In this issue of Blood, Palomero et al elucidate a mechanism whereby SOX11 (SRY [Sex determining region-Y]-box 11) promotes angiogenesis in mantle cell lymphoma (MCL).1
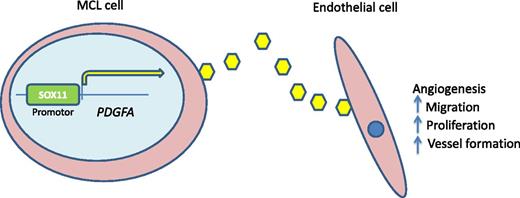
SOX11-PDGFA paracrine axis. SOX11 serves to transcriptionally regulate PDGFA. Secretion of PDGFA promotes angiogenesis to support lymphomagenesis. Inhibition of this pathway by agents such as imatinib mesylate may be an avenue for clinical trial investigation.
SOX11-PDGFA paracrine axis. SOX11 serves to transcriptionally regulate PDGFA. Secretion of PDGFA promotes angiogenesis to support lymphomagenesis. Inhibition of this pathway by agents such as imatinib mesylate may be an avenue for clinical trial investigation.
MCL is a mature CD5-positive B-cell lymphoma, thought to be derived from a pregerminal center antigen naive B cell, although a subset of cases appear to show evidence (immunoglobulin heavy chain somatic hypermutation) of germinal center passage.2 The genetic hallmark is the IGH/CCND1 fusion that leads to overexpression of cyclin D1.2 At first thought to be an aggressive lymphoma with poor prognosis, an indolent nonnodal form has been recognized with disease mostly limited to blood, bone marrow, and spleen.3,4 SOX11, a member of the C subgroup of the SOX family of genes, has recently been shown to be expressed in virtually all cases of aggressive mantle cell lymphoma (MCL) whereas indolent forms appear to have decreased expression.2,4 In contrast, it is not expressed in other mature small B-cell lymphomas but can be seen in a subset of aggressive B-cell lymphomas such as Burkitt lymphoma and B-lymphoblastic lymphoma.5 SOX11 is not expressed in normal lymphoid progenitors or mature B cells. As a diagnostic marker, SOX11 has been used to recognize cyclin D1–negative MCL.6 Although we have learned how to use SOX11 diagnostically, we are only beginning to understand the biology behind the expression of SOX11 in MCL. Earlier work by the same group demonstrated a role for SOX11 in MCL by upregulating PAX5 and blocking B-cell maturation by maintaining PRDM1/BLIMP1 repression.7
In the current work, tools developed from these earlier studies, such as cell lines with SOX11 knockdown, enabled further examination of the role of SOX11 in MCL pathogenesis. Gene expression studies comparing SOX11 knockdown and SOX11-positive xenografts showed enrichment of genes involved in angiogenesis in the SOX11-positive tumors. This was confirmed at the protein level with antibody arrays and supported by increased microvessel density in the SOX11-positive xenograft tumors. Functional evidence of a secreted factor was shown in conditioned media from SOX11-positive cell lines, which promoted angiogenesis in tube formation, endothelial proliferation, and migration assays.
The authors identified platelet-derived growth factor alpha polypeptide (PDGFA) as the responsible factor in a series of elegant experiments in which a limited set of candidate proangiogenic factors was identified by comparing the gene expression profiles of SOX11-positive and SOX11-silenced cell lines. They then interrogated conditioned media for evidence of secretion of the corresponding proteins by antibody arrays, and PDGFA was the only one of these candidates found to be present in the medium. PDGFA was further validated based on prior genome-wide chromatin immunoprecipitation-on-chip studies that identified blood vessel development as an important process that was overrepresented by SOX11-bound genes. Interestingly, of the candidate genes identified in the aforementioned gene expression studies, PDGFA was the only SOX11-bound gene found in these chromatin immunoprecipitation studies. As further confirmation, the authors show that SOX11 was capable of upregulating PDGFA expression by luciferase reporter assays.
Furthering the functional and potential clinical importance of these findings, the authors demonstrate that conditioned media from SOX11-positive cell lines resulted in phosphorylation of PDGFRA but not PDGFRB in human vascular endothelial cells. Importantly, inhibition of PDGFA signaling with imatinib or PDGFA-neutralizing antibodies inhibited angiogenesis in response to conditioned media from SOX11-positive cell lines. Thus, a SOX11-PDGFA paracrine angiogenesis axis has been discovered (see figure). In vivo modeling supported these results. In a final set of experiments, the authors show that SOX11-positive MCL primary tumors overexpress PDGFA and have increased microvessel densities, compared with SOX11-negative lymphomas. The therapeutic implications were shown in xenograft studies in which imatinib treatment reduced tumor growth and angiogenesis.
Taken together, these data demonstrate a new biologic role for SOX11 in the pathogenesis of MCL. SOX11 induces transcription of PDGFA which, in a paracrine manner, promotes angiogenesis and thus growth of the lymphoma. This may also explain the clinical features of indolent, nonnodal forms of MCL because the lack of SOX11 in these cases may be the reason for prolonged localization in blood, bone marrow, and spleen. These cells may still retain biologic characteristics of normal mantle cells because at least some cases of monoclonal B-cell lymphocytosis–like nonnodal MCL appear to have an “in situ” MCL pattern when gastrointestinal staging biopsies are performed.8
Although our understanding of the biology of SOX11 in MCL is significantly advanced through this article,1 questions still of course remain. Some controversy does exist regarding the prognostic relevance of SOX11 because some groups have reported SOX11 as a favorable factor, whereas others report it as an adverse factor.9,10 Careful analysis of inclusion criteria in such studies as well as further integrative studies to include the genomic mutational landscape will be required. However, from a therapeutic perspective, this opens a new avenue of investigation. The activity of lenalidomide in non-Hodgkin lymphomas including MCL may provide hints that angiogenesis is a relevant target given antiangiogenesis is one possible effect; however, the immunomodulatory and other putative actions of this drug cloud the issue. In light of this study, the inhibition of PDGFRA signaling via any number of biological drugs is a logical next step. Such studies may help define the importance of this pathway in MCL, explore the relevance of assessing SOX11 expression as a predictive biomarker, and hopefully improve the outcome for patients with MCL.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal