Key Points
Presence of either ActR2a or BMPR2 in hepatocytes is sufficient to maintain hepatic hepcidin gene expression and iron metabolism.
Deficiency of both BMP type II receptors in hepatocytes induces iron overload.
Abstract
Expression of hepcidin, the hepatic hormone controlling iron homeostasis, is regulated by bone morphogenetic protein (BMP) signaling. We sought to identify which BMP type II receptor expressed in hepatocytes, ActR2a or BMPR2, is responsible for regulating hepcidin gene expression. We studied Bmpr2 heterozygous mice (Bmpr2+/−), mice with hepatocyte-specific deficiency of BMPR2, mice with global deficiency of ActR2a, and mice in which hepatocytes lacked both BMPR2 and ActR2a. Hepatic hepcidin messenger RNA (mRNA) levels, serum hepcidin and iron levels, and tissue iron levels did not differ in wild-type mice, Bmpr2+/− mice, and mice in which either BMPR2 or ActR2a was deficient. Deficiency of both BMP type II receptors markedly reduced hepatic hepcidin gene expression and serum hepcidin levels leading to severe iron overload. Iron injection increased hepatic hepcidin mRNA levels in mice deficient in either BMPR2 or ActR2a, but not in mice deficient in both BMP type II receptors. In addition, in mouse and human primary hepatocytes, deficiency of both BMPR2 and ActR2a profoundly decreased basal and BMP6-induced hepcidin gene expression. These results suggest that BMP type II receptors, BMPR2 and ActR2a, have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism.
Introduction
The hepatic hormone, hepcidin, is a critical regulator of iron metabolism. Hepcidin binds to ferroportin, the exclusive iron exporter localized on the membranes of enterocytes, macrophages, and hepatocytes.1 Once bound, hepcidin induces the degradation and internalization of ferroportin, leading to intracellular sequestration of iron.2 An excess of hepcidin decreases iron absorption and induces retention of iron in macrophages, leading to iron deficiency, a reduction of iron available for erythropoiesis, and eventually anemia (eg, iron refractory iron deficiency anemia).1-3 On the other hand, hepcidin deficiency induces iron overload (eg, hemochromatosis).4,5
Hepcidin synthesis occurs predominantly in hepatocytes and is primarily regulated at the level of transcription.1,6 Hepcidin gene expression is increased by iron, inflammation, and bone morphogenetic protein (BMP) signaling, and it is decreased by anemia and hypoxia.1,6-8 BMP6 is thought to be the predominant BMP ligand regulating hepatic hepcidin gene expression, and synthesis of BMP6 is increased in response to elevated serum iron levels.9-11 BMP ligands act by binding to a heteromeric receptor composed of BMP type I receptors (ALK1, ALK2, ALK3, or ALK6) and BMP type II receptors (BMPR2, ActR2a, or ActR2b). Binding of BMPs induces BMP type II receptors to phosphorylate and activate BMP type I receptors, which, in turn, phosphorylate cytoplasmic regulatory SMADs (SMADs 1, 5, and 8).12 Phosphorylated SMADs bind to SMAD4, translocate to the nucleus, and activate the transcription of target genes including those encoding the inhibitor of DNA binding 1 (Id1) and hepcidin. We have previously shown that, in mice, hepatocyte-specific deletion of ALK2 and ALK3 induces moderate and severe iron overload, respectively, and that both receptors are required to respond to BMP2, in vitro, and to increased iron levels, in vivo.13
Of the 3 BMP type II receptors, ActR2a and BMPR2 are expressed in the liver.14,15 Xia et al16 reported that, in vitro, hemojuvelin activated BMP signaling and hepcidin gene expression, preferentially through ActR2a. However, the role of BMP type II receptors in the regulation of hepatic hepcidin gene expression remains incompletely elucidated.
Patients with idiopathic pulmonary arterial hypertension (IPAH) often develop unexplained iron deficiency.15,17-19 Iron-deficient IPAH patients have lower cardiac indices, reduced exercise tolerance, and higher pulmonary arterial resistances compared with IPAH patients with normal serum iron levels.17,19 The iron deficiency reported in IPAH patients is associated with inappropriately high levels of circulating hepcidin.17,18 A quarter of IPAH patients harbor heterozygous germline mutations of BMPR2.20 Soon et al18 reported that the prevalence of iron deficiency was higher in IPAH patients who carry BMPR2 mutations, suggesting the possibility that BMPR2 deficiency could lead to the abnormal regulation of hepcidin expression and iron deficiency.
We sought to characterize the contributions of BMPR2 and ActR2a to the regulation of hepatic hepcidin gene expression and iron metabolism. In BMPR2 heterozygous mice, mice with hepatocyte-specific deficiency of BMPR2, mice with global deficiency of ActR2a, and mice deficient in both BMP type II receptors, we measured serum and tissue iron levels, serum hepcidin levels, and hepatic hepcidin gene expression. In addition, we measured hepcidin messenger RNA (mRNA) levels at baseline and after stimulation with BMP6 in cultured primary mouse and human hepatocytes with reduced BMPR2 and/or ActR2a mRNA levels. We report that, in mice, the presence of either ActR2a or BMPR2 in hepatocytes is sufficient to maintain normal serum and tissue iron levels and hepcidin synthesis. In contrast, deficiency of both BMP type II receptors dramatically reduces hepcidin mRNA levels in vitro and in vivo and induces marked iron overload in vivo.
Methods
Animals
All mouse experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
We studied the phenotype of mice carrying a mutant Bmpr2 allele (Bmpr2+/−), on a C57BL/6 background.21,22
Bmpr2fl/fl mice23 were bred to B6.Cg-Tg(Alb-Cre)21Mgn/J mice (The Jackson Laboratory) to obtain animals with or without the Alb-Cre transgene, all on a C57BL/6 background.
Mice with global deficiency of ActR2a (ActR2a−/−)24 were bred with Bmpr2fl/fl mice carrying 1 copy of the Alb-Cre transgene to obtain ActR2a−/−;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice on a mixed Sv129/C57BL/6 background.
Female mice fed a standard, iron-replete diet (Prolab 5P75 Isopro 3000, 380 ppm iron) were used for measurement of basal serum hepcidin and iron levels, tissue iron levels, and hepatic hepcidin gene expression.
Iron challenge
Male mice, Bmpr2fl/fl, Bmpr2fl/fl;Alb-Cre, ActR2a+/+;Bmpr2fl/fl, ActR2a−/−;Bmpr2fl/fl, and ActR2a−/−;Bmpr2fl/fl;Alb-Cre were studied after 2 weeks on a low-iron diet (Teklab, 2-6 ppm iron). Mice were injected via the tail vein with iron-dextran or dextran (0.2 g/kg; Sigma-Aldrich). Four hours after injection, blood was collected, mice were euthanized, and liver was harvested.
Iron parameters
Blood was obtained by retro-orbital puncture. Serum iron levels and transferrin saturations were determined using the Iron/UIBC Kit (Genzyme) following the manufacturer’s protocol.
After euthanasia, liver and spleen tissues were harvested, and nonheme tissue iron was measured, as previously described.13
Mouse serum hepcidin-1 levels were measured using the Hepcidin Elisa Kit, following the manufacturer’s instructions (Intrinsic LifeSciences).
Prussian blue staining
Livers and spleens were fixed in 10% formalin and embedded in paraffin. Deparaffinized tissue sections were stained with Prussian blue and counterstained with nuclear fast red (Polysciences). Sections were photographed by light microscopy with a Nikon Eclipse 80i microscope (Nikon Instruments).
Mouse primary hepatocytes
Isolation of hepatocytes from livers of ActR2a+/+;Bmpr2fl/fl, ActR2a+/+;Bmpr2fl/fl;Alb-Cre, ActR2a−/−;Bmpr2fl/fl, and ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice was performed by the Cell Resource Core at the Shriners Hospitals for Children (Boston, MA), using a 2-step collagenase digestion.25 Cells were plated in 6-well, collagen-coated plates and cultured in serum-free Williams’ Medium E. After 2 days, hepatocytes were incubated with BMP6 (10 ng/mL) for 30 minutes or 4 hours, after which cells were harvested to extract proteins or RNA, respectively.
Depletion of BMP type II receptors in human primary hepatocytes
Human primary hepatocytes were obtained from Gibco (Life Technologies) and cultured according to the manufacturer’s protocol. Four hours after plating, cells were cultured in serum-free Williams’ Medium E supplemented with hepatocyte maintenance supplement (Gibco). After 24 hours, cells were trypsinized and reverse-transfected with small interfering RNA (siRNA), using Lipofectamine RNAi-Max (Invitrogen). To deplete ActR2a and BMPR2, hepatocytes were transfected with corresponding siRNAs (30 nM; Silencer Select, and S980 and S2044, respectively [Ambion]). As a negative control, cells were transfected with a nontargeting siRNA (Silencer Select Negative Control No. 1; Ambion). Forty-eight hours after transfection, hepatocytes were incubated without or with BMP6 (10 ng/mL; R&D Systems) for 4 hours, after which cells were harvested to extract RNA.
Quantitation of mRNA levels
Total RNA was extracted from primary mouse hepatocytes and liver tissues using TRIzol (Invitrogen). Reverse RNA transcription was accomplished using MMLV-RT (Applied Biosystems). Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed on a Mastercycler Realplex2 (Eppendorf), using KAPA SYBR FAST (for SYBR primers) or KAPA PROBE FAST (for TaqMan primers). Levels of 18S ribosomal RNA and mRNAs encoding the TATA box-binding protein (TBP), hepcidin, BMPR2, ActR2a, Id1, ALK2, ALK3, BMP6, and the transferrin receptor protein 1 (Tfrc) were measured.
Total RNA was extracted from human primary hepatocytes and reverse transcribed using the Cell-to-CT Kit (Ambion). qRT-PCR was performed using Cell-to-CT TaqMan reagents. Levels of mRNAs encoding TBP, hepcidin, BMPR2, and ActR2a were measured.
Sequences of the primers used for qRT-PCR are listed in supplemental Table 1. Changes in the relative gene expression normalized to levels of 18S ribosomal RNA or TBP mRNA were determined using the relative cycle threshold method.
Statistical methods
All values were expressed as mean ± standard deviation. When applicable, data were analyzed using the Student t test or 1-way analysis of variance (ANOVA) with post hoc testing using the least-squares method. Statistical significance was considered for P < .05.
Results
Mice heterozygous for a BMPR2 mutation have normal hepcidin mRNA and iron levels
Iron deficiency in IPAH patients is common and associated with increased levels of circulating hepcidin.15,18 Germline mutations of BMPR2 are reported in IPAH patients with a decrease of BMPR2 expression in the lung.26 Although levels of BMPR2 expression in the liver of IPAH patients are unknown, we hypothesized that BMPR2 haploinsufficiency could lead to abnormal regulation of hepcidin gene expression. Therefore, we studied the phenotype of mice globally deficient in 1 copy of the Bmpr2 gene (Bmpr2+/− mice).21 As expected, hepatic BMPR2 mRNA levels in Bmpr2+/− mice were 50% of those in wild-type mice (Bmpr2+/+; supplemental Figure 1A, available at the Blood Web site). Hepatic hepcidin gene expression and serum and liver iron levels did not differ in Bmpr2+/− and Bmpr2+/+ mice (supplemental Figure 1B-D). These results suggest that, in mice, Bmpr2 haploinsufficiency does not alter hepatic hepcidin gene expression or iron metabolism.
Hepatocyte-specific deletion of BMPR2 does not cause iron overload
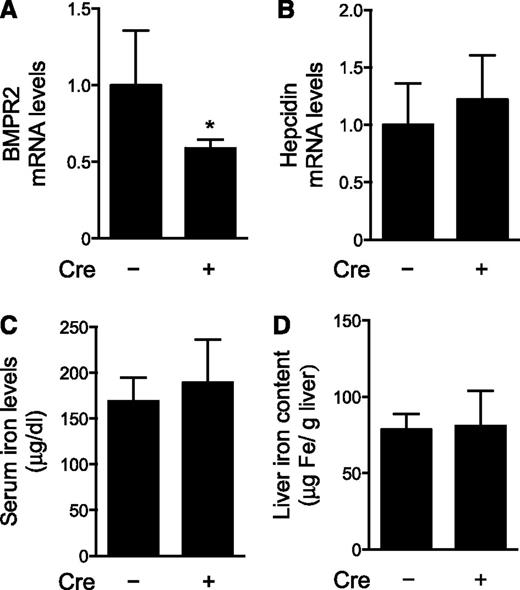
Because BMPR2 has multiple roles in early embryogenesis, mice that are globally deficient for BMPR2 do not survive past gastrulation.22 To further investigate the role of BMPR2 in the regulation of hepatic hepcidin gene expression, we generated mice homozygous for the Bmpr2 alleles flanked by loxP sequences (Bmpr2fl/fl), which also carry a transgene specifying Cre recombinase under the control of the hepatocyte-specific albumin gene promoter (Alb-Cre). Mice with hepatocyte-specific deficiency of BMPR2 (Bmpr2fl/fl;Alb-Cre) were compared with Bmpr2fl/fl littermates that do not carry the Alb-Cre transgene. Bmpr2fl/fl;Alb-Cre mice had reduced hepatic BMPR2 mRNA levels (Figure 1A). Residual hepatic BMPR2 mRNA levels in Bmpr2fl/fl;Alb-Cre mice may be attributable, in part, to liver cell types other than hepatocytes, such as endothelial cells. Hepatic hepcidin mRNA levels, serum hepcidin levels, and serum and liver iron concentrations did not differ in Bmpr2fl/fl;Alb-Cre and Bmpr2fl/fl mice (Figure 1B-D and supplemental Figure 2A). Additionally, ActR2a, BMP6, and Id1 mRNA levels did not differ between Bmpr2fl/fl;Alb-Cre and Bmpr2fl/fl mice (supplemental Figure 3). Similar Id1 mRNA levels in mice with or without hepatocyte-specific deletion of BMPR2 suggest that basal BMP signaling is preserved in BMPR2-deficient hepatocytes. Taken together, these results suggest that, in mice, deficiency of BMPR2 in hepatocytes does not alter basal BMP signaling, hepatic hepcidin synthesis, or iron metabolism. Moreover, in response to hepatocyte-specific deficiency of BMPR2, there is no compensatory increase in ActR2a or BMP6 mRNA levels.
Hepatocyte-specific deficiency of BMPR2 does not induce iron overload. Hepatic levels of mRNAs encoding BMPR2 (A) and hepcidin (B), as well as serum iron levels (C) and liver iron content (D), were measured in 10-week-old female mice with hepatocyte-specific deficiency of BMPR2 (Bmpr2fl/fl;Alb-Cre, n = 7) and age-matched control mice (Bmpr2fl/fl, n = 12). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. Student t test, *P < .009 vs Bmpr2fl/fl mice.
Hepatocyte-specific deficiency of BMPR2 does not induce iron overload. Hepatic levels of mRNAs encoding BMPR2 (A) and hepcidin (B), as well as serum iron levels (C) and liver iron content (D), were measured in 10-week-old female mice with hepatocyte-specific deficiency of BMPR2 (Bmpr2fl/fl;Alb-Cre, n = 7) and age-matched control mice (Bmpr2fl/fl, n = 12). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. Student t test, *P < .009 vs Bmpr2fl/fl mice.
Global deficiency of ActR2a does not alter hepcidin gene expression
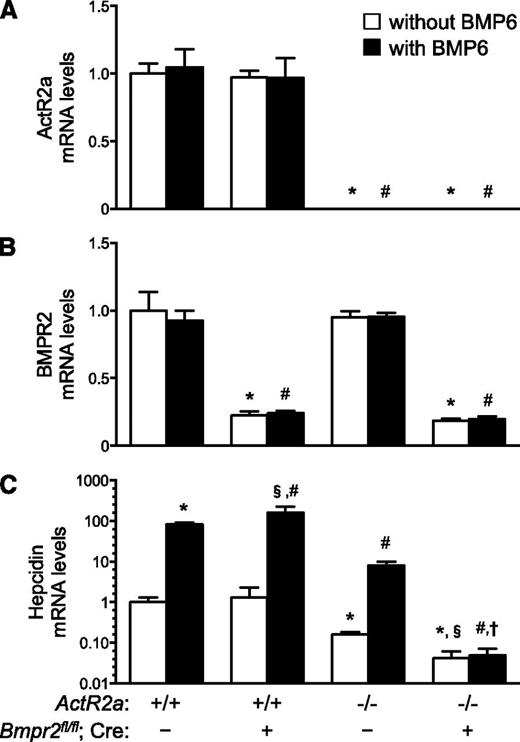
To assess the role of ActR2a in the regulation of hepatic hepcidin gene expression, we studied the phenotype of mice that are globally deficient for ActR2a (ActR2a−/−). Because ActR2a−/− female mice on a C57BL/6 background are sterile, we maintained ActR2a−/− mice on an Sv129 background.24 As a first step toward producing mice lacking both BMP type II receptors in hepatocytes, we generated ActR2a−/−;Bmpr2fl/fl mice by breeding ActR2a−/− mice with Bmpr2fl/fl mice. We compared ActR2a−/−;Bmpr2fl/fl mice with their ActR2a+/+;Bmpr2fl/fl littermates. ActR2a mRNA levels were not detected in the livers of ActR2a−/−;Bmpr2fl/fl mice (Figure 2A). Hepatic BMPR2 and BMP6 mRNA levels did not differ between mice with or without global deficiency of ActR2a (Figure 2B and supplemental Figure 4C). Hepatic hepcidin gene expression (Figure 2C), serum hepcidin levels (supplemental Figure 2B), serum iron levels, transferrin saturations, and liver and spleen iron contents (Figure 3) were similar in ActR2a−/−;Bmpr2fl/fl and ActR2a+/+;Bmpr2fl/fl mice. Hepatic Id1 mRNA levels did not differ between ActR2a−/−;Bmpr2fl/fl and ActR2a+/+;Bmpr2fl/fl mice (Figure 2D), suggesting that basal BMP signaling is preserved in ActR2a-deficient hepatocytes. Taken together, these results suggest that, in mice, ActR2a deficiency does not alter basal hepatic BMP signaling, hepatic hepcidin synthesis, or iron metabolism. Moreover, in response to ActR2a deficiency, there is no compensatory increase in hepatic BMPR2 or BMP6 mRNA levels.
Hepatic hepcidin gene expression is preserved in mice with global deficiency of ActR2a but is markedly reduced in mice lacking both ActR2a and BMPR2. Hepatic levels of mRNAs encoding ActR2a (A), BMPR2 (B), hepcidin (C), and Id1 (D) were measured in 10- to 12-week-old female mice, globally deficient for ActR2a, without or with hepatocyte-specific deficiency of BMPR2 (ActR2a−/−;Bmpr2fl/fl [n = 23] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 13], respectively), as well as control mice (ActR2a+/+;Bmpr2fl/fl [n = 7]). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. ANOVAs P < .009; *P < .0001 vs ActR2a+/+;Bmpr2fl/fl mice; #P < .003 vs ActR2a−/−;Bmpr2fl/fl mice; §P < .05 vs ActR2a+/+;Bmpr2fl/fl mice.
Hepatic hepcidin gene expression is preserved in mice with global deficiency of ActR2a but is markedly reduced in mice lacking both ActR2a and BMPR2. Hepatic levels of mRNAs encoding ActR2a (A), BMPR2 (B), hepcidin (C), and Id1 (D) were measured in 10- to 12-week-old female mice, globally deficient for ActR2a, without or with hepatocyte-specific deficiency of BMPR2 (ActR2a−/−;Bmpr2fl/fl [n = 23] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 13], respectively), as well as control mice (ActR2a+/+;Bmpr2fl/fl [n = 7]). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. ANOVAs P < .009; *P < .0001 vs ActR2a+/+;Bmpr2fl/fl mice; #P < .003 vs ActR2a−/−;Bmpr2fl/fl mice; §P < .05 vs ActR2a+/+;Bmpr2fl/fl mice.
