In this issue of Blood, Yeung et al provide evidence of a role for 12-lipoxygenase in platelet activation through FcγRIIa, which may provide a therapeutic target in heparin-induced thrombocytopenia (HIT).1
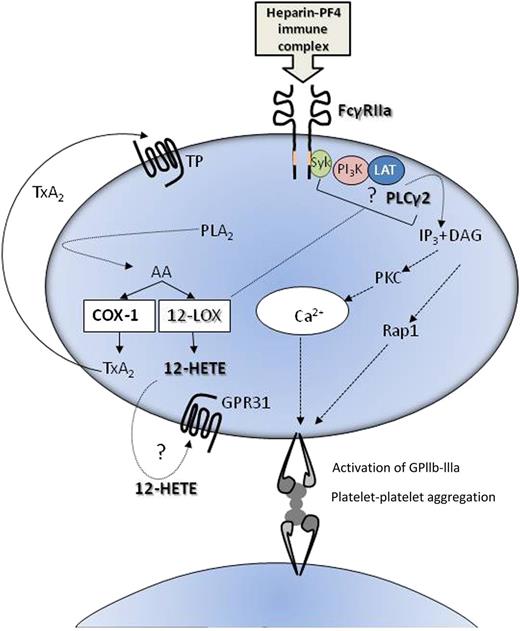
Possible roles of 12-LOX in platelet activation through FcγRIIa. 12-LOX may enhance platelet activation induced by ligation of FcRγIIa through either 12-HETE generated from AA, through its receptor GPR31, or via an interaction involving PLCγ2 and/or upstream signaling and adaptor molecules such as Syk (spleen tyrosine kinase), PI3K (phosphinositide 3-kinase), and LAT (linker for activation of T cells). DAG, diacyl glycerol; IP3, inositol triphosphate; PLA2, phospholipase A2.
Possible roles of 12-LOX in platelet activation through FcγRIIa. 12-LOX may enhance platelet activation induced by ligation of FcRγIIa through either 12-HETE generated from AA, through its receptor GPR31, or via an interaction involving PLCγ2 and/or upstream signaling and adaptor molecules such as Syk (spleen tyrosine kinase), PI3K (phosphinositide 3-kinase), and LAT (linker for activation of T cells). DAG, diacyl glycerol; IP3, inositol triphosphate; PLA2, phospholipase A2.
HIT can be a serious complication for patients receiving heparin, particularly in the context of surgery. Although relatively rare (affecting up to 5% of patients), the apparently paradoxical combination of thrombocytopenia and thrombosis seen in HIT can be life threatening. It is caused by heparin binding to platelet factor 4 (PF4) released from platelets, which in some patients can result in immune recognition of the structurally modified heparin-PF4 complexes. Binding of these immune complexes to the immunoglobulin (Ig)G Fc receptor on human platelets, FcγRIIa (CD32), results in their activation.2
FcγRIIa is 1 of 3 members of the immunoreceptor-based activatory motif (ITAM) family of tyrosine kinase signaling receptors in human platelets; the others are the glycoprotein VI collagen receptor and the recently discovered C-type lectin receptor 2.3 These differ in their signaling pathway from the G protein-coupled receptors (GPCRs) for all other platelet agonists such as thrombin, adenosine 5′-diphosphate (ADP), and thromboxane A2 (TXA2).
Activation via ITAMs leads not just to platelet aggregation and degranulation but also to formation of procoagulant platelets and the release of procoagulant microparticles (MPs). Hence, HIT is characterized by a fall in platelet numbers as they disintegrate and a release of platelet-derived MPs into the circulation that promote thrombin generation.2
The focus of the paper from Yeung et al is the role of platelet 12(S)-lipoxygenase (12-LOX) in platelet activation through FcγRIIa. Platelets have long been known to contain 12-LOX, but its role in platelet biology has remained elusive, unlike that of the other main platelet oxygenase cyclooxygenase-1 (COX-1). As is well known, COX-1 converts the arachidonic acid (AA) that is liberated from membrane phospholipids following activation, to the platelet agonist TXA2, which on release activates platelets in an autocrine and paracrine manner through their thromboxane prostanoid (TP) receptors. Specific and irreversible inhibition of COX-1 by aspirin is a mainstay of antiplatelet therapy.
