C) CCN1 levels in BM sera of patients who progressed from MGUS/AMM to MM in <36 months (n = 34; circles) to those who progressed in ≥36 months (n = 55; triangles). SEM, standard error of the mean.
Elevated CCN1 at MGUS/AMM diagnosis is associated with longer time until progression to MM. (A) Time for overt MM to progress from MGUS/AMM, according to high (≥401 pg/mL, n = 60; blue) or low (<401 pg/mL, n = 29; red) levels of CCN1. (B) Time for overt MM to progress from AMM alone, according to high (≥614 pg/mL, n = 13; blue) or low (<614 pg/mL, n = 39; red) levels of CCN1. (C) CCN1 levels in BM sera of patients who progressed from MGUS/AMM to MM in <36 months (n = 34; circles) to those who progressed in ≥36 months (n = 55; triangles). SEM, standard error of the mean.
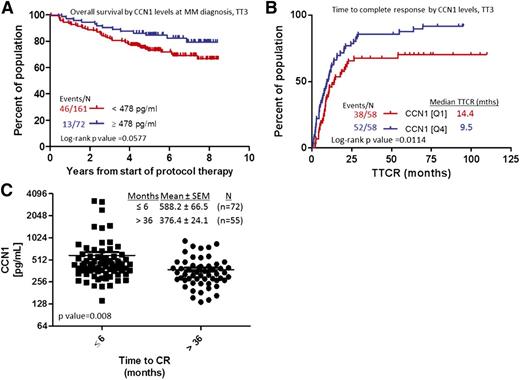
High levels of circulating CCN1 in MM patients at diagnosis or during remission are associated with longer time to progression and superior survival
Associations between baseline CCN1 level and MM patients’ outcome (overall survival and CR) were analyzed. Baseline CCN1 ≥478 pg/mL trended toward better overall survival, nearing statistical significance (P = .0577, Figure 3A). When analyzed against GEP-defined high-risk MM, upper-quartile levels of CCN1 (≥519.7 pg/mL) were associated with longer progression-free survival (4.78 years) and overall survival (6.87 years) compared with patients with lower CCN1 levels (Q1 and Q3, 2.63 and 4.01 years, respectively; data not shown). CCN1 levels did not stratify GEP-defined low-risk patients. CR was reached significantly sooner (9.5 months) for patients with high levels of CCN1 than for those with low levels of CCN1 (14.4 months, P = .01; Figure 3A). Similarly, patients who did not achieve CR within 3 years had significantly lower levels of CCN1 at diagnosis than those who achieved CR in <6 months. These analyses showed that shorter time to reach CR was associated with elevated CCN1 at diagnosis (376 vs 588 pg/mL, respectively, P = .008; Figure 3B).
MM patients with elevated CCN1 at baseline have shorter time to CR and longer overall survival. (A) Overall survival of MM patients with high (≥478 pg/mL, n = 72; blue) or low (<478 pg/mL; red) CCN1 levels at diagnosis. (B) Time to reach CR for patients with high (Q4, n = 58; blue) or low (Q1, n = 58; red) levels of CCN1 at the time of MM diagnosis. (C) CCN1 levels in BM of patients who achieved CR in ≤6 months (n = 72; squares) and those who achieved CR in >36 months (n = 55; circles).
MM patients with elevated CCN1 at baseline have shorter time to CR and longer overall survival. (A) Overall survival of MM patients with high (≥478 pg/mL, n = 72; blue) or low (<478 pg/mL; red) CCN1 levels at diagnosis. (B) Time to reach CR for patients with high (Q4, n = 58; blue) or low (Q1, n = 58; red) levels of CCN1 at the time of MM diagnosis. (C) CCN1 levels in BM of patients who achieved CR in ≤6 months (n = 72; squares) and those who achieved CR in >36 months (n = 55; circles).