Deficiency of both ActR2a and BMPR2 in hepatocytes induces iron overload. Serum iron levels (A) and transferrin saturations (B), as well as liver (C) and spleen (D) iron content, were measured in 10- to 12-week-old female control mice (ActR2a+/+;Bmpr2fl/fl [n = 7]) and mice globally deficient for ActR2a without or with hepatocyte-specific deletion of BMPR2 (ActR2a−/−;Bmpr2fl/fl [n = 23] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 13], respectively). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. ANOVAs P < .0001; *P < .0001 vs ActR2a+/+;Bmpr2fl/fl mice; #P < .0001 vs ActR2a−/−;Bmpr2fl/fl mice.
Deficiency of both ActR2a and BMPR2 in hepatocytes induces iron overload. Serum iron levels (A) and transferrin saturations (B), as well as liver (C) and spleen (D) iron content, were measured in 10- to 12-week-old female control mice (ActR2a+/+;Bmpr2fl/fl [n = 7]) and mice globally deficient for ActR2a without or with hepatocyte-specific deletion of BMPR2 (ActR2a−/−;Bmpr2fl/fl [n = 23] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 13], respectively). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. ANOVAs P < .0001; *P < .0001 vs ActR2a+/+;Bmpr2fl/fl mice; #P < .0001 vs ActR2a−/−;Bmpr2fl/fl mice.
Deficiency of both ActR2a and BMPR2 in hepatocytes decreases hepcidin gene expression
As deficiency of either ActR2a or BMPR2 in hepatocytes did not alter hepcidin gene expression, we tested whether absence of both BMP type II receptors would reduce hepatic hepcidin mRNA levels. We generated mice globally deficient in ActR2a with hepatocyte-specific deficiency of BMPR2 (ActR2a−/−;Bmpr2fl/fl;Alb-Cre). ActR2a mRNA levels were not detected in the livers of ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice, and BMPR2 mRNA levels were less than those in ActR2a+/+;Bmpr2fl/fl mice (Figure 2A-B). Deficiency of both ActR2a and BMPR2 was associated with a profound decrease in hepatic hepcidin gene expression (Figure 2C) and serum hepcidin levels (supplemental Figure 2B), as well as a reduction in Id1 mRNA levels (Figure 2D). The expression of the genes encoding the BMP type I receptors expressed in the liver, ALK2 and ALK3, did not differ in ActR2a−/−;Bmpr2fl/fl;Alb-Cre and ActR2a+/+;Bmpr2fl/fl mice (supplemental Figure 4A-B). These results suggest that the absence of both BMP type II receptors in hepatocytes reduces BMP signaling leading to a marked decrease of hepatic hepcidin synthesis.
Hepatocyte deficiency of ActR2a and BMPR2 induces iron overload
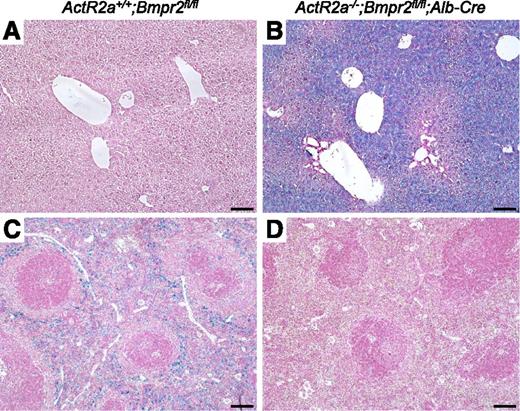
We measured the impact of the deficiency of both BMP type II receptors on iron metabolism. ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice had severe iron overload with increased serum iron levels, transferrin saturations, and liver iron contents (Figure 3A-C). Prussian blue staining of liver sections of ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice showed iron accumulation predominantly in centrilobular areas (Figure 4A-B). In contrast, lack of hepcidin decreased macrophage iron levels, as demonstrated by decreased spleen iron content (Figure 3D), and reduced Prussian blue staining in spleen tissue sections (Figure 4C-D). As has been described in other states of iron overload associated with hepcidin deficiency,13,27 we found increased hepatic BMP6 mRNA levels and reduced Tfrc gene expression in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice (supplemental Figure 4C-D). These results suggest that the profound decrease of hepatic hepcidin synthesis caused by the absence of ActR2a and BMPR2 in hepatocytes induces severe iron overload.
Iron accumulation in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. Livers (A-B) and spleens (C-D) from ActR2a+/+;Bmpr2fl/fl (A,C) and ActR2a−/−;Bmpr2fl/fl;Alb-Cre (B,D) mice were fixed in formalin, paraffin embedded, sectioned, and stained with Prussian blue. Sections were prepared from at least 4 animals in each group, and representative photomicrographs are shown. Scale bars represent 100 µm.
Iron accumulation in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. Livers (A-B) and spleens (C-D) from ActR2a+/+;Bmpr2fl/fl (A,C) and ActR2a−/−;Bmpr2fl/fl;Alb-Cre (B,D) mice were fixed in formalin, paraffin embedded, sectioned, and stained with Prussian blue. Sections were prepared from at least 4 animals in each group, and representative photomicrographs are shown. Scale bars represent 100 µm.
Iron challenge in mice deficient in BMPR2 and/or ActR2a in hepatocytes
Injection of iron increases hepatic hepcidin mRNA levels in a BMP-dependent manner.13,28 To test whether iron challenge requires BMPR2 to induce hepcidin gene expression, we measured hepatic hepcidin mRNA levels in male mice with and without hepatocyte-specific deficiency of BMPR2, 4 hours after intravenous injection of iron-dextran or dextran, as a control. In pilot studies, we observed that the induction of hepcidin gene expression in response to iron-dextran was similar in age-matched male and female mice. Compared with injection of dextran, injection of iron-dextran markedly increased serum iron levels (supplemental Figure 5A) associated with an induction of hepatic hepcidin gene expression in both Bmpr2fl/fl;Alb-Cre and Bmpr2fl/fl mice (Figure 5A).
