In this issue of Blood, Low and colleagues show that reactive oxygen species (ROS)–mediated phosphorylation of Bcl-2 overcomes chemoresistance in hematopoietic malignancies and other cancers and that this function is mediated by selective nitration of Y289 on the Bcl-2–bound B56δ subunit of protein phosphatase 2A (PP2A), which interferes with the interaction of Bcl-2 with the PP2A catalytic core, leading to increased Bcl-2 S70 phosphorylation.1
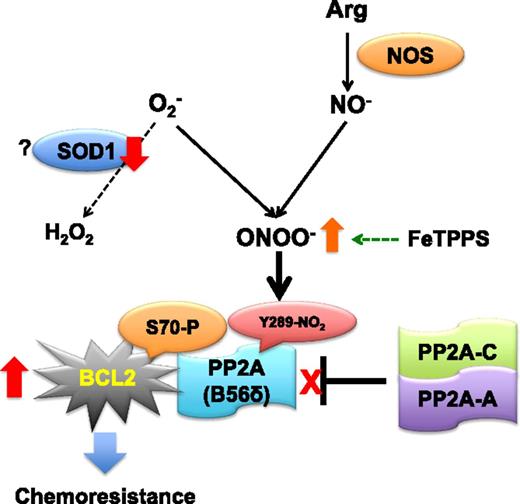
ROS generation begins with the formation of O2−, which is produced by 1-electron reduction of molecular oxygen (O2). The first line of cellular defense against high O2− levels is dismutation of O2− into O2 and H2O2 by SOD. In the presence of nitric oxide (NO), O2− can more rapidly react with NO than with SOD to form ONOO−, a highly reactive ROS species. In lymphoma cells, downregulation of SOD1 expression increases intracellular ONOO− formation by reacting with NO, which is formed from arginine by NOSs. ONOO− can nitrate Y289 of B56 and leads to the dissociation of the PP2A-AC heterodimer from the Bcl-2–bound B56δ subunit of PP2A, resulting in the enhanced phosphorylation of Bcl-2 S70. This may confer resistance to drug-induced cell death. NOS, NO synthase.
ROS generation begins with the formation of O2−, which is produced by 1-electron reduction of molecular oxygen (O2). The first line of cellular defense against high O2− levels is dismutation of O2− into O2 and H2O2 by SOD. In the presence of nitric oxide (NO), O2− can more rapidly react with NO than with SOD to form ONOO−, a highly reactive ROS species. In lymphoma cells, downregulation of SOD1 expression increases intracellular ONOO− formation by reacting with NO, which is formed from arginine by NOSs. ONOO− can nitrate Y289 of B56 and leads to the dissociation of the PP2A-AC heterodimer from the Bcl-2–bound B56δ subunit of PP2A, resulting in the enhanced phosphorylation of Bcl-2 S70. This may confer resistance to drug-induced cell death. NOS, NO synthase.
ROS are involved in many vital physiological processes such as host defense and biosynthesis at low to moderate concentrations; however, high levels of ROS can cause biological damage termed oxidative stress and are thereby implicated in many pathophysiological conditions, including cardiovascular diseases, neurological disorders, and cancers.2 Under oxidative stress conditions, excessive ROS can damage cellular proteins, lipids, and DNA, leading to genomic instability, genetic mutation, and modulation of gene expression that may contribute to tumorigenesis.3 Because cancer cells often display increased ROS generation and disturbed redox regulation, the increase of ROS in cancer cells often induces redox adaptation in response to the sustained oxidative stress, leading to upregulation of antioxidant molecules, such as reduced glutathione, which may confer resistance toward anticancer agents. One of the most important ways that ROS function in cancer is by activating various intracellular signaling pathways. Hydrogen peroxide (H2O2) can also function as a second messenger, activating intracellular pathways such as mitogen-activated protein kinases (MAPKs), phosphoinositide 3-kinase/serine-threonine kinase, and nuclear factor-κB pathways. H2O2 can activate protein kinase and protein phosphatase through oxidative modification of key cysteine residues.4 Tyrosine nitration is also one of the important posttranscriptional modifications of proteins that may interfere with signaling pathways. Peroxynitrite (ONOO–) reacts with tyrosine residues in proteins to form nitrotyrosine and often changes protein structure and function. Aberrant protein tyrosine nitration is associated with different diseases, including inflammatory diseases and neurodegenerative diseases.5 In cancers, there are only a few reports identifying molecules that are targeted for tyrosine nitration. These include tumor suppressor p53,6 the xenobiotic-metabolizing enzyme, arylamine N-acetyltransferase 1,7 cytochrome c, and procaspase 38 ; however, the functional significance of these nitration events remains unknown. Besides protein tyrosine nitration being an important biomarker for oxidative stress, ONOO– is now being recognized as an important modulator of various cell signaling pathways. Therefore, of particular interest is the discovery of specific protein targets for nitration in various signal transduction pathways.
In a previous study, the Pervaiz group demonstrated that the antiapoptotic activity of Bcl-2, first discovered in t(14:18) follicular lymphomas, is related to intracellular superoxide (O2−) levels in human acute lymphoblastic leukemia CEM cells.9 Because Bcl-2 overexpression has been implicated in the growth and chemoresistance of tumor cells, Low et al evaluated the mechanisms by which cellular ROS can strengthen the antiapoptotic function of Bcl-2 and whether the mechanisms are clinically relevant. A mild increase in intracellular O2− concentration by pharmacological inhibition (diethyldithiocarbamate [DDC]) or knockdown of superoxide dismutase 1 [SOD1] augmented S70 phosphorylation of Bcl-2, and this phosphorylation was essential for O2−-mediated chemoresistance in Jurkat T-cell leukemia and other cancer cells. Because the phosphorylation status of Bcl-2 is regulated by MAPKs (extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38) and/or heterotrimers of PP2A, the effects of DDC on the functions of these signaling proteins were evaluated. The results show that inactivation of PP2A phosphatase is the main cause of the upregulation of Bcl-2 S70 phosphorylation. The elevated O2− concentration inhibited the recruitment of PP2A to mitochondrial Bcl-2 by preventing the holoenzyme assembly of PP2A. By coimmunoprecipitation experiments, B56δ was identified as an interacting partner of Bcl-2 and the binding of Bcl-2 to PP2A-AC heterodimer was significantly inhibited by increased intracellular O2−. Furthermore, Low et al show that reduction of SOD1 expression in Jurkat cells enhances the production of ONOO− by unknown mechanisms, and that ONOO− nitrates Y289 of B56δ and inhibits PP2A holoenzyme assembly, leading to enhanced S70 phosphorylation of Bcl-2 and apoptotic resistance to anticancer drug (see figure). Importantly, elevated phosphorylation of Bcl-2 S70 was shown to be associated with O2−-induced nitration of B56δ Y289 in primary cells derived from clinical human lymphomas.
PP2A is a well-known tumor suppressor that is frequently mutated in a variety of human malignancies.10 Posttranslational modifications, including phosphorylation, nitration, and methylation, can also affect the function of PP2A. This is the first report of the association between ROS and promotion of chemoresistance via nitration of PP2A. This study also suggests that nitration of PP2A-B56δ and phosphorylation of S70 Bcl-2 could serve as biomarkers for diagnosis or molecular targets for therapeutic intervention, particularly in ROS-driven neoplastic processes.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal