Key Points
No evidence was found for regulated ATP release from erythrocytes other than by cell lysis with all stimuli tested: mechanical, hypoxia, and cAMP.
The results point to intravascular hemolysis as a primary mechanism governing ATP-dependent regulation of local blood flow.
Abstract
The hypothesis that regulated ATP release from red blood cells (RBCs) contributes to nitric oxide-dependent control of local blood flow has sparked much interest in underlying release mechanisms. Several stimuli, including shear stress and hypoxia, have been found to induce significant RBC ATP release attributed to activation of ATP-conducting channels. In the present study, we first evaluated different experimental approaches investigating stimulated RBC ATP release and quantifying hemolysis. We then measured ATP and free hemoglobin in each and every RBC supernatant sample to directly assess the contribution of hemolysis to ATP release. Hypotonic shock, shear stress, and hypoxia, but not cyclic adenosine monophosphate agonists, significantly enhanced ATP release. It tightly correlated, however, with free hemoglobin in RBC supernatants, indicating that lysis was responsible for most, if not all, ATP release. Luminescence ATP imaging combined with simultaneous infrared cell imaging showed that ATP was released exclusively from lysing cells with no contribution from intact cells. In summary, with all stimuli tested, we found no evidence of regulated ATP release from intact RBCs other than by cell lysis. Such a release mechanism might be physiologically relevant in vivo, eg, during exercise and hypoxia where intravascular hemolysis, predominantly of senescent cells, is augmented.
Introduction
Matching oxygen supply with demand requires specific mechanism(s) to increase blood flow in response to reduced tissue oxygen level. In addition to serving as major oxygen carriers, red blood cells (RBCs) have been proposed to function as oxygen sensors that respond to local tissue hypoxia with controlled ATP release.1 Subsequent activation of vascular endothelial P2Y purinergic receptors stimulates the release of nitric oxide and other mediators of vasodilation, leading to vessel caliber alteration and enhanced blood flow.2-4 In vitro studies have shown that shear stress and mechanical deformation are major stimuli of RBC ATP release, which is also induced by hypoxia.5-8 Elevated ATP levels have been found in vivo in venous effluent from exercising forearm muscle1,9 and further augmented by exercise performed in hypoxia.2,10 Moreover, it has been demonstrated that only when the vessels were perfused with RBCs did the venous effluent ATP level increase and vessels dilate in response to low extraluminal O2.2 All these results implicated RBCs in sensing low extraluminal O2 and contributing to ATP-dependent local blood flow regulation. It has been hypothesized that shear stress- and hypoxia-induced ATP release involves activation of the cyclic adenosine monophosphate (cAMP) signaling pathway, opening up ATP-conducting channels.11 Consistent with this hypothesis, ATP release has also been observed in response to other stimuli that elevate cAMP, such as agonists of prostacyclin,12,13 or β-adrenergic receptors14 (for a review, see Ellsworth and Sprague4 ). Other stimulators of ATP release from RBCs include hypotonic shock15 and elevated temperature.16
Because mature mammalian RBCs are devoid of intracellular organelles, the only plausible pathways for such release appear to be limited to nonvesicular mechanisms, such as ATP-conducting channels.17 Pannexin-1,18 voltage-dependent anion channels, and cystic fibrosis transmembrane conductance regulator have been implicated in conductive ATP release.13,19 However, hemolysis also is an important source of extracellular ATP, and intravascular hemolysis occurs in vivo as a consequence of hypoxia and mechanical trauma to RBCs.20-22 Some degree of hemolysis is unavoidable during in vitro studies. Although it has been considered to be a potential factor contributing to stimulated ATP release in most previous investigations, its actual involvement has not yet been systematically investigated, eg, by paired measurement of free hemoglobin and ATP in each and every sample.
In the present study, we began by evaluating different experimental approaches to determine stimulated ATP release from RBCs and quantify hemolysis to determine their limitations and identify potential pitfalls. The most accurate methods were thus used to measure hemoglobin and ATP content in RBC supernatant samples. To directly assess the contribution of hemolysis to ATP release, free hemoglobin was always quantified in the same supernatant samples for which ATP content was reported.
Materials and methods
Preparation and handling of erythrocytes
Human blood was collected from hemochromatosis patients undergoing phlebotomy treatment. After obtaining informed verbal consent with institutional Ethic Committee approval in accordance with the Declaration of Helsinki, blood was drawn by venipuncture, anticoagulated with citrate phosphate dextrose solution, and stored at 4°C. Although most experiments were performed with fresh or 1- to 2-day-old blood samples, qualitatively similar results were obtained with cells that had been stored for up to 14 days. All samples were centrifuged (500g, 10 minutes, 4°C) prior to the experimental procedure. Blood plasma and buffy coat were removed by aspiration, and erythrocytes were washed 3 times with physiological salt solution (PSS) containing (in mM) 140.5 NaCl, 4.7 KCl, 2 CaCl2, 1.2 MgSO4, 21 Tris-base, 11.1 dextrose, and 0.5% bovine serum albumin (pH 7.4, osmolality 307 mOsm/kg). Unless stated otherwise, the cell count in all experiments was adjusted to 106 cells/µL (∼10% hematocrit). Osmolality of the solutions was measured with a freezing point osmometer (Model 3300, Micro Osmometer; Advanced Instruments Inc., Norwood, MA).
Measurement of hemolysis
In preliminary experiments, we evaluated several commonly used methods of hemolysis determination, including Drabkin23 and luminol assays,24 and found that hemolysis readings based on hemoglobin absorbance measurements, such as the Harboe method, are most accurate, reproducible, and less susceptible to the presence of interfering substances, in agreement with a previous report.25 In our hands, absorbance measurements allowed reliable detection of 20 to 30 hemolyzed cells/µL, corresponding to 0.002% to 0.003% hemolysis at our standard cell density of 106 cells/µL (supplemental Methods; supplemental Figure 1, available on the Blood Web site).