Iron does not induce hepatic hepcidin gene expression in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. (A) Male mice 10 to 12 weeks old with hepatocyte-specific deficiency of BMPR2 (Bmpr2fl/fl;Alb-Cre, n = 8) and control mice (Bmpr2fl/fl, n = 11) received an intravenous injection of dextran or iron-dextran (0.2 g/kg). After 4 hours, mice were sacrificed, livers were harvested, and RNA was extracted to measure hepcidin mRNA levels. ANOVAs P < .0001; *P < .0001 vs Bmpr2fl/fl mice injected with dextran; #P < .0001 vs Bmpr2fl/fl;Alb-Cre mice injected with dextran. (B) Male mice 10 to 12 weeks old globally deficient for ActR2a without or with hepatocyte-specific BMPR2 deficiency (ActR2a−/−;Bmpr2fl/fl [n = 10] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 11], respectively) and control mice (ActR2a+/+;Bmpr2fl/fl [n = 8]) received an intravenous injection of dextran or iron-dextran (0.2 g/kg). After 4 hours, mice were sacrificed, livers were harvested, and RNA was extracted to measure hepcidin mRNA levels. Hepcidin mRNA levels in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice injected with dextran or iron-dextran are highlighted in an inset. ANOVAs P < .008; *P < .009 vs ActR2a+/+;Bmpr2fl/fl mice injected with dextran; #P < .008 vs ActR2a−/−;Bmpr2fl/fl mice injected with dextran; †P < .05 vs ActR2a+/+;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl mice, injected with dextran; ††P < .001 vs ActR2a+/+;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl mice, challenged with iron-dextran.
Iron does not induce hepatic hepcidin gene expression in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. (A) Male mice 10 to 12 weeks old with hepatocyte-specific deficiency of BMPR2 (Bmpr2fl/fl;Alb-Cre, n = 8) and control mice (Bmpr2fl/fl, n = 11) received an intravenous injection of dextran or iron-dextran (0.2 g/kg). After 4 hours, mice were sacrificed, livers were harvested, and RNA was extracted to measure hepcidin mRNA levels. ANOVAs P < .0001; *P < .0001 vs Bmpr2fl/fl mice injected with dextran; #P < .0001 vs Bmpr2fl/fl;Alb-Cre mice injected with dextran. (B) Male mice 10 to 12 weeks old globally deficient for ActR2a without or with hepatocyte-specific BMPR2 deficiency (ActR2a−/−;Bmpr2fl/fl [n = 10] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 11], respectively) and control mice (ActR2a+/+;Bmpr2fl/fl [n = 8]) received an intravenous injection of dextran or iron-dextran (0.2 g/kg). After 4 hours, mice were sacrificed, livers were harvested, and RNA was extracted to measure hepcidin mRNA levels. Hepcidin mRNA levels in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice injected with dextran or iron-dextran are highlighted in an inset. ANOVAs P < .008; *P < .009 vs ActR2a+/+;Bmpr2fl/fl mice injected with dextran; #P < .008 vs ActR2a−/−;Bmpr2fl/fl mice injected with dextran; †P < .05 vs ActR2a+/+;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl mice, injected with dextran; ††P < .001 vs ActR2a+/+;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl mice, challenged with iron-dextran.
To test whether iron requires ActR2a or both BMP type II receptors to induce hepcidin gene expression, we measured hepatic hepcidin mRNA levels in ActR2a+/+;Bmpr2fl/fl, ActR2a−/−;Bmpr2fl/fl, and ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice after intravenous injection of dextran or iron-dextran. Compared with injection of dextran, injection of iron-dextran markedly increased serum iron levels (supplemental Figure 5B) associated with an induction of hepatic hepcidin gene expression in both ActR2a+/+;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl mice (Figure 5B). In contrast, iron-dextran injection increased serum iron levels but did not increase hepatic hepcidin mRNA levels in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice (Figure 5B and supplemental Figure 5B).
Taken together, these results suggest that ActR2a and BMPR2 have redundant roles in the induction of hepatic hepcidin gene expression by iron.
The ability of BMP signaling to induce hepcidin gene expression is blocked in mouse primary hepatocytes deficient in both BMPR2 and ActR2a
To further assess the roles of BMP type II receptors in the regulation of hepatic hepcidin gene expression, we studied the ability of BMP6 to increase hepcidin mRNA levels in primary hepatocytes isolated from livers of ActR2a+/+;Bmpr2fl/fl, ActR2a+/+;Bmpr2fl/fl;Alb-Cre, ActR2a−/−;Bmpr2fl/fl, and ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice (Figure 6). As expected, BMPR2 and ActR2a mRNA levels were decreased in hepatocytes isolated from mice with hepatocyte-specific deficiency of BMPR2 and global deficiency of ActR2a, respectively. Basal hepcidin mRNA levels were not altered in BMPR2-deficient hepatocytes, decreased in ActR2a-deficient hepatocytes, and markedly decreased in hepatocytes deficient in both BMP type II receptors. The ability of BMP6 to induce hepcidin gene expression was preserved in hepatocytes deficient in either BMP type II receptor and was blocked in hepatocytes deficient in both BMP type II receptors. After incubation with BMP6, hepcidin mRNA levels were greater in BMPR2-deficient hepatocytes and less in ActR2a-deficient hepatocytes than in control hepatocytes.
In mouse primary hepatocytes, deficiency of both ActR2a and BMPR2 markedly decreased basal hepcidin mRNA levels and blocked the ability of BMP6 to induce hepcidin gene expression. Hepatocytes were isolated from livers of ActR2a+/+;Bmpr2fl/fl, ActR2a+/+;Bmpr2fl/fl;Alb-Cre, ActR2a−/−;Bmpr2fl/fl, and ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. Two days later, cells were incubated without or with BMP6 (10 ng/ml) for 4 hours. RNA was extracted to measure the expression of the genes encoding ActR2a (A), BMPR2 (B), and hepcidin (C, with a logarithmic y-axis). ANOVAs P < .002; *P < .03 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl mice, not stimulated with BMP6; §P < .003 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl;Alb-Cre mice, not stimulated with BMP6; #P < .03 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl mice, stimulated with BMP6; †P < .001 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl;Alb-Cre mice, stimulated with BMP6.
In mouse primary hepatocytes, deficiency of both ActR2a and BMPR2 markedly decreased basal hepcidin mRNA levels and blocked the ability of BMP6 to induce hepcidin gene expression. Hepatocytes were isolated from livers of ActR2a+/+;Bmpr2fl/fl, ActR2a+/+;Bmpr2fl/fl;Alb-Cre, ActR2a−/−;Bmpr2fl/fl, and ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. Two days later, cells were incubated without or with BMP6 (10 ng/ml) for 4 hours. RNA was extracted to measure the expression of the genes encoding ActR2a (A), BMPR2 (B), and hepcidin (C, with a logarithmic y-axis). ANOVAs P < .002; *P < .03 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl mice, not stimulated with BMP6; §P < .003 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl;Alb-Cre mice, not stimulated with BMP6; #P < .03 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl mice, stimulated with BMP6; †P < .001 vs hepatocytes from ActR2a+/+;Bmpr2fl/fl;Alb-Cre mice, stimulated with BMP6.
In the absence of BMPR2, the persistence of BMP signaling has been extensively described.29,30 To study the impact of ActR2a deficiency, alone or combined with BMPR2 deficiency, on BMP signaling, we measured the levels of Smad1/5 phosphorylation in primary mouse hepatocytes isolated from livers of ActR2a+/+;Bmpr2fl/fl, ActR2a−/−;Bmpr2fl/fl, and ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice, incubated without or with BMP6 (supplemental Figure 6). As anticipated, the ability of BMP6 to induce Smad1/5 phosphorylation was preserved in ActR2a-deficient hepatocytes but abrogated in hepatocytes deficient in both ActR2a and BMPR2.
These results suggest that, in mouse primary hepatocytes, deficiency of both ActR2a and BMPR2 is required to maximally suppress basal hepcidin gene expression and abrogate responsiveness to BMP6 as well as BMP signaling.
The ability of BMP signaling to induce hepcidin gene expression is blocked in human primary hepatocytes deficient in both BMPR2 and ActR2a
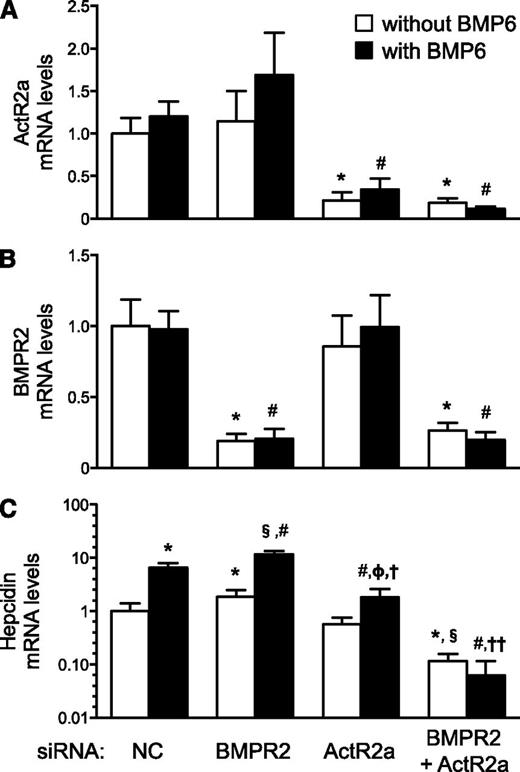
To confirm that the redundancy of BMP type II receptors is not species dependent and to exclude effects from lifelong receptor deficiency, we studied the ability of BMP6 to increase hepcidin mRNA levels in human primary hepatocytes, in which BMPR2 and/or ActR2a were depleted using siRNAs (Figure 7). Basal hepcidin mRNA levels were increased in BMPR2-depleted hepatocytes, not altered in ActR2a-depleted hepatocytes, and profoundly decreased in hepatocytes depleted in both BMP type II receptors. The ability of BMP6 to induce hepcidin gene expression was preserved in hepatocytes depleted in either BMP type II receptor but was blocked in hepatocytes depleted in both BMP type II receptors. After incubation with BMP6, hepcidin mRNA levels were greater in BMPR2-depleted hepatocytes and less in ActR2a-depleted hepatocytes than in hepatocytes treated with control siRNA. These results suggest that, in human primary hepatocytes, ActR2a and BMPR2 have redundant roles in the regulation of hepcidin gene expression. Moreover, the similarity of observations from human and mouse primary hepatocytes suggests that findings in mouse models deficient in BMP type II receptors are applicable to human pathophysiology.
In human primary hepatocytes, depletion of both ActR2a and BMPR2 markedly decreased basal hepcidin mRNA levels and blocked the ability of BMP6 to induce hepcidin gene expression. Human primary hepatocytes were transfected with nontargeting siRNA (siNC) or siRNAs directed against BMPR2, ActR2a, or both. Forty-eight hours later, cells were stimulated without or with BMP6 (10 ng/mL) for 4 hours. RNA was extracted to measure the expression of the genes encoding ActR2a (A), BMPR2 (B), and hepcidin (C, with a logarithmic y-axis). ANOVAs P < .0001; *P < .007 vs siNC without BMP6; #P < .0002 vs siNC with BMP6; §P < .0001 vs siBMPR2 without BMP6; ΦP < .05 vs siActR2a without BMP6; †P < .0001 vs siBMPR2 with BMP6; ††P < .005 vs siBMPR2 and siActR2a, with BMP6.
In human primary hepatocytes, depletion of both ActR2a and BMPR2 markedly decreased basal hepcidin mRNA levels and blocked the ability of BMP6 to induce hepcidin gene expression. Human primary hepatocytes were transfected with nontargeting siRNA (siNC) or siRNAs directed against BMPR2, ActR2a, or both. Forty-eight hours later, cells were stimulated without or with BMP6 (10 ng/mL) for 4 hours. RNA was extracted to measure the expression of the genes encoding ActR2a (A), BMPR2 (B), and hepcidin (C, with a logarithmic y-axis). ANOVAs P < .0001; *P < .007 vs siNC without BMP6; #P < .0002 vs siNC with BMP6; §P < .0001 vs siBMPR2 without BMP6; ΦP < .05 vs siActR2a without BMP6; †P < .0001 vs siBMPR2 with BMP6; ††P < .005 vs siBMPR2 and siActR2a, with BMP6.
Discussion
BMP signaling is a critical regulator of hepatic hepcidin gene expression, which requires the association of 2 BMP type I and 2 BMP type II receptors.12 Although the contributions of ALK2 and ALK3 to the regulation of hepcidin gene expression have been described,13,31 the roles of BMPR2 and ActR2a have remained unclear. We report that iron status is normal in Bmpr2 heterozygous mice, mice with hepatocyte-specific deficiency of BMPR2, and mice with a global deficiency of ActR2a. In contrast, mice lacking both BMP type II receptors in hepatocytes manifest markedly decreased hepatic hepcidin gene expression and serum hepcidin levels associated with severe iron overload. Injection of iron induced hepatic hepcidin gene expression in Bmpr2fl/fl;Alb-Cre and ActR2a−/− mice, but not in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. Moreover, in mouse and human primary hepatocytes, the ability of BMP6 to increase hepcidin mRNA levels was blocked in cells deficient in both BMP type II receptors. These results suggest that presence of either ActR2a or BMPR2 is sufficient for iron and BMP ligands to regulate hepatic hepcidin gene expression.
To investigate the role of BMPR2 haploinsufficiency in the development of iron deficiency seen in patients with IPAH, we studied Bmpr2 heterozygous mice. Hepatic hepcidin mRNA and serum and tissue iron levels did not differ in Bmpr2+/− and wild-type mice. These results do not support the hypothesis that BMPR2 haploinsufficiency, observed in IPAH patients, contributes to the development of iron deficiency.
To further investigate the role of BMPR2, one of the 2 BMP type II receptors expressed in the liver,14 in the regulation of hepcidin gene expression, we studied mice with hepatocyte-specific deficiency of BMPR2. Hepatic hepcidin gene expression, serum hepcidin and iron levels, and tissue iron levels were unchanged in the absence of BMPR2. Moreover, loss of BMPR2 in hepatocytes did not alter responsiveness of hepcidin gene expression to iron challenge. These results suggest that, in vivo, ActR2a alone is sufficient to maintain basal hepatic hepcidin gene expression and responsiveness to increased serum iron levels.
ActR2a is the second BMP type II receptor expressed in hepatocytes.14 Because hepatocyte-specific deficiency of BMPR2 did not alter hepcidin gene expression or iron levels, we studied the iron status in mice with global deficiency of ActR2a. Hepatic hepcidin gene expression, serum hepcidin and iron levels, and tissue iron levels were preserved in the absence of ActR2a. These results are consistent with the findings of Dussiot et al,32 who reported that administration of RAP011, an ActR2a ligand trap, to wild-type mice did not alter iron metabolism. We also observed that loss of ActR2a did not alter the ability of iron challenge to induce hepatic hepcidin gene expression. These results suggest that, in vivo, BMPR2 alone is sufficient to maintain basal hepatic hepcidin mRNA levels, and responsiveness to increased serum iron levels.
In isolated mouse and human primary hepatocytes, BMPR2 deficiency did not reduce basal hepcidin mRNA levels, and the ability of BMP6 to stimulate hepcidin gene expression was preserved. However, hepcidin mRNA levels were greater in BMPR2-deficient hepatocytes than in control hepatocytes incubated with BMP6. We chose to incubate primary hepatocytes with BMP6, because BMP6 has been reported to be the BMP ligand predominantly responsible for regulating hepatic hepcidin gene expression.1,9,33 Consistent with our findings, Rhodes and colleagues15 reported that the ability of BMP6 to induce hepcidin gene expression was increased in human hepatoma (HepG2) cells, in which BMPR2 was depleted using siRNA. The importance of BMPR2 in BMP ligand-specific signaling has previously been studied in mouse pulmonary arterial smooth muscle cells (PaSMCs). Yu and colleagues30 reported that responsiveness to BMP6, as well as the closely related BMP ligand BMP7, was markedly enhanced in mouse Bmpr2−/− PaSMCs. They suggested that, in the presence of BMPR2, BMP signaling via ActR2a was inhibited and that, in the absence of BMPR2, BMP6/7 responsiveness was enhanced because ActR2a has a higher affinity for these BMP ligands than does BMPR2.12,34
In isolated mouse and human hepatocytes, the ability of BMP6 to stimulate hepcidin gene expression was preserved in the absence of ActR2a. However, after incubation with BMP6, hepcidin mRNA levels were lower in ActR2a-deficient hepatocytes than in control hepatocytes. These observations are consistent with the findings of Baragova et al29 and Leyton et al,35 who reported that BMP7 signaling was decreased in ActR2a-deficient PaSMCs. The residual ability of ActR2a-deficient hepatocytes to respond to BMP6 suggests that BMPR2 can compensate, in part, for the absence of ActR2a.
Although incubation with BMP6 led to higher hepcidin mRNA levels in the absence of BMPR2 and lower levels in the absence of ActR2a in cultured hepatocytes compared with control hepatocytes, hepatic hepcidin gene expression, serum hepcidin levels, and iron metabolism were not altered in mice with deficiency of a single BMP type II receptor. Unlike in vitro experiments, hepcidin gene expression in vivo may be regulated by multiple stimuli, including BMP ligands other than BMP6. Moreover, whereas BMPR2 interacts specifically with BMPs, ActR2a can also bind to activins that regulate hepcidin gene expression.12,36
As the presence of one BMP type II receptor is sufficient to maintain iron homeostasis, we studied mice in which both ActR2a and BMPR2 were deficient in hepatocytes. Deficiency of both BMP type II receptors was associated with a marked decrease of hepatic hepcidin gene expression and serum hepcidin levels, as well as severe iron overload similar to that observed in other mouse models of iron overload induced by hepcidin deficiency.4,5 Loss of both ActR2a and BMPR2 abrogated responsiveness of hepatic hepcidin gene expression to iron challenge. Of note, these findings provide further support for the concept that iron induces hepcidin gene expression in a BMP-signaling–dependent manner.13,28 Findings in primary cultures of mouse and human hepatocytes were consistent with results obtained in vivo: deficiency of both BMP type II receptors profoundly decreased basal hepcidin mRNA levels and blocked the ability of BMP6 to induce hepcidin gene expression.
Taken together, these results suggest that ActR2a and BMPR2 have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism. The ability of either BMP type II receptor, alone, to maintain BMP signaling in hepatocytes is consistent with results reported in other cell types including mouse PaSMCs,29,30 human mesenchymal stem cells,37 and human pulmonary endothelial cells.38 Moreover, the redundancy of BMP type II receptors has also been described, in vivo, in retention and pigmentation of hair.39
The contribution of BMP type II receptors to BMP signaling and the regulation of hepcidin gene expression differs from that of BMP type I receptors. Hepatocyte-specific deficiency of either ALK2 or ALK3 reduces basal hepcidin gene expression, as well as responsiveness to increased iron levels and BMP ligands.13 We and others hypothesized that the 2 BMP type I receptors assemble together into heteromeric BMP receptor complexes.37,40 The redundancy of the BMP type II receptors in the regulation of hepatic hepcidin gene expression suggests the possibility that heteromeric BMP receptor complexes in hepatocytes may contain both BMP type I receptors and either one of the BMP type II receptors.
In summary, the presence of either BMP type II receptor, ActR2a or BMPR2, is sufficient to maintain hepatic hepcidin synthesis and iron homeostasis in mice. On the other hand, deficiency of both BMP type II receptors abrogates the ability of BMP6 in vitro or iron in vivo to increase hepcidin mRNA levels and markedly reduces basal hepcidin gene expression leading to iron overload.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bote Bruisna for his help in isolating mouse hepatocytes; Donald D. Bloch, Nicholas W. Morrell, Randall T. Peterson, and Paul B. Yu for their scientific advice; Rajeev Malhotra for his statistics expertise; and Mark Westerman, who provided the hepdicin-1 enzyme-linked immunosorbent assay kits.
This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01DK082971) and by a grant from the Fondation LeDucq.
Authorship
Contribution: C.M., P.A.L., and K.D.B. designed the research; C.M., P.A.L., S.A.K., and B.Y. performed the experiments; C.M., P.A.L., and K.D.B. analyzed the results; and C.M. and K.D.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claire Mayeur, Anesthesia Center for Critical Care Research, Massachusetts General Hospital, 55 Fruit St, Thier 503, Boston, MA 02114; e-mail: cmayeur@mgh.harvard.edu.
References
Author notes
C.M. and P.A.L. contributed equally to this study.

![Figure 2. Hepatic hepcidin gene expression is preserved in mice with global deficiency of ActR2a but is markedly reduced in mice lacking both ActR2a and BMPR2. Hepatic levels of mRNAs encoding ActR2a (A), BMPR2 (B), hepcidin (C), and Id1 (D) were measured in 10- to 12-week-old female mice, globally deficient for ActR2a, without or with hepatocyte-specific deficiency of BMPR2 (ActR2a−/−;Bmpr2fl/fl [n = 23] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 13], respectively), as well as control mice (ActR2a+/+;Bmpr2fl/fl [n = 7]). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. ANOVAs P < .009; *P < .0001 vs ActR2a+/+;Bmpr2fl/fl mice; #P < .003 vs ActR2a−/−;Bmpr2fl/fl mice; §P < .05 vs ActR2a+/+;Bmpr2fl/fl mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/13/10.1182_blood-2014-04-572644/4/m_2116f2.jpeg?Expires=1769155623&Signature=rCCtkrRUIvrd0CYSqOBUW9nrvDD9t7ydb080FanXjFK6AWH4GOkte8Mm47iX-5BixT8mVqrkfJaaXyiPKa~abPS-dsMoZ4JQu1I1Z6-sQRujXg9kYOBpc~EWvuvfLX~GMGe0-FMUr-41c8oveWVhwaK6HjN4Mbpv9f3hwxC4ZXn34vVfsznxp56lrhgQS6ybnhiQIcrgGXauMZ492~F1v7-JuHxkJ4t8kGyNDYjV4b8dIzK6SWnhsnRhgfoLmfi691uFnht8ZCA7ZqkvnuXeMdhCfnmypJT61umKS3mJO5AvG1CdAk6aJiiuhtXFB7k4Dz665imspKZmJAhl9oTMxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Deficiency of both ActR2a and BMPR2 in hepatocytes induces iron overload. Serum iron levels (A) and transferrin saturations (B), as well as liver (C) and spleen (D) iron content, were measured in 10- to 12-week-old female control mice (ActR2a+/+;Bmpr2fl/fl [n = 7]) and mice globally deficient for ActR2a without or with hepatocyte-specific deletion of BMPR2 (ActR2a−/−;Bmpr2fl/fl [n = 23] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 13], respectively). Mice carrying the Cre recombinase transgene are indicated by “Cre +”. ANOVAs P < .0001; *P < .0001 vs ActR2a+/+;Bmpr2fl/fl mice; #P < .0001 vs ActR2a−/−;Bmpr2fl/fl mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/13/10.1182_blood-2014-04-572644/4/m_2116f3.jpeg?Expires=1769155623&Signature=BOOPYDoULTPaIzTEj0yw3I3DRj81ZX7jwqhKIOODM7rDhbazwlNOTHfdlQVoKmusxK-Q4uou~If9i91Nv4loFhPie7o83dkjcEP3E3gDcpstO~UgynI5qj1awPqtlZxAQqsdvgbAmDX73ydzrnKa0h-5dYkuXHWml-YZ-jjHpmd4RpJGIpngI4Z9BLsdHbfN0pBpQIyg~5JtNwkaGa8RwOLjopouTPgkJwXSVtcR0-YEGtrQyc7pIdfQ9O~Sqqhwvn-2utyUMooWmZVI1WndoWls18TTyFiDEuvWSLaR-oHjf5ttSyvP79Qe20~HJDawPRU4pPgr4dSA2Y-3BvqkDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Iron does not induce hepatic hepcidin gene expression in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice. (A) Male mice 10 to 12 weeks old with hepatocyte-specific deficiency of BMPR2 (Bmpr2fl/fl;Alb-Cre, n = 8) and control mice (Bmpr2fl/fl, n = 11) received an intravenous injection of dextran or iron-dextran (0.2 g/kg). After 4 hours, mice were sacrificed, livers were harvested, and RNA was extracted to measure hepcidin mRNA levels. ANOVAs P < .0001; *P < .0001 vs Bmpr2fl/fl mice injected with dextran; #P < .0001 vs Bmpr2fl/fl;Alb-Cre mice injected with dextran. (B) Male mice 10 to 12 weeks old globally deficient for ActR2a without or with hepatocyte-specific BMPR2 deficiency (ActR2a−/−;Bmpr2fl/fl [n = 10] and ActR2a−/−;Bmpr2fl/fl;Alb-Cre [n = 11], respectively) and control mice (ActR2a+/+;Bmpr2fl/fl [n = 8]) received an intravenous injection of dextran or iron-dextran (0.2 g/kg). After 4 hours, mice were sacrificed, livers were harvested, and RNA was extracted to measure hepcidin mRNA levels. Hepcidin mRNA levels in ActR2a−/−;Bmpr2fl/fl;Alb-Cre mice injected with dextran or iron-dextran are highlighted in an inset. ANOVAs P < .008; *P < .009 vs ActR2a+/+;Bmpr2fl/fl mice injected with dextran; #P < .008 vs ActR2a−/−;Bmpr2fl/fl mice injected with dextran; †P < .05 vs ActR2a+/+;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl mice, injected with dextran; ††P < .001 vs ActR2a+/+;Bmpr2fl/fl and ActR2a−/−;Bmpr2fl/fl mice, challenged with iron-dextran.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/13/10.1182_blood-2014-04-572644/4/m_2116f5.jpeg?Expires=1769155623&Signature=Dx6SW8dcDAqMfn87-NYXHnnXZwQAIozQk2eQEZVQtds4WKDkBQHmwOZAf0iqOOyKKhEIXJBdOxKMLRvX1v3qInKdfHrdXEVTMUOQYRDIyjMoapacoZAGy8~LeTfKRa6XTzRTIr1RBOgzr1sogDPCz2UIiq19619-xOVkKvyPeStfVF1Q4aoNLgbgbNMLV6YR2DhTMNE7-~KQFCcVhQ7PNwITnjI5CJQ~JUseo7JWZjyqQOYBVHNo0Aaty24I36gJFRgHObFRMEjcq0UefnliB0jGZ59u-CSOVE4qBUerCxMjMpA2imK3zgVdeGEeprJ0LfyyAHgGdYNncF55n9ghIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)