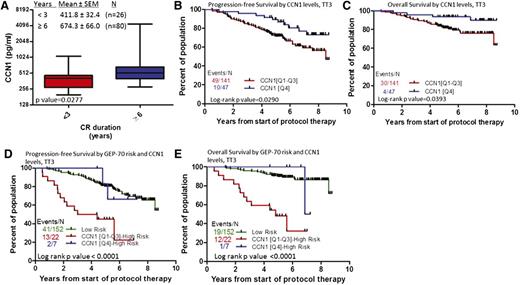
We also examined whether CCN1 levels during CR (between onset of CR and first relapse) were associated with outcomes. Patients who remained in CR <3 years had lower levels of CCN1 (412 pg/mL) during CR than those who remained in CR ≥6 years (674 pg/mL, P = .0277) (Figure 3C). In terms of survival, upper-quartile levels of CCN1 (≥642.6 pg/mL) during CR were associated with superior progression-free and overall survival (Figure 4A-B). If CCN1 levels during CR are separated by GEP70-risk, CCN1 levels can stratify cases of GEP70-defined high-risk MM (P < .0001, Figure 4C-D) but not those of low-risk MM (data not shown). For patients with GEP70-defined high-risk MM whose CCN1 levels were in the lower 3 quartiles during CR, median survival was 4.8 years, but for those whose CCN1 levels were in the highest quartile, median survival was 7 years. These data indicate higher levels of CCN1 at diagnosis or during remission are associated with shorter time to achieve CR and prolonged CR and further stratify patients with high-risk disease.
MM patients with elevated CCN1 during CR have prolonged duration of CR and longer progression-free and overall survival. (A) CCN1 levels during CR in patients who remained in CR <3 years (n = 26) compared with that of those who remained in CR ≥6 years (n = 80) is significantly different. (B) Progression-free survival and (C) overall survival of patients with high (Q4, n = 47; blue) and low (Q1-Q3, n = 141; red) levels of CCN1. (D) Progression-free survival and (E) overall survival of patients with GEP-defined low-risk MM (n = 152; green), high-risk MM and high levels of CCN1 (Q4, n = 7; blue), and high-risk MM and low levels of CCN1 (Q1-Q3, n = 22; red).
MM patients with elevated CCN1 during CR have prolonged duration of CR and longer progression-free and overall survival. (A) CCN1 levels during CR in patients who remained in CR <3 years (n = 26) compared with that of those who remained in CR ≥6 years (n = 80) is significantly different. (B) Progression-free survival and (C) overall survival of patients with high (Q4, n = 47; blue) and low (Q1-Q3, n = 141; red) levels of CCN1. (D) Progression-free survival and (E) overall survival of patients with GEP-defined low-risk MM (n = 152; green), high-risk MM and high levels of CCN1 (Q4, n = 7; blue), and high-risk MM and low levels of CCN1 (Q1-Q3, n = 22; red).
Functional analysis of the role of CCN1 in MM biology
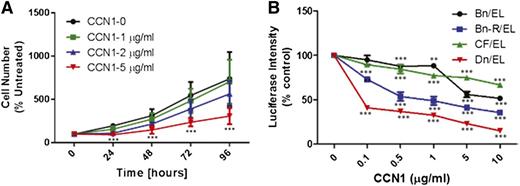
To shed light on the role of CCN1 in MM growth, H929 cells were incubated for 24 to 96 hours with different concentrations (0-5 μg/mL) of recombinant human CCN1. At concentrations ≥2 µg/mL, CCN1 suppressed growth of H929 cells (Figure 5A). We also examined the effects of recombinant CCN1 on growth of luciferase-expressing stroma-dependent cell lines (n = 4) that are capable of survival and slow growth in coculture with MSCs,30 which secrete 6 to 10 ng/mL of CCN1, as determined by ELISA. Nevertheless, addition of as little as 100 ng/mL recombinant CCN1 into the coculture system inhibited MM cell growth. Inhibition was specific to MM cells and occurred in a cell line–specific and dose-dependent manner (Figure 5B). To test whether CCN1 produced by MSCs affects MM growth, CYR61 was knocked down in MSCs by 50% before initiation of cocultures (supplemental Figure 4A). Growth of stroma-dependent MM lines (n = 3) or H929 cells in coculture with scramble siRNA- or CYR61 siRNA-treated MSCs was similar suggesting that the level of CCN1 produced by MSCs is insufficient to directly suppress MM growth (supplemental Figure 4B). These data suggest that MM cells heterogeneously respond to CCN1 and that growth of H929 MM cells is inhibited by relatively high concentrations of recombinant CCN1.