ATP luminometry assay
ATP was quantified by luciferin-luciferase (LL) bioluminescence assay, with ATP Assay Mix and ATP Assay Mix Dilution Buffer supplied by Sigma-Aldrich Canada (Oakville, ON, Canada). Luminescence was measured by Turner TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Released ATP was expressed in fmol × (106 cells)−1. A calibration curve of LL luminescence (in arbitrary light units) vs standard ATP concentrations was produced before and after each experiment, and a separate calibration curve was plotted for each hypotonic and isotonic solution.
Following preliminary experiments, we chose to analyze ATP in RBC supernatants after centrifugation of RBC suspension samples (supplemental Methods; supplemental Figure 2A). With this approach, each and every supernatant sample underwent paired measurements of extracellular ATP and free hemoglobin, allowing direct monitoring of hemolysis contribution to ATP release.
Hypotonic shock-induced ATP release
Most ATP efflux experiments were performed in 6- or 12-well plate format. Each well contained ∼3 mL of RBC suspension in 6-well plates placed inside a 37°C incubator (Ambio High-Low Chamber; Laboratory-Line Instruments, Melrose Park, IL) on a 3D rotator platform (10° angle, 30 rpm; Laboratory-Line Instruments) for gentle mixing. A hypotonic stimulus was applied by diluting concentrated, washed erythrocyte suspensions (5 × 106 cells/µL) to a final density of 106 cells/µL by mixing appropriate volumes of concentrated cell suspension with saline solution of a reduced (eg, by 20%, 25%, 30%, and 35%) NaCl concentration. Cells were incubated in hypotonic solutions for 5 or 15 minutes with gentle mixing, centrifuged, and the supernatants analyzed for extracellular ATP and free hemoglobin (see above). All experiments were conducted at 37°C.
Pharmacological stimulation
To study pharmacological stimulation of ATP release, experiments were performed in 6-well plate format, as described in the preceding section. Unless stated otherwise, before the experiments, forskolin (an adenylate cyclase activator) and papaverin (a phosphodiesterase inhbitor) stocks were prediluted with PSS to contain <10% dimethylsulfoxide (DMSO) and were added directly to RBC suspensions (final DMSO concentration <0.01%).
Mechanical stimulation
To apply shear stress, 2-mL samples of RBC suspensions (106 cells/µL) were vigorously mixed in a 15-mL conical polypropylene tube by a VX100 vortex mixer (Labnet, Woodbridge, NJ) at ∼1000 rpm for 15 to 300 seconds. Supernatants were collected by centrifugation (1800g 10 minutes, 4°C) and kept on ice until ATP and free hemoglobin determinations. Computational fluid dynamics modeling with a similar vortex mixing system showed that at 1000 rpm agitation frequency, steady-state volume-averaged shear stress was ∼4 dyne/cm.2,26
Exposure of erythrocytes to low oxygen conditions
RBC samples (in 6- or 12-well plate format) were placed in an airtight flow-through box with a continuously injected gas mixture containing 0% O2 and 5% CO2 balanced with N2. After 30 minutes of equilibration with gentle mixing on the 3D rotator platform, hemoglobin oxygen saturation, measured by blood gas analyzer (ABL800 FLEX; Radiometer, London, ON, Canada), was within the 40% to 80% range compared with 97% to 99% in the controls.
Luminescence imaging of ATP release
To image ATP release from cells, a fresh droplet of RBCs was collected from healthy donors by finger prick and washed 2 to 3 times with PSS. Low-density RBC suspensions were deposited on poly-lysine–coated glass coverslips (0.1 or 0.01 mg/mL poly-lysine, molecular weight 2.7 kDa). To visualize cellular ATP release in real time, we deployed an imaging system that combined simultaneous high-sensitivity bioluminescence detection of ATP and infrared (IR) differential interference contrast (DIC) images of cells to identify ATP-releasing cells27,28 (supplemental Methods).
Statistical analysis
Statistical significance was assessed by nonparametric Mann-Whitney U test (STATISTICA 10 software, StatSoft, Tulsa, OK). Differences were considered to be significant at P < .05. All data were expressed as mean ± standard error of the mean (SEM).
Results
Hypotonic shock-induced ATP release
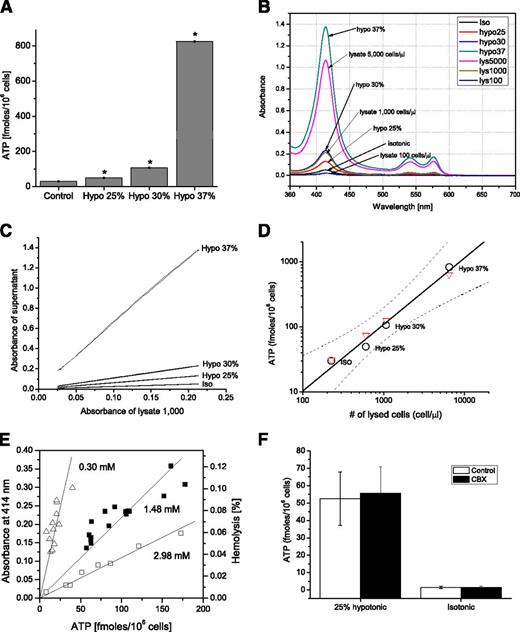
Figure 1A depicts an experimental example where RBC suspension samples were exposed for 5 minutes to isotonic PSS or 25%, 30%, or 37% hypotonic PSS (ie, containing 140.5, 105.38, 98.35, or 88.52 mM NaCl, respectively). Extracellular ATP measured in supernatants significantly increased with rising hypotonicity. However, elevated hemolysis was also noted with RBCs exposed to hypotonic solutions, as revealed by hemoglobin absorbance measurement of the same supernatant samples (Figure 1B-C). By plotting released ATP against the number of lysed cells found in supernatant samples, we determined that released ATP (black circles) increases with the number of hemolyzed cells, and this dependence is well fitted by a linear relationship (correlation coefficient R = 0.9880, Figure 1D). Such linear dependence strongly suggests that hemolysis contributes, at least in part, to hypotonic shock-induced ATP release. To verify the extent of this contribution, Figure 1 also compares ATP released by hypotonic shock with ATP released by lysing the corresponding cell number of the same RBC batch with Triton X-100 (red triangles). The lysate data points closely overlap with hypotonic shock-induced ATP release, demonstrating that all ATP released by hypotonic shock originates from cell lysis. The slope of the linear relationships in Figure 1D corresponds to an intracellular ATP concentration of 1.42 mM, but it may vary between different RBC batches. This is illustrated in Figure 1E, where examples of similar hypotonic shock experiments performed with 3 different blood batches are presented. In all such experiments (n = 8), despite up to ∼10-fold variability of intracellular ATP concentration, we observed a tight, linear relationship between hemolysis level and released ATP (P < .0001). As might be expected for release mechanisms involving cell lysis, the pannexin channel inhibitor carbenoxolone (CBX) showed no effect on hypotonic shock-induced ATP release (Figure 1F).
Hypotonic shock-induced ATP release tightly correlates with cell lysis. (A) ATP content in RBC supernatants exposed for 5 minutes to isotonic (control) or 25%, 30%, or 37% hypotonic solutions in 6-well plate format. The data are average ± SEM (n = 3 determinations), and, for hypotonic conditions, they are significantly different from control values (P < .05, Mann-Whitney U test, indicated by *). (B) Absorbance spectra (λ = 360-700 nm) of RBC supernatants exposed to different hypotonic solutions from the experiment reported in A. Absorbance of supernatant from cell lysates equivalent to 100, 1000, and 5000 cells/µL is also shown. Note that the absorbance spectra of 30% hypotonic stress supernatants overlap with those of lysates of 1000 cells/µL. From the calibration curve of peak absorbance (at 414 nm) vs cell lysate density, as illustrated in supplemental Figure 1B, we could calculate the number and percentage of lysed cells in each experimental condition. (C) For low hemolysis levels, lysed cell number was evaluated more precisely by plotting the entire absorbance spectra of the supernatant vs the absorbance spectra of the reference lysate. y-axis: Absorbance spectra of RBC supernatants exposed to different hypotonic solutions from the experiments reported in A are plotted against absorbance of reference lysate of 1000 cells/µL (x-axis) for the entire spectrum between 380 and 620 nm. From the slope of linear fit, the exact number of lysed cells in the supernatants was determined for each experimental condition: Iso, Hypo 25%, 30%, and 37%. The corresponding number of lysed cells was 226, 603, 1073, and 6470/µL, respectively. (D) Hypotonic shock-induced ATP release vs number of lysed cells. The number of lysed cells in control (Iso) and hypotonic conditions was calculated by fitting their corresponding absorbance spectra to those of a reference cell lysate of 1000 cells/µL (white circles), shown in C. The solid line is a least square linear fit to the hypotonic shock-induced ATP release data with 95% confidence bands indicated by dashed lines and correlation coefficient R = 0.988. Red triangles indicate ATP measured in cell lysates prepared from the same batch of blood and adjusted to corresponding numbers of lysed cells found under Iso and hypotonic conditions. The slope of the linear relationship corresponds to an intracellular ATP concentration of 1.42 mM. (E) Correlation between extent of hemolysis (shown as absorbance – left axis, or % hemolysis – right axis) and ATP release induced by hypotonic shock. Paired values of extracellular ATP and free hemoglobin in the supernatant samples were fitted by linear regression with Origin Laboratory 7.5. The data are examples of 3 independent experiments (each indicated by a different symbol) similar to those in A-D but performed in 12-well plate format. In each experiment, RBC suspensions were incubated in either isotonic or hypotonic (20%, 25%, 30%, 35%, or 40%) solution. The slopes of the fitted lines correspond to the intracellular ATP concentration and are indicated on the graph. (F) Effect of CBX (100 μM) on ATP release stimulated by 5-minute exposure to 25% hypotonic shock. Cells were preincubated for 10 minutes with CBX in isotonic PSS, then transferred to a Petri dish containing CBX in hypotonic medium. Average of n = 3 experiments ± SEM.
Hypotonic shock-induced ATP release tightly correlates with cell lysis. (A) ATP content in RBC supernatants exposed for 5 minutes to isotonic (control) or 25%, 30%, or 37% hypotonic solutions in 6-well plate format. The data are average ± SEM (n = 3 determinations), and, for hypotonic conditions, they are significantly different from control values (P < .05, Mann-Whitney U test, indicated by *). (B) Absorbance spectra (λ = 360-700 nm) of RBC supernatants exposed to different hypotonic solutions from the experiment reported in A. Absorbance of supernatant from cell lysates equivalent to 100, 1000, and 5000 cells/µL is also shown. Note that the absorbance spectra of 30% hypotonic stress supernatants overlap with those of lysates of 1000 cells/µL. From the calibration curve of peak absorbance (at 414 nm) vs cell lysate density, as illustrated in supplemental Figure 1B, we could calculate the number and percentage of lysed cells in each experimental condition. (C) For low hemolysis levels, lysed cell number was evaluated more precisely by plotting the entire absorbance spectra of the supernatant vs the absorbance spectra of the reference lysate. y-axis: Absorbance spectra of RBC supernatants exposed to different hypotonic solutions from the experiments reported in A are plotted against absorbance of reference lysate of 1000 cells/µL (x-axis) for the entire spectrum between 380 and 620 nm. From the slope of linear fit, the exact number of lysed cells in the supernatants was determined for each experimental condition: Iso, Hypo 25%, 30%, and 37%. The corresponding number of lysed cells was 226, 603, 1073, and 6470/µL, respectively. (D) Hypotonic shock-induced ATP release vs number of lysed cells. The number of lysed cells in control (Iso) and hypotonic conditions was calculated by fitting their corresponding absorbance spectra to those of a reference cell lysate of 1000 cells/µL (white circles), shown in C. The solid line is a least square linear fit to the hypotonic shock-induced ATP release data with 95% confidence bands indicated by dashed lines and correlation coefficient R = 0.988. Red triangles indicate ATP measured in cell lysates prepared from the same batch of blood and adjusted to corresponding numbers of lysed cells found under Iso and hypotonic conditions. The slope of the linear relationship corresponds to an intracellular ATP concentration of 1.42 mM. (E) Correlation between extent of hemolysis (shown as absorbance – left axis, or % hemolysis – right axis) and ATP release induced by hypotonic shock. Paired values of extracellular ATP and free hemoglobin in the supernatant samples were fitted by linear regression with Origin Laboratory 7.5. The data are examples of 3 independent experiments (each indicated by a different symbol) similar to those in A-D but performed in 12-well plate format. In each experiment, RBC suspensions were incubated in either isotonic or hypotonic (20%, 25%, 30%, 35%, or 40%) solution. The slopes of the fitted lines correspond to the intracellular ATP concentration and are indicated on the graph. (F) Effect of CBX (100 μM) on ATP release stimulated by 5-minute exposure to 25% hypotonic shock. Cells were preincubated for 10 minutes with CBX in isotonic PSS, then transferred to a Petri dish containing CBX in hypotonic medium. Average of n = 3 experiments ± SEM.
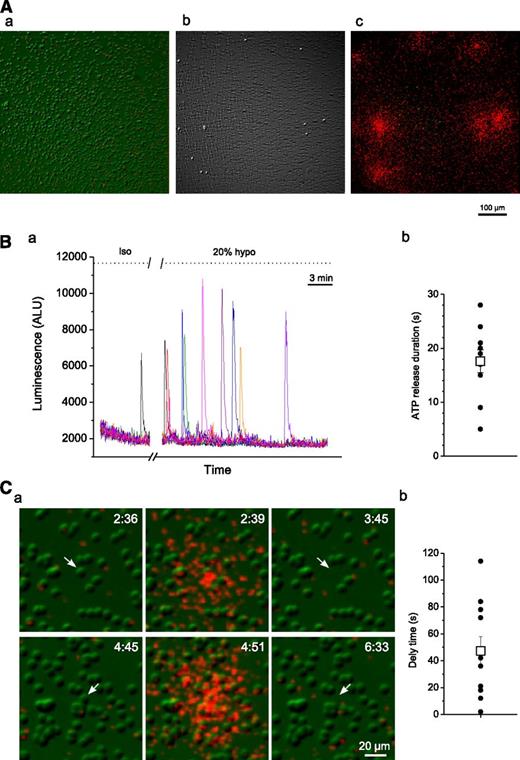
The above experiments revealed that, besides hemolysis, there was no evidence of additional ATP release that could be attributed to other mechanisms triggered by hypotonic stress. To further confirm this observation, we performed luminescence ATP imaging, as described by Grygorczyk et al and Furuya et al.27,28 RBCs were deposited on poly-lysine–coated glass coverslips and bathed in LL containing PSS (supplemental Methods). IR-DIC and luminescence images were simultaneously captured for the duration of the experiment. Figure 2A shows that all regions displaying ATP release perfectly matched sites where cells had lysed, demonstrating that ATP release occurred exclusively from lysed cells and was not detected from cells that remained intact. The time-course of luminescence responses in this experiment is depicted in Figure 2B, which discloses brief spikes of luminescence (average duration of 17.6 ± 6.7 seconds) due to ATP release from lysing cells. Although isolated lysis events could occasionally be seen under isotonic conditions, their frequency increased dramatically during 20% hypotonic shock. Interestingly, after ATP release, cell collapse/lysis was apparent after a delay of 47 ± 10.6 seconds (average ± SEM) (Figure 2C). The results confirm that cell lysis was the sole mechanism of ATP release in these experiments. It should be noted that spontaneous hemolysis of RBCs attached to the glass surface coated with high poly-lysine concentration may also contribute to RBC lysis, even in the absence of hypotonic stimulation.29
Luminescence imaging of ATP release from RBCs. (A) Image a is an IR-DIC image of RBCs before hypotonic shock application. Image b was obtained by subtracting control image a (before hypotonic shock) from that acquired ∼25 minutes after 20% hypotonic shock. The resulting white dots correspond to cells that lysed and were missing in images captured after hypotonic stimulation. c is an overlay of image b and cumulative ATP-dependent luminescence (in red) observed during the entire experiment. Note that regions of ATP release coincide with spots where cells lysed. (B) (a) Time-course of luminescence responses due to lysis of single RBCs in the experiment shown in A. Luminescence responses were measured at individual release sites seen in A and are indicated by traces of different colors. See supplemental Movie 1 for the entire time course of ATP release. (b) ATP release durations for the responses depicted in a measured from the start of release until the luminescence response decayed to 1/e of its peak value (black circles). Average (±SEM) release time was 17.6 ± 2.1 seconds (white square). (C) (a) Two examples (upper and lower row images) illustrating ATP release due to lysis of single RBCs. IR images of RBCs (in green) are overlaid with extracellular ATP-dependent luminescence (in red). 20% hypotonic shock-induced ATP release (center image) occurs shortly before lysis/collapse of a single RBC (indicated by a white arrow; see also supplemental Movie 2). No ATP release from intact RBCs is evident. The elapsed time (minutes:seconds) is shown in the upper-right corner. (b) Average delay time between start of ATP release and cell lysis for the responses shown in B (black circles). Average (±SEM) delay time was 47 ± 10.6 seconds (white square).
Luminescence imaging of ATP release from RBCs. (A) Image a is an IR-DIC image of RBCs before hypotonic shock application. Image b was obtained by subtracting control image a (before hypotonic shock) from that acquired ∼25 minutes after 20% hypotonic shock. The resulting white dots correspond to cells that lysed and were missing in images captured after hypotonic stimulation. c is an overlay of image b and cumulative ATP-dependent luminescence (in red) observed during the entire experiment. Note that regions of ATP release coincide with spots where cells lysed. (B) (a) Time-course of luminescence responses due to lysis of single RBCs in the experiment shown in A. Luminescence responses were measured at individual release sites seen in A and are indicated by traces of different colors. See supplemental Movie 1 for the entire time course of ATP release. (b) ATP release durations for the responses depicted in a measured from the start of release until the luminescence response decayed to 1/e of its peak value (black circles). Average (±SEM) release time was 17.6 ± 2.1 seconds (white square). (C) (a) Two examples (upper and lower row images) illustrating ATP release due to lysis of single RBCs. IR images of RBCs (in green) are overlaid with extracellular ATP-dependent luminescence (in red). 20% hypotonic shock-induced ATP release (center image) occurs shortly before lysis/collapse of a single RBC (indicated by a white arrow; see also supplemental Movie 2). No ATP release from intact RBCs is evident. The elapsed time (minutes:seconds) is shown in the upper-right corner. (b) Average delay time between start of ATP release and cell lysis for the responses shown in B (black circles). Average (±SEM) delay time was 47 ± 10.6 seconds (white square).
Mechanical stimulation
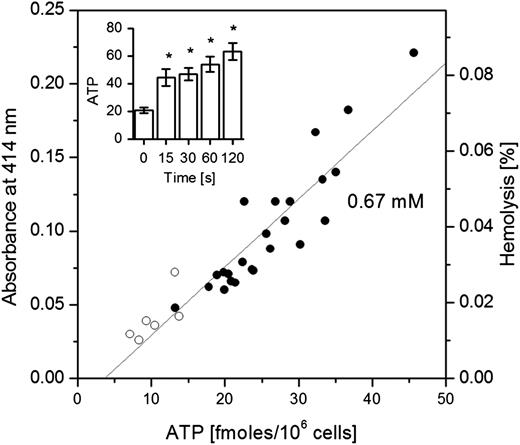
When shear stress was applied to stimulate RBCs, ATP in cell supernatants progressively increased with the duration of stimulation (Figure 3 inset). It was accompanied, however, by elevated cell hemolysis showing a tight correlation between ATP and free hemoglobin in supernatant samples (Figure 3). Thus, the data are consistent with hemolysis as the primary ATP release mechanism in RBCs subjected to shear stress.
Mechanically stimulated ATP release and hemolysis. Inset: The amount of ATP (fmol/106 cells) detected in supernatants depends on the duration of mechanical shear stress stimulation. Each bar represents the average of 6 measurements ± SEM. The data on stimulated release are significantly different from control values (P < .05, Mann-Whitney U test, indicated by *). The main graph shows tight, linear correlation (R = 0.923; P < .0001) between ATP release induced by shear stress and extent of hemolysis (depicted as absorbance – left axis, or % hemolysis – right axis) for all data points (n = 30) of the experiment reported in the inset. Open symbols refer to the controls and solid symbols to stimulated RBC samples. The slope of linear fit corresponds to intracellular ATP concentration of 0.67 mM. The data are representative of n = 4 similar experiments. The estimated volume averaged shear stress was ∼4 dyne/cm2 (see “Materials and methods”).
Mechanically stimulated ATP release and hemolysis. Inset: The amount of ATP (fmol/106 cells) detected in supernatants depends on the duration of mechanical shear stress stimulation. Each bar represents the average of 6 measurements ± SEM. The data on stimulated release are significantly different from control values (P < .05, Mann-Whitney U test, indicated by *). The main graph shows tight, linear correlation (R = 0.923; P < .0001) between ATP release induced by shear stress and extent of hemolysis (depicted as absorbance – left axis, or % hemolysis – right axis) for all data points (n = 30) of the experiment reported in the inset. Open symbols refer to the controls and solid symbols to stimulated RBC samples. The slope of linear fit corresponds to intracellular ATP concentration of 0.67 mM. The data are representative of n = 4 similar experiments. The estimated volume averaged shear stress was ∼4 dyne/cm2 (see “Materials and methods”).
Pharmacological stimulation
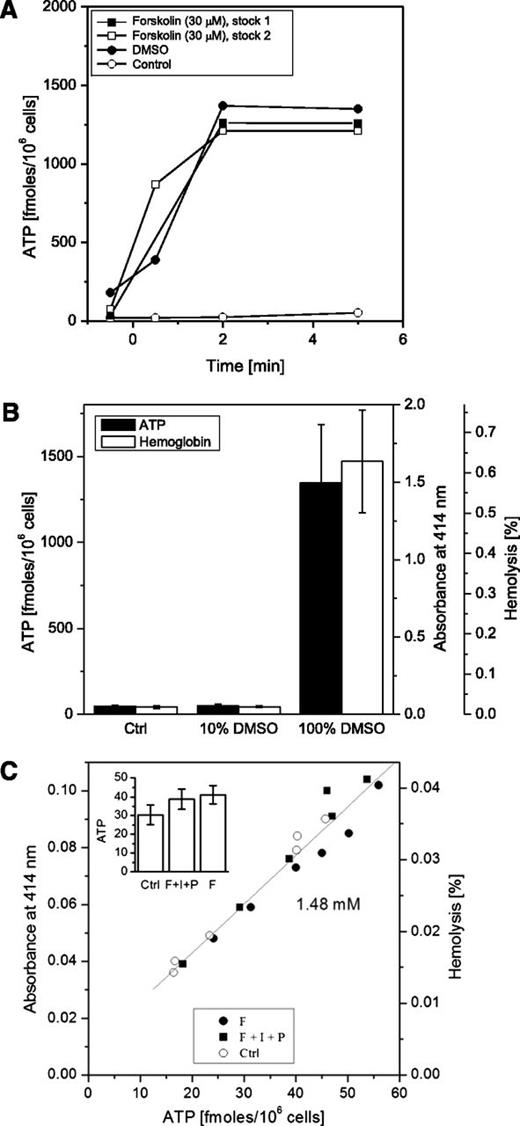
Forskolin, a potent adenylate cyclase activator, was reported in several previous studies to stimulate ATP release from RBCs. In an attempt to replicate these experiments, we tested the effect of forskolin on ATP release and found that adding a small aliquot of concentrated forskolin stock solution in DMSO directly to erythrocyte suspensions markedly increased extracellular ATP (Figure 4A). It also illustrates that ATP responses were indistinguishable from those evoked by an equivalent volume of DMSO alone, indicating that even when final DMSO concentration (∼0.27%) was harmless, some cells, presumably those in close proximity to the pipette tip, might have been exposed to high solvent levels causing their lysis. Indeed, Figure 4B reports that DMSO-evoked elevation of extracellular ATP closely followed extracellular hemoglobin. When the same amount of DMSO was prediluted 10-fold in PSS and then added to the cells, no increase in hemolysis or ATP release was seen. A similar result was obtained with forskolin, when its DMSO stock solution was prediluted 10-fold in PSS before addition to RBC suspension: neither extracellular ATP nor hemoglobin showed any increment, the latter remaining <0.05%, as in control supernatant samples.
Effect of cAMP agonists and DMSO on ATP release. (A) Two separately prepared concentrated forskolin stock solutions in DMSO (final concentration 30 µM), or equivalent volume of DMSO, were directly applied to cell suspensions at time 0, and ATP release was quantified at different time points, with the controls being nonstimulated cells (representative of n = 4 experiments). (B) Low DMSO concentration (<10%) does not induce RBC ATP release. The same amount of DMSO was added to RBC suspension either as a small aliquot of 100% concentrated stock or after 10-fold dilution (to 10% DMSO in PSS), giving the same final concentration of 0.27%. Black bars (scale on the left) – extracellular ATP, white bars (scale on the right) – hemoglobin released. Each bar represents an average of n = 4 experiments ± SEM. No statistically significant difference was found in either ATP or hemolysis between the controls and samples treated with 10% DMSO. The difference between the controls and samples treated with 100% concentrated stock was statistically significant (P < .05, Mann-Whitney U test). (C) Effect of cAMP stimulation on extracellular ATP. Cells were incubated for 5 minutes at 37°C with forskolin (F, 30 µM), or a mixture of F (30 µM) plus isoproterenol (I, 10 µM) and papaverine (P, 100 µM). Extracellular ATP level (x-axis) was plotted against absorbance at λ = 414 nm (y-axis) measured in the same supernatant samples. Extracellular ATP showed strong linear correlation with hemolysis (P < .0001; R = 0.975). The slope of linear fit (gray line) corresponded to intracellular ATP concentration of 1.48 mM. Open circles, control, nonstimulated cells; closed circles, cells stimulated by F; closed squares, cells stimulated by F+I+P cocktail. Inset: ATP (fmol/106 cells) in RBC supernatants treated with F or F+I+P mixture (average ± SEM, n = 6). Average ATP release was not significantly different for all 3 conditions (P = .23). The data are representative of n = 4 similar experiments.
Effect of cAMP agonists and DMSO on ATP release. (A) Two separately prepared concentrated forskolin stock solutions in DMSO (final concentration 30 µM), or equivalent volume of DMSO, were directly applied to cell suspensions at time 0, and ATP release was quantified at different time points, with the controls being nonstimulated cells (representative of n = 4 experiments). (B) Low DMSO concentration (<10%) does not induce RBC ATP release. The same amount of DMSO was added to RBC suspension either as a small aliquot of 100% concentrated stock or after 10-fold dilution (to 10% DMSO in PSS), giving the same final concentration of 0.27%. Black bars (scale on the left) – extracellular ATP, white bars (scale on the right) – hemoglobin released. Each bar represents an average of n = 4 experiments ± SEM. No statistically significant difference was found in either ATP or hemolysis between the controls and samples treated with 10% DMSO. The difference between the controls and samples treated with 100% concentrated stock was statistically significant (P < .05, Mann-Whitney U test). (C) Effect of cAMP stimulation on extracellular ATP. Cells were incubated for 5 minutes at 37°C with forskolin (F, 30 µM), or a mixture of F (30 µM) plus isoproterenol (I, 10 µM) and papaverine (P, 100 µM). Extracellular ATP level (x-axis) was plotted against absorbance at λ = 414 nm (y-axis) measured in the same supernatant samples. Extracellular ATP showed strong linear correlation with hemolysis (P < .0001; R = 0.975). The slope of linear fit (gray line) corresponded to intracellular ATP concentration of 1.48 mM. Open circles, control, nonstimulated cells; closed circles, cells stimulated by F; closed squares, cells stimulated by F+I+P cocktail. Inset: ATP (fmol/106 cells) in RBC supernatants treated with F or F+I+P mixture (average ± SEM, n = 6). Average ATP release was not significantly different for all 3 conditions (P = .23). The data are representative of n = 4 similar experiments.
In subsequent experiments, only prediluted stock solutions containing DMSO <10% in PSS were tested to avoid hemolysis when adding dissolved agents directly to cell suspensions. With this approach, in our hands, neither forskolin alone nor forskolin in combination with papaverine and isoproterenol had any significant effect on ATP release (Figure 4C inset). Extracellular ATP levels in individual samples varied within the same range as in untreated control cells and strongly correlated with hemolysis (P < .0001, R = 0.975), which remained <0.05%, comparable with control values obtained in our other experiments (Figure 4C). Luminescence ATP imaging experiments confirmed that forskolin did not stimulate ATP release from intact cells (supplemental Movie 3); it occurred only from cells that lysed either because of mechanical perturbations or coapplied hypotonic shock (supplemental Movie 4). Thus, ATP luminometry and luminescence ATP imaging experiments demonstrated that in response to forskolin or other cAMP agonists, there was no additional mechanism of ATP release beside hemolysis.
Effect of hypoxia on ATP release
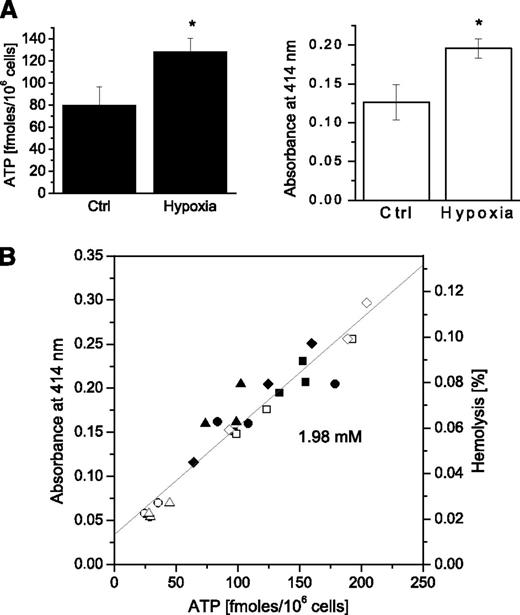
Exposure of RBC suspensions to low oxygen conditions for 30 minutes led to significantly increased extracellular ATP and heightened free hemoglobin levels in RBC supernatants (Figure 5A). Hemolysis and extracellular ATP varied between individual experiments, but in each case, they were significantly higher in hypoxia-treated samples than in the controls. Moreover, a tight linear relationship was observed between the 2 variables, demonstrating that hemolysis was the main mechanism responsible for ATP release in these experiments (Figure 5B).
Effect of hypoxia on ATP release and cell lysis. (A) Average ATP (left) and hemolysis (right) in control and hypoxia-treated RBCs. Each bar represents an average of 12 or 13 samples ± SEM from 4 independent experiments performed with the same blood batch, *Statistically significant difference compared with the controls (P < .05, Mann-Whitney U test). (B) Relationship between extracellular ATP and hemolysis. The graph shows tight, linear correlation (P < .0001) between hemolysis extent (depicted as absorbance – left axis, or % hemolysis – right axis) and ATP release induced by hypoxia for all data points of the 4 independent experiments reported in A. Each experiment is represented by a different symbol, with black symbols referring to hypoxia and open symbols indicating the control (normoxia) condition. The slope of linear fit corresponds to intracellular ATP concentration of 1.98 mM.
Effect of hypoxia on ATP release and cell lysis. (A) Average ATP (left) and hemolysis (right) in control and hypoxia-treated RBCs. Each bar represents an average of 12 or 13 samples ± SEM from 4 independent experiments performed with the same blood batch, *Statistically significant difference compared with the controls (P < .05, Mann-Whitney U test). (B) Relationship between extracellular ATP and hemolysis. The graph shows tight, linear correlation (P < .0001) between hemolysis extent (depicted as absorbance – left axis, or % hemolysis – right axis) and ATP release induced by hypoxia for all data points of the 4 independent experiments reported in A. Each experiment is represented by a different symbol, with black symbols referring to hypoxia and open symbols indicating the control (normoxia) condition. The slope of linear fit corresponds to intracellular ATP concentration of 1.98 mM.
Discussion
In this study, we attempted to identify cell-regulated ATP release pathway(s) in human erythrocytes. By paired measurements of ATP and free hemoglobin in each and every sample of RBC supernatants, we found that basal and stimulated ATP levels always correlated tightly with extracellular hemoglobin, a marker of cell lysis. Unexpectedly, this was seen with all stimuli tested, strongly indicating that, for each stimulus, the only source of extracellular ATP was cell lysis. In the case of hypotonic shock and cAMP/forskolin stimulation, we confirmed our observations more directly by simultaneous luminescence ATP imaging and IR-DIC imaging of substrate-attached RBCs. These experiments identified single ATP-releasing cells and revealed that only lysing cells contributed to ATP release. Thus, despite our efforts, we obtained no evidence of regulated, nonlytic ATP release in RBCs.
Basal hemolysis levels in our experiments varied between different blood donors and were elevated after prolonged blood storage. Typically between 0.01% and 0.05%, they were somewhat augmented in stimulated RBC samples subjected to shear stress, hypoxia, or hypotonic shock, but remained ≤0.1%. Such hemolysis values are similar to or below those reported by other investigators.5,12,13 In several studies, hemolysis was stated to have negligibly contributed to stimulated ATP release, and samples with heightened hemolysis were simply excluded from the analysis. In other investigations, released ATP was corrected by subtracting average ATP attributable to hemolysis. The latter was often found to be not different or comparable in stimulated and control RBC samples. To the best of our knowledge, in most previous studies, hemolysis was not systematically measured or analyzed, ie, in all individual samples for which ATP content was reported. As a result, its contribution to ATP release might have been underestimated or, in some cases, entirely overlooked.
In the present investigation, we tested several stimuli, including hypotonic shock, previously demonstrated to induce regulated RBC ATP release. Although not exactly a physiological stimulus, hypotonic shock is often considered as a surrogate of mechanical stimulation,30,31 and ATP release can be extrapolated to responses evoked by mechanical perturbations. We noted heightened hemolysis at ∼25% hypotonicity, which increased with augmented hypoosmotic shock and strongly correlated with extracellular ATP, as expected for lytic ATP release mechanism. Apart from hemolysis, nonlytic mechanism(s) may contribute to ATP release from intact erythrocytes. This would be seen as a “surplus” of released ATP above the hemolysis “background.” However, ATP found in lysates of known cell density (eg, 1000 cells/µL) was almost exactly the same as that actually released by the same number of cells when they lysed during hypotonic shock. Furthermore, despite different intracellular ATP concentrations and the resulting variability of ATP release observed with various blood batches (Figure 1E), it always increased linearly with rising hemolysis level, demonstrating that there was no “surplus” ATP above the hemolysis “background” in all such experiments. Because hemoglobin is a major protein constituent of the RBC cytoplasm, its leakage eventually leads to cell collapse. Interestingly, after ATP release, cell collapse/lysis occurred with an average delay that was ∼2.7-fold longer than ATP release duration (compare Figure 2Bb, Cb). Slower hemoglobin release might be expected due to its ∼3.7-fold smaller diffusion coefficient in water vs ATP (6.9 × 10−7 and 2.6 × 10−6 cm2/s, respectively). The data support the view that ATP release occurs simultaneously with hemoglobin leakage during RBC lysis and involves the same release pathway.
An interesting finding in our study was the effect of DMSO on ATP release. Because of its amphiphilic properties and high polarity, DMSO is widely used as a solvent, easily permeating biological membranes and facilitating drug transport.32,33 However, in high concentrations, DMSO imposes osmotic stress and is toxic to cells, affecting water flow through membranes and other cell functions via incompletely understood mechanisms.34 When added to RBC suspensions, DMSO readily diffuses into cells, resulting in cell swelling or eventual rupture (supplemental Figure 3; supplemental Movie 5). We noticed a strong effect of DMSO on ATP release when concentrated stocks of test compounds, dissolved in DMSO, were added directly to RBC suspensions. Even if the final DMSO concentration in a given volume of RBC suspension is harmless, some cells may undergo lysis, presumably exposed to the highest solvent concentration, in proximity to the pipette tip.33,35 To exclude a direct effect of DMSO on cell lysis, we tested prediluted stocks of compounds containing <10% DMSO. In our hands, forskolin and other cAMP agonists did not stimulate ATP release when precautions were taken to avoid DMSO-induced osmotic stress and cell lysis. Interestingly, in several previous studies, cAMP agonists (forskolin 10 µM, and 3-isobutyl-1-methylxanthine 100 µM) were included in “stop” solution designed to prevent ATP release and hydrolysis in human blood samples, consistent with lack of cAMP-stimulated ATP release in these investigations.16,36
Recently, Mairbäurl et al (2013)37 addressed the role of hemolysis in shear stress- and hypoxia-induced ATP release in more detail in their in vitro study. They noted that, in shear stress experiments under normoxia, up to 50% of ATP release was due to hemolysis. Its contribution, however, decreased to <5% when RBCs were exposed to shear stress and hypoxia at the same time. Although precise correlations between hemolysis and ATP levels were not investigated, most ATP release observed in the hypoxia experiments was attributed to a cell-regulated mechanism. This was not confirmed in our study: we saw that shear stress or hypoxia alone could induce dose-dependent elevation of both extracellular ATP and free hemoglobin in cell supernatants, with strong, positive correlations between the 2 variables. Interestingly, hypoxia was shown to decrease deformability of RBCs and increase susceptibility to cell damage.22,38 Thus, the increment of extracellular ATP induced by hypoxia and hypoxia together with shear stress could be attributed to augmented cell lysis.
The results presented here contradict much previously published experimental data on ATP-release mechanism(s) in RBCs. Owing to the absence of intracellular organelles, mature mammalian RBCs were considered as the ultimate example of a cell system where ATP release occurs exclusively via conductive pathways. Several channels were implicated in ATP release, including cystic fibrosis transmembrane conductance regulator, voltage-dependent anion channels, and pannexins based on observed stimulation of ATP release, eg, by cAMP agonists, or its inhibition by various compounds, such as DIDS, flufenamic acid, glibenclamide, CBX, or probenicid. All these compounds have a wide spectrum of effects, blocking other channels, transporters, or processes besides putative ATP channels.39-42 Some inhibitors may directly interfere with the bioluminescence reaction used to measure ATP. For example, the stretch-activated ion channel blocker gadolinium (at 10 µM) and the anion channel blocker 5-nitro-2-[(3-phenylpropyl)amino]benzoic acid (at >250 µM) were found to inhibit LL bioluminescence.31,43 Certain inhibitors, including CBX and glibenclamide, were found to deplete cellular ATP by interfering with cell metabolism in other cell types.39,40 The effects of test compounds, or their solvents, on cell membrane stiffness/fragility, resulting in altered susceptibility to hemolysis, may also contribute to apparent ATP release modulation under specific experimental conditions.44 Thus, in addition to tight hemolysis control, several other control experiments are needed to support the validity of RBC ATP release inhibition data.
The major finding of our study is that ATP released from RBCs by various stimuli correlated tightly with free hemoglobin, clearly demonstrating an important yet often underestimated role of hemolysis in RBC ATP release. Luminescence imaging of ATP release and IR imaging of RBCs provided direct evidence that ATP is released exclusively from lysing cells. Our data show that extreme caution must be taken when studying ATP release from RBCs. In in vitro experiments, from the moment of blood collection through storage, centrifugation, mixing, and pipetting to final assessment, erythrocytes are exposed to various physical perturbations that unavoidably cause rupture and hemolysis of some cells. Furthermore, cell lysis can be induced by several stimuli intended to trigger regulated ATP release, as we have demonstrated for hypoxia, mechanical stimulation, hypotonic shock, or the addition of test compounds dissolved in concentrated DMSO.
Importantly, hemolysis also occurs in vivo, and such ATP release mechanisms may have physiological and pathophysiological roles in local purinergic signaling and blood flow regulation. Intravascular hemolysis is particularly profound during intense exercise and mainly affects senescent RBCs, which are mechanically ruptured when they pass through capillaries in contracting muscles.20,45 Furthermore, exercise training under hypoxia was shown to cause RBC senescence, reduce deformability under shear flow, and thus increase susceptibility to membrane rupture. Altered RBC fragility is observed in a number of disease conditions, including hereditary spherocytosis, chronic liver diseases, and thalassemia. Our study therefore suggests that mechanisms controlling RBC deformability and membrane fragility will also contribute to modulation of ATP release via hemolysis. For example, the Ca2+-activated K+ channel (Gardos channel), by controlling RBC dehydration, participates in the regulation of erythrocyte deformability and its activity is suppressed in senescent cells.22 Moreover, elevated intracellular calcium is known to increase RBCs’ osmotic fragility, which may in part involve specific Ca2+-induced lytic vulnerability of membrane,46 eg, via activation of scramblase and calpain and subsequent lysis of cytoskeletal proteins.22,47
In summary, the results presented here call into question current views on the mechanism of stimulated ATP release from RBCs and show that hemolysis is the primary release mechanism in these cells. In vivo, such an ATP release mechanism, involving lysis of senescent cells, might have physiologically relevant roles, eg, matching oxygen supply with demand during exercise and hypoxia, where elevated intravascular hemolysis is observed.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (MOP64364 to R.G.). The authors thank Dr R. Beaulieu, Hematology Department, Centre Hospitalier de l'Université de Montréal – Hôtel-Dieu, Montreal, for help in obtaining blood samples, and Dr J. W. Hanrahan, McGill University, for helpful discussions and comments on the manuscript.
Authorship
Contribution: R.G., principal investigator in this study, contributed to the study design, its conduct, data analysis, and drafting the manuscript; J.S. performed all luminometry experiments and data analysis and drafted the graphs and “Results”; K.F. and R.G. contributed to luminescence imaging experiments and their analysis; and S.N.O. contributed to the study plan and research direction. All authors contributed to data review and provided comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ryszard Grygorczyk, CRCHUM, Tour Viger, 900, rue St-Denis, Montreal, QC, Canada H2X 0A9; e-mail: ryszard.grygorczyk@umontreal.ca.