However, there is an alternative pathway for the metabolism of AA in the platelet, via 12-LOX, which metabolizes AA to 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid (12-HpETE).4 This is rapidly reduced to12-hydroxyeicosatretraenoic acid (12-HETE) and released from the platelets in nanomolar quantities. 12-HETE is known to play a role in trans-cellular communication, but its role in platelets in less clear. Reports over the last 30 years have variously suggested that 12-HpETE and 12-HETE can either augment or inhibit platelet activation.4 Studies with 12-LOX inhibitors have also proved inconsistent, largely due to the lack of specificity.
Recently, medicinal chemists in Rockville and University of California, Santa Cruz have developed inhibitors that are selective for 12(S)-LOX over other lipoxygenases and cyclooxygenases.5 Holinstat’s group6,7 used these inhibitors to provide clear evidence for the involvement of 12-LOX in aggregation and degranulation of platelets activated by collagen as well as by ADP and thrombin, the latter via the protease-activated receptor (PAR)4 receptor, but interestingly, not via PAR1. In the current paper, this group use one of these new inhibitors (ML355) to provide evidence that 12-LOX also plays an important role in the activation of platelets through FcγRIIa. They use 2 ligands known to activate platelets through FcγRIIa; a cross-linked anti-CD32 monoclonal antibody (mAb) and a CD9 mAb that binds to the CD9 antigen via its immunoglobulin Fab regions and to FcγRIIa via its Fc domain. Through a series of in vitro experiments in human platelets, they demonstrate that activation of GPIIb-IIIa (the platelet fibrinogen receptor) and consequent aggregation and degranulation are attenuated by ML355 via a mechanism that also affects phosphorylation of phospholipase C γ2 (PLCγ2), calcium mobilization, and phosphorylation of protein kinase C (PKC) and Ras-proximate-1 or Ras-related protein 1 (Rap1; key to activation of GPIIb-IIIa), while having no direct effect on phosphorylation of FcγRIIa itself. The reduced phosphorylation of Rap1 was confirmed in platelets from 12-LOX−/− mice, indicating that the effects were specific to 12-LOX.
Taken together, the data suggest that inhibition of 12-LOX may be a viable therapeutic target in patients with HIT. Preclinical testing of ML355 indicates a favorable pharmacological profile, and as 12-LOX appears, like TXA2 and ADP, to augment platelet activation, inhibiting 12-LOX may reduce rather than ablate the platelet response, predicting a favorable balance between thrombotic and bleeding risk. In addition, data from this group and others suggest a wider therapeutic potential because 12-LOX can also affect the response to collagen ADP and thrombin.
Many questions still remain. Importantly, although ML355 is shown to block generation of 12-HETE, it is unclear whether the effect of inhibiting 12-LOX on the platelet response to FcγRIIIa is via 12-HETE interacting with its recently identified receptor (GPCR31)8 (analogous to the role of TXA2) or via protein-protein interactions within the platelets, which is a reported mechanism of 12-LOX activity in other cells.9-12 It is known that platelet activation causes translocation of 12-LOX from the cytoplasm to the membrane,13 but its interacting partners are yet to be fully characterized. However, the rate of effect of ML355 on 12-LOX activity and the effects on the downstream signaling pathways of FcγRIIa favor an intracellular mechanism for 12-LOX either directly through PLCγ2 or via an upstream ITAM signaling complex (see figure).
What is important is that this is the first demonstration that 12-LOX is involved in platelet activation through the immune Fc receptor FcγRIIa and as such may provide a viable therapeutic target for patients with HIT. It is also worth noting our own observation that following infusion of heparin, there is a significant release of 12-HETE in vivo, despite aspirin therapy,14 suggesting 12-LOX may be even more intrinsically involved in the pathology of HIT. However, because 12-HETE has roles in other cells, the effect of inhibiting 12-LOX may have wider ranging effects that will be revealed through in vivo studies with inhibitors such as ML355.
Conflict-of-interest disclosure: The author declares no competing financial interests.