Recombinant CCN1 inhibits MM cell growth in vitro. (A) H929 MM cells were incubated for indicated times alone (control; black) with recombinant CCN1 at 1 μg/mL (green), 2 μg/mL (blue), or 5 μg/mL (red). Cells exposed to 2 μg/mL CCN1 at 24 hours and 5 μg/mL CCN1 at 24, 48, 72, and 96 hours had significantly lower cell numbers than untreated controls (P < .01 and P < .001, respectively) (B) Luciferase-expressing stroma-dependent MM cell lines were cocultured with MSCs with increasing doses (μg/mL) of recombinant CCN1 for 7 days, and MM cell growth was determined every 24 hours from bioluminescence intensity.
Recombinant CCN1 inhibits MM cell growth in vitro. (A) H929 MM cells were incubated for indicated times alone (control; black) with recombinant CCN1 at 1 μg/mL (green), 2 μg/mL (blue), or 5 μg/mL (red). Cells exposed to 2 μg/mL CCN1 at 24 hours and 5 μg/mL CCN1 at 24, 48, 72, and 96 hours had significantly lower cell numbers than untreated controls (P < .01 and P < .001, respectively) (B) Luciferase-expressing stroma-dependent MM cell lines were cocultured with MSCs with increasing doses (μg/mL) of recombinant CCN1 for 7 days, and MM cell growth was determined every 24 hours from bioluminescence intensity.
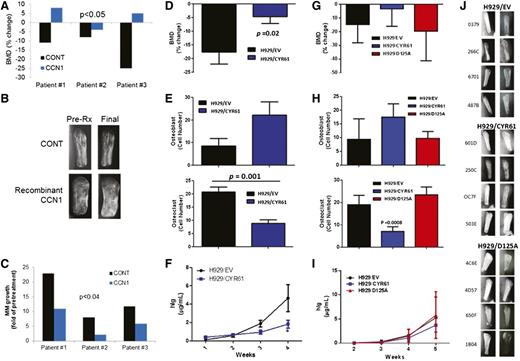
To characterize the effects of CCN1 on bone metabolism and MM growth in myelomatous bone, SCID-hu mice engrafted with MM cells from 3 patients were treated with saline or recombinant CCN1 for 4 weeks. BMD of the myelomatous bones in saline-treated hosts was reduced by 14.7% ± 5.2% and was increased by 3.0% ± 3.6% in CCN1-treated hosts (P < .05 pre-Rx vs final, Figure 6A). Radiographic analysis of the bones showed that, as expected, saline-treated bones were severely resorbed, whereas CCN1-treated bones exhibited decreased resorption (Figure 6B). As a marker of MM growth, human immunoglobulin (hIg) levels were measured in sera of mice and revealed that hIg levels were reduced in CCN1-treated mice (P < .04; Figure 6C).
CYR61 prevents bone loss and reduces tumor burden in the SCID-hu mouse model. In the first experiment (A-C), CD138-selected primary MMPCs from 3 patients were injected (100 000/mouse) into implanted bones in SCID-hu mice and were treated with recombinant CCN1. Pretreatment (CONT) and post-CCN1 treatment (CCN1) groups were compared. In the second set of experiments (D-F; 10 mice/group) and (G-J; 8 mice/group), H929 cells transduced with empty vector (EV), CYR61 complementary DNA (CYR61) or CYR61 with mutated αvβ3-binding domain (D125A) were injected (100 000 cells/mouse) into implanted bones in SCID-hu mice. (A,D,G) Change in BMD of the myelomatous bones from preengraftment levels (B,J) Radiographs of human bones implanted in SCID-hu mice before start of therapy (column 1) and at the end of the study (column 2) for each treatment group. (C,F,I) Serum hIg was quantitated by ELISA as a measure of tumor growth. (E,H) Numbers of osteoblasts (positive for osteocalcin staining) and osteoclasts (positive for TRAP staining).
CYR61 prevents bone loss and reduces tumor burden in the SCID-hu mouse model. In the first experiment (A-C), CD138-selected primary MMPCs from 3 patients were injected (100 000/mouse) into implanted bones in SCID-hu mice and were treated with recombinant CCN1. Pretreatment (CONT) and post-CCN1 treatment (CCN1) groups were compared. In the second set of experiments (D-F; 10 mice/group) and (G-J; 8 mice/group), H929 cells transduced with empty vector (EV), CYR61 complementary DNA (CYR61) or CYR61 with mutated αvβ3-binding domain (D125A) were injected (100 000 cells/mouse) into implanted bones in SCID-hu mice. (A,D,G) Change in BMD of the myelomatous bones from preengraftment levels (B,J) Radiographs of human bones implanted in SCID-hu mice before start of therapy (column 1) and at the end of the study (column 2) for each treatment group. (C,F,I) Serum hIg was quantitated by ELISA as a measure of tumor growth. (E,H) Numbers of osteoblasts (positive for osteocalcin staining) and osteoclasts (positive for TRAP staining).
To further examine the effects of CCN1 on MM growth and bone disease in vivo, H929 cells were stably transduced with a human CYR61 construct. H929 cells carrying the CYR61 construct (H929/CYR61 cells) expressed and secreted CCN1 (1000 ng/mL), but control H929/EV cells did not (supplemental Figure 5A-C). In vitro growth of H929/CYR61 cells and control cells was not different when monitored for 5 days (supplemental Figure 5D), indicating that CCN1 secretion by H929/CYR61 cells is insufficient to directly impact their growth. H929/CYR61 or H929/EV was engrafted in SCID-hu mice and their growth was restricted to the implanted bone area during the experimental period. BMD in bones engrafted with H929/CYR61 cells was reduced by only 4.62% ± 2.46%) from pretreatment level, but BMD of bones engrafted with H929/EV cells was reduced by 17.66% ± 4.37% from pretreatment levels (P < .05, H929/EV vs H929/CYR61 groups; Figure 6A).
Because CCN1 directly affects osteoblastogenesis and osteoclastogenesis,19,22 we evaluated the numbers of osteoblasts and osteoclasts in MM-bearing bones. Histologic analyses showed that surfaces of bones engrafted with H929/CYR61 cells had more osteocalcin-expressing osteoblasts and fewer multinucleated TRAP-expressing osteoclasts (P = .001) than those engrafted with H929/EV cells (Figure 6B). Although equal numbers of cells were injected into the implanted bones, hIg levels (marker for MM growth) in sera of H929/CYR61-bearing mice were lower than in sera of H929/EV-bearing mice (P < .05; Figure 6C). Taken together, CCN1 moderately attenuated in vivo growth of H929 MM cells directly and indirectly through its inhibitory effects on MM-induced bone disease.
To determine whether the CCN1 effect on MM bone disease may occur through αvβ3 signaling, as reported,19 we engrafted SCID-hu mice with H929 cells transduced with a CCN1 mutant harboring a D125A substitution (ie, H929/D125A cells) that prevents it from binding to αvβ3,20 along with engraftment of H929/EV and H929/CYR61 cells (Figure G-J). Lack of CCN1 binding to αvβ3 results in inhibition of αvβ3-mediated activities, including cell adhesion, stimulation of cell migration, and enhancement of DNA synthesis.20 Furthermore, CCN1-mediated upregulation of BMP-2, resulting in enhanced cell proliferation and osteoblastic differentiation, is abrogated in the presence of αvβ3 neutralizing antibodies.19 At the end of the experiment, BMD in bones engrafted with H929/D125A or H929/EV cells was lower than that of bones engrafted with H292/CYR61 cells (Figure 6G), indicating that inhibition of CCN1 interaction with αvβ3 integrin attenuated CCN1-mediated reduction of osteolysis. The surfaces of bones engrafted with H929/CYR61 cells had more osteocalcin-expressing osteoblasts and fewer multinucleated TRAP-expressing osteoclasts (P = .008) than those engrafted with H929/EV or H929/D125A cells (Figure 6H). Radiographic analysis of the bones showed that, as expected, bones injected with H929/EV control cells were severely resorbed, as were those injected with H929/D125A; in contrast, bones injected with H929/CYR61 cells were preserved (Figure 6J). Reduced tumor growth was also attenuated by inhibiting αvβ3 binding by CYR61 (Figure 6I), underlining the important role of αvβ3 integrin in mediating CCN1 activities in myelomatous bone.
Discussion
We showed that CYR61, which encodes the secreted protein CCN1, is mainly expressed by mesenchymal cells and is overexpressed in the BM of a subset of patients with MGUS, AMM, and MM. CYR61 expression is significantly lower in focal lesions compared with random BM, reflecting alteration of osteoblast/MSC numbers or properties in focal lesions.
Elevated CCN1 is associated with longer times for MGUS/AMM to progress to overt MM. Overexpression of CYR61 or high CCN1 level at diagnosis is each an independent prognostic factor associated with superior progression-free and overall survival and with sustained remission of MM patients enrolled in the TT3 protocol. High CCN1 levels during remission were associated with superior progression-free and overall survival and stratified patients with GEP70-defined high-risk MM. Exogenous CCN1 inhibited in vitro growth of MM cells and overexpression of CYR61 in MM cells delayed tumor growth and attenuated bone destruction in vivo. Collectively, these data suggest that therapies that induce CCN1 may help control MM bone disease and that factors such as CCN1, decorin,31 and adiponectin32 that are expressed in the BM microenvironment and are involved in bone remodeling may be used to predict outcome and progression of MM patients.
Forced expression of CYR61 in H929 cells had no effect on their in vitro growth, but growth was inhibited when relatively high concentrations of recombinant CCN1 were added to culture medium. Similarly, a stroma-dependent MM line grown in coculture with MSCs that secrete low levels of CCN1 (<10 ng/mL) was inhibited by addition of recombinant CCN1 in high concentrations (>100 ng/mL). In contrast, in SCID-hu mice, forced expression of CYR61 in engrafted H929 cells resulted in reduced bone disease, suggesting that CCN1 is a microenvironmental factor influenced by the presence of and conditions induced by MM and that expression of CYR61/CCN1 has an effect on disease progression. Because the growth characteristics of H929/EV compared with H929/CYR61 showed no difference in vitro, it is unlikely the tumors had different growth characteristics in vivo, leading to the observed results. As further evidence, the D125A mutant cells and the control H929/EV cells produced similar results on BMD, osteoblast and osteoclast numbers, and tumor growth in our experimental model.
Understanding the role of the microenvironment in progression from MGUS/AMM to MM and achieving rapid, sustained long-term CR is clinically important. MM cells from patients with MGUS/AMM possess cytogenetic abnormalities and molecular subsets typically identified in MM patients,26 yet only approximately 1% of MGUS and 10% of AMM cases progress to MM every year.33,34 This supports the notion that, in addition to MM burden parameters (eg, M-protein and free-light chain levels, percent BM plasmacytosis, GEP70 risk scores),26,33,34 other host factors (eg, reduced circulating adiponectin levels,32 suppression of uninvolved immunoglobulins,26,35 other immune cell components36 ) are also associated with and may contribute to disease transformation in these patients. We now provide evidence that lower expression of CYR61 and BM plasma levels of CCN1 are additional risk factors for progression of MGUS and AMM to MM. Our findings that CCN1 levels are not significantly correlated with other risk parameters that predict MGUS/AMM progression supports reports indicating poor concordance between the current models and the need for a better definition of high-risk MGUS/AMM patients.34 In addition to incorporation of GEP70 and novel imaging,26 circulating CCN1 levels may serve as an additional, convenient, and independent risk parameter.
The role of the BM microenvironment in MM relapse is poorly understood. Higher CYR61 expression and CCN1 levels during CR suggest that the BM microenvironment remained abnormal in a significant number of patients who achieved CR. Our data also suggest that sustaining high levels of CCN1 during remission may contribute to the prolonged duration of CR and superior overall survival that is observed among these patients.23 Moreover, our findings that higher CCN1 levels were associated with superior survival of patients with molecularly defined high-risk MM suggest that CCN1 levels can be used to stratify these patients and that high CCN1 or induction of CCN1 production might be beneficial for patients with high-risk disease.
The observation that patients with MM have high levels of CCN1 is intriguing because CCN1 activity is associated with WNT signaling, which is thought to be suppressed in the MM BM microenvironment.37,38 CYR61 is transiently upregulated at the early stage of osteoblastogenesis during Wnt3a-induced osteogenesis and is indispensable for this process.15 WNT antagonist sclerostin directly binds CCN1,39 although the consequences on WNT signaling or bone formation are unclear. In nonmalignant conditions, CCN1 may enhance Wnt3a signaling to increase BMP2 and allow osteoblast differentiation to proceed.19 However, in MM, osteoblast differentiation is suppressed by osteoblast-deactivating factors, including WNT inhibitors (eg, DKK1, SFRP2)40,41 and other factors (eg, hepatocyte growth factor, Activin A),42,43 resulting in accumulation of osteoblast precursors and reactive MSCs. These cells may express CYR61 as a compensatory mechanism against MM, along with factors that promote osteoclastogenesis (eg, RANKL)38 and MM growth (eg, IL-6).44 Lack of CYR61 overexpression in a subset of MM patients with inferior outcome could be a reflection of genetic and epigenetic alteration of stromal cells45 and/or reduced numbers of reactive MSCs and osteoblast precursors. Our current data and previous reports suggest that high levels of CCN1 induced by overexpression of CYR61 or WNT3A13 in MM cells or treatment with PTH14 overcome the suppressive effects of osteoblast-inactivating agents, resulting in induction of bone formation and prevention of bone disease. Furthermore, the pro-angiogenic properties46 and induction of cell adhesion by CCN117 may promote MM cell quiescence, resulting in prevention of disease reoccurrence. Indeed, our findings indicate that low CYR61/CCN1 may be predictive of MGUS/AMM progression to overt MM and of MM relapse. The paradoxical overexpression of CYR61/CCN1 in myelomatous bone of MM patients requires further investigation.
CCN1 function can vary, depending on integrin combinations and cell context. For example, CCN1 enhances osteoblastogenesis in a αvβ3/integrin linked kinase/extracellular signal-regulated kinase–dependent manner,19 but inhibits osteoclastogenesis independently of αvβ3 and αvβ5.22 With a mutated αVβ3-binding site construct of CYR61, we determined that CCN1 effects on osteolysis and its inhibitory effects on MM cell growth were transmitted via αVβ3. CCN1 has previously been demonstrated to inhibit formation of αvβ3-positive or TRAP-positive osteoclasts, reduce the expression of osteoclast phenotypic markers TRAP, matrix metalloproteinase-9, calcitonin receptor, and cathepsin K but did not affect the formation of multinucleated osteoclasts when added to osteoclast precursors or affect the number or resorptive activity of osteoclasts cultured on dentine discs, indicating that CCN1 affects early osteoclast precursors but not mature osteoclasts.22 Interestingly, when a mutant form of CCN1 defective in αvβ3 binding was used, CCN1 still directly inhibited osteoclastogenesis,22 suggesting that stimulation of osteoclastogenesis by H292/D125A cells in myelomatous bones was indirectly mediated by suppression of osteoblastogenesis.
In summary, we showed that CCN1, a small soluble factor with many different biological roles, is overexpressed in the BM microenvironment of patients with MGUS and MM. Its level is associated with disease progression, onset of CR, duration of CR, and overall survival. The exact function of this molecule in MM remains unclear, but we showed that it may have anti-MM and bone-anabolic effects; therefore, its upregulation is a protective response against the presence of MM. Our data also supports the notion that osteoblast-richer and MSC-poorer BM would be associated with better prognosis in MM. Modulating microenvironmental levels of CCN1 with osteoblast-activating factors such as Wnt3a and parathyroid hormone14,15 may be useful not only in treating MM but also in preventing MGUS from progressing to MM.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Peggy Brenner, PhD, Editor in the Life Sciences (Office of Grants and Scientific Publications, University of Arkansas for Medical Sciences), provided editorial assistance to the authors during preparation of this manuscript. The authors wish to thank the faculty, staff, and patients of the Myeloma Institute for Research and Therapy for their support.
This work was supported by a grant from the National Cancer Institute (CA55819).
Authorship
Contribution: S.K.J. performed the studies, conceptualized the work, analyzed and interpreted the data, and wrote the article; J.P.S. performed in vitro studies and cell transfection; R.B. performed in vitro studies; P.Q. analyzed the data and performed statistical analyses; J.D.S. performed gene expression profiling; J.E. helped design the experimental studies and analyzed and interpreted the data; B.B. and F.v.R. provided patient materials and interpreted the data; and S.Y. designed and performed the research, conceptualized the work, analyzed and interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: J.D.S. is the founder of Myeloma Health, LLC, a genomics-based predictive medicine company; he holds stock options in Myeloma Health. The remaining authors declare no competing financial interests.
Correspondence: Sarah K. Johnson, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, 4301 W Markham #776, Little Rock, AR 72205; e-mail: johnsonsarah@uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal