Key Points
In this phase 1/2 study, obinutuzumab (GA101) monotherapy was active in patients with relapsed or refractory CLL.
Best overall response was lower in phase 2 vs phase 1, possibly due to higher baseline tumor burden resulting in lower treatment exposure.
Abstract
GAUGUIN evaluated the safety and efficacy of obinutuzumab (GA101) monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia (CLL). In phase 1 (dose escalation), 13 patients received obinutuzumab 400 to 1200 mg (days 1 and 8 of cycle 1; day 1 of cycles 2-8). In phase 2, 20 patients received a fixed dose of 1000 mg (days 1, 8, and 15 of cycle 1; day 1 of cycles 2-8). Infusion-related reactions occurred in nearly all patients, but few were grade 3/4. Grade 3/4 neutropenia occurred in 7 patients in phase 1 (but was not dose-related) and in 4 patients in phase 2. Overall end-of-treatment response (all partial responses) was 62% (phase 1) and 15% (phase 2); best overall response was 62% and 30%, respectively. Phase 2 median progression-free survival was 10.7 months and median duration of response was 8.9 months. In summary, obinutuzumab monotherapy is active in patients with heavily pretreated relapsed/refractory CLL. GAUGUIN was registered at www.clinicaltrials.gov as #NCT00517530.
Introduction
The anti-CD20 monoclonal antibody (mAb) rituximab (Rituxan; MabThera) dramatically improved outcomes in B-cell non-Hodgkin lymphoma (NHL) and, combined with chemotherapy, is standard care for chronic lymphocytic leukemia (CLL). Adding rituximab to fludarabine plus cyclophosphamide significantly improved progression-free survival (PFS) and overall survival in young, fit patients with untreated CLL1 ; adding rituximab to chlorambucil improved complete response (CR) rates compared with historical chlorambucil monotherapy in older, less fit patients.2,3 However, response rates in elderly patients remain low and virtually all patients will relapse4 ; improved response rates and disease outcomes in CLL patients are needed, and may be addressed by novel anti-CD20 antibodies.
Obinutuzumab (GA101) is the first glycoengineered type II anti-CD20 mAb in clinical testing.5 It is a humanized IgG1 antibody with low-fucose Fc oligosaccharides, and enhanced binding to immune cell-surface FcγRIIIa, leading to increased in vitro antibody-dependent cellular cytotoxicity.5
Type I and II mAbs differ in their CD20 binding and activation of cell death pathways. Type I antibodies (rituximab-like) induce migration and stabilization of CD20-mAb complexes into lipid rafts and activate complement-dependent cytotoxicity (CDC). Type II antibodies (obinutuzumab-like) do not induce this translocation or considerable CDC; instead, they stimulate increased direct cell death5,6 associated with actin reorganization and homotypic adhesion,7 distinct from classical apoptosis. Obinutuzumab has superior killing activity vs rituximab in nonmalignant and CLL cells in in vitro and ex vivo whole-blood assays5,8,9 and xenograft models.5 Obinutuzumab also induces greater B-cell depletion than rituximab in cynomolgus monkeys, particularly in the spleen and lymph nodes.5
Encouraging preclinical data led to obinutuzumab clinical evaluation in B-cell malignancies.10-13 GAUGUIN phase 1 investigated the safety and tolerability of obinutuzumab monotherapy in heavily pretreated relapsed/refractory CLL, and established a dose for phase 2 examination. We report final safety and efficacy data from CLL patients participating in both GAUGUIN study phases. Data from patients with indolent or aggressive NHL in GAUGUIN have been published previously.10,14,15
Methods
Patients
Eligible patients had relapsed/refractory CD20-positive CLL (per International Workshop criteria for CLL),16 confirmed by flow cytometry, for which anti-CD20 antibody treatment was considered appropriate with no higher priority therapy available. Relapse required a documented history of relapse after a response lasting ≥6 months to all prior fludarabine and/or rituximab-containing regimens. Refractory was defined as progression on treatment, stable disease (SD), or relapse after partial response (PR) or better <6 months after any prior fludarabine and/or rituximab-containing regimen.
Patients were aged ≥18 years with a life expectancy >3 months and an Eastern Cooperative Oncology Group performance status 0 to 2, with ≥1 bidimensionally measurable lesion (>1.5 cm by computerized tomography scan). Exclusion criteria included anti-leukemia therapy/radiation in the 4 weeks prior to enrollment, rituximab within 56 days of study entry, or radioimmunotherapy in the 3 months prior to study entry.
Patients with abnormal blood chemistry (aspartate aminotransferase or alanine aminotransferase levels >2.5 times the upper limit of normal, platelet counts <75 × 109 cells per liter, neutrophil counts <1.5 × 109 cells per liter, hemoglobin concentrations <10 g/dL) or positivity for HIV, hepatitis B, or hepatitis C were excluded. All patients provided written informed consent. GAUGUIN was conducted in accordance with the Declaration of Helsinki; the protocol was approved by local ethics committees. The authors had access to the primary data.
Study design
GAUGUIN was open label. Phase 1 investigated the safety and tolerability of escalating IV doses of obinutuzumab monotherapy (400/800 mg, 800/1200 mg, and 1200/2000 mg) given in 8 3-weekly cycles to patients with CLL in a standard 3 + 3 design with ≥3 patients per regimen. The lower dose of each regimen was given as a single infusion on day 1, cycle 1; if this infusion was well tolerated, the higher dose was given for the remainder of the regimen (8 infusions: day 8, cycle 1 and day 1, cycles 2-8: Figure 1A). A fourth regimen, with a fixed dose (1000 mg) for all infusions over the same 8-cycle schedule, was added subsequently (Figure 1A). Obinutuzumab was diluted to 10 mg/mL and administered initially at 50 mg per hour. In the absence of infusion-related reactions (IRRs), infusion rate was increased by 50 mg per hour every 30 minutes to 400 mg per hour. Patients were premedicated with oral acetaminophen/paracetamol (650-1000 mg) and an antihistamine 30 minutes before first infusion and before subsequent infusions in patients at high risk of tumor lysis syndrome (TLS) or in those experiencing IRRs during previous infusions. Corticosteroid premedication was considered in patients at high risk of severe IRRs or those previously requiring corticosteroid premedication for rituximab-associated IRRs. Use of granulocyte colony-stimulating factor (G-CSF) was permitted per institutional practice for severe hematological toxicity (eg, common terminology criteria grade 4 neutropenia). General prophylactic G-CSF was not permitted.
Study design and patient disposition in the GAUGUIN study. (A) Phase 1. (B) Phase 2. *One patient enrolled in phase 2 of GAUGUIN did not enter follow-up because of a new anti-leukemia therapy.
Study design and patient disposition in the GAUGUIN study. (A) Phase 1. (B) Phase 2. *One patient enrolled in phase 2 of GAUGUIN did not enter follow-up because of a new anti-leukemia therapy.
It was calculated that the sample size of 20 for phase 2 would enable the response rate to be estimated with a precision of 14% (80% confidence intervals) assuming a response rate of 40%. Phase 2 dose selection was based on safety and preliminary efficacy data and on modeling and simulation of pharmacokinetic data. The latter showed faster elimination of obinutuzumab in the first cycle than later cycles, indicating a need for a more dose-dense regimen in the first cycle. A fixed-dose regimen of obinutuzumab 1000 mg was selected, administered in the same dose schedule as in phase 1, with an additional infusion on day 15, cycle 1. Phase 2 participants therefore received up to 10 doses of 1000 mg of obinutuzumab (days 1, 8, and 15, cycle 1 and day 1, cycles 2-8; Figure 1B). The phase 2 primary objective was to investigate the efficacy and safety of obinutuzumab at this chosen dose. The primary efficacy end points in phase 2 were end-of-treatment response (ETR) and best overall response (BOR) rates.
Safety assessments
Adverse events (AEs) were assessed per National Cancer Institute Common Terminology Criteria (version 3.0). During phase 1, safety assessments were performed on days 1 and 8, cycle 1, every 3 weeks thereafter, and 28 days after last obinutuzumab dose. The primary safety end point was incidence of dose-limiting toxicities, defined as obinutuzumab-related grade 3/4 AEs (other than B-cell depletion, lymphopenia, neutropenia that was not febrile and improved to grade ≤2 within 1 week, and thrombocytopenia that improved to grade 3 within 1 week and grade ≤2 within a further week without platelet transfusion) occurring within 28 days of each administration. Dose-limiting toxicities for the first dose of obinutuzumab were considered to be any grade 4 infusion-related toxicity or any grade 3 infusion-related toxicity that did not resolve with a reduced infusion rate, dose interruption/discontinuation, or corticosteroids.
During phase 2, safety was examined throughout treatment, and 28 days and 2 months after last infusion. After treatment completion, patients entered a 2-year follow-up.
Efficacy assessments
Tumor response was assessed after cycle 4 and 1 month after cycle 8 (or 28 days after last infusion for those who withdrew) by clinical examination, laboratory assessment, and computed tomography scan per International Workshop criteria for CLL guidelines.16 ETR was measured again 2 months after last dose to confirm response (CR or PR). Response was then assessed every 3 months over 6 to 18 months after treatment. CR, incomplete CR, and PR were considered treatment responses; BOR was defined as the best response experienced during the study prior to new anti-leukemia therapy.
Pharmacokinetic and pharmacodynamic assessments
A description of the timing of assessments for pharmacokinetic and pharmacodynamic investigations is given in supplemental material (see supplemental Methods available at the Blood Web site).
Statistics
The safety and efficacy populations comprised patients who received ≥1 dose of obinutuzumab. Baseline and demographic characteristics, AE frequency, and response rates are presented using descriptive statistics. Survival curves and median time to event for PFS were estimated using Kaplan-Meier methodology. Nonlinear mixed effects modeling was used to determine dose concentration-time data for obinutuzumab.
Results
Patients
In phase 1, 13 patients with CLL were recruited from 7 centers across France. Table 1 shows demographic and baseline disease characteristics. Eight patients previously received rituximab, but none were refractory; 6 (75%) had CR to last treatment. No patient withdrew from phase 1 (Figure 1A).
Baseline demographics and disease characteristics
| . | Phase 1, n = 13* . | Phase 2, n = 20 . |
|---|---|---|
| Demographics | ||
| Median age, y (range) | 64.0 (46-81) | 62.5 (36-81) |
| ≤70 y, n (%) | 9 (69) | 15 (75) |
| >70 y, n (%) | 4 (31) | 5 (25) |
| Male, n (%) | 9 (69) | 12 (60) |
| Disease characteristics | ||
| Median time from diagnosis, y (range) | 7.6 (2.6-15.2) | 8.7 (2.0-22.6) |
| Median no. of previous treatments, n (range) | 3 (1-8) | 3 (1-7) |
| Prior rituximab treatment, n (%) | 8 (62) | 10 (50) |
| Prior fludarabine treatment, n (%) | 13 (100) | 18 (90) |
| Rituximab refractory, n (%) | 0 (0) | 3 (15) |
| Screening assessment | ||
| Binet stage, n (%) | ||
| A | 5 (38) | 7/19 (37) |
| B | 6 (46) | 7/19 (37) |
| C | 1 (8) | 2/19 (11) |
| Unknown | 1 (8) | 3/19 (16) |
| Hematologic parameters | ||
| Median hemoglobin, g/dL (range) | 12.6 (9.4-14.9) | 12.2 (9.6-15.9) |
| Median platelets × 109 (range) | 191 (48-404) | 120 (38-246) |
| Median neutrophils × 109 (range) | 4.0 (1.3-8.6) | 2.6 (0-15) |
| Median lymphocytes × 109 (range) | 47.4 (7.0-119.3) | 58 (0.6-134.0) |
| Lymph nodes | ||
| ≥5 cm | 4 (31) | 7 (35) |
| ≥10 cm | 2 (15) | 1 (5) |
| Patients with splenomegaly, n (%) | 3 (23) | 8 (40) |
| Median SPD, mm2 (min-max) | 2124 (1068-26 732) | 3138 (330-6399) |
| . | Phase 1, n = 13* . | Phase 2, n = 20 . |
|---|---|---|
| Demographics | ||
| Median age, y (range) | 64.0 (46-81) | 62.5 (36-81) |
| ≤70 y, n (%) | 9 (69) | 15 (75) |
| >70 y, n (%) | 4 (31) | 5 (25) |
| Male, n (%) | 9 (69) | 12 (60) |
| Disease characteristics | ||
| Median time from diagnosis, y (range) | 7.6 (2.6-15.2) | 8.7 (2.0-22.6) |
| Median no. of previous treatments, n (range) | 3 (1-8) | 3 (1-7) |
| Prior rituximab treatment, n (%) | 8 (62) | 10 (50) |
| Prior fludarabine treatment, n (%) | 13 (100) | 18 (90) |
| Rituximab refractory, n (%) | 0 (0) | 3 (15) |
| Screening assessment | ||
| Binet stage, n (%) | ||
| A | 5 (38) | 7/19 (37) |
| B | 6 (46) | 7/19 (37) |
| C | 1 (8) | 2/19 (11) |
| Unknown | 1 (8) | 3/19 (16) |
| Hematologic parameters | ||
| Median hemoglobin, g/dL (range) | 12.6 (9.4-14.9) | 12.2 (9.6-15.9) |
| Median platelets × 109 (range) | 191 (48-404) | 120 (38-246) |
| Median neutrophils × 109 (range) | 4.0 (1.3-8.6) | 2.6 (0-15) |
| Median lymphocytes × 109 (range) | 47.4 (7.0-119.3) | 58 (0.6-134.0) |
| Lymph nodes | ||
| ≥5 cm | 4 (31) | 7 (35) |
| ≥10 cm | 2 (15) | 1 (5) |
| Patients with splenomegaly, n (%) | 3 (23) | 8 (40) |
| Median SPD, mm2 (min-max) | 2124 (1068-26 732) | 3138 (330-6399) |
SPD, sum of the products of the longest perpendicular dimensions of the six largest lymph nodes.
One patient in cohort 1200/2000 mg received 1600 mg as the first infusion instead of the assigned 1200 mg, therefore an extra patient was enrolled into the 1200/2000 mg cohort per protocol specifications, to confirm the safety and tolerability of the 1200/2000 mg dose in 3 patients.
In phase 2, 20 patients with CLL were recruited from 20 centers across France and Germany. Thirteen (65%) completed the treatment period (Figure 1B). During cycles 1 through 4, 7 patients withdrew due to AEs (n = 3), insufficient therapeutic response (n = 2), treatment refusal (n = 1), and inclusion criteria violation (n = 1).
Median time from diagnosis to study participation was 8.7 vs 7.6 years for phase 2 and 1 participants, respectively. Ten participants (50%) previously received rituximab, 3 were refractory. Phase 2 participants had higher median baseline sums of the products of the longest perpendicular dimensions (SPDs) of the 6 largest lymph nodes than phase 1 participants. More phase 2 than phase 1 participants had extranodal involvement (55% vs 15%).
Safety: treatment period
All phase 1 participants initiated all 9 planned obinutuzumab infusions. The first infusion was slowed, interrupted until IRR resolution, or permanently discontinued 4, 9, and 1 time(s) in 11 patients, respectively. Infusion duration was <8 hours, except for 3 infusions in 2 patients that were split over 2 days. All patients experienced ≥1 AE, most commonly IRRs, neutropenia, lymphopenia, and thrombocytopenia (Table 2). Most AEs were grade 1/2 (93 of 113, 82%). All patients experienced IRRs, with hypotension and pyrexia the most common symptoms. Ten patients (77%) experienced 20 grade 3/4 AEs. Seven patients (54%) experienced ≥1 episode of grade 3/4 neutropenia; 3 episodes had onset during cycle 1. The median duration of grade 3/4 neutropenia was 10 days (range, 7-62 days). All patients with grade 3/4 neutropenia received all 9 scheduled obinutuzumab infusions without dose reduction. All received G-CSF per institutional practice. One patient (400/800 mg dose group) experienced grade 4 febrile neutropenia (onset day 137).
Most common AEs during the treatment period
| . | Phase 1, n = 13 . | Phase 2, n = 20 . |
|---|---|---|
| Patients with at least 1 AE (all grades), n (%) | 13 (100) | 20 (100) |
| IRR* | 13 (100) | 19 (95) |
| Hypotension | 10 (77) | 12 (60) |
| Pyrexia | 8 (62) | 13 (65) |
| Chills | 7 (54) | 6 (30) |
| Vomiting | 6 (46) | 11 (55) |
| Neutropenia | 7 (54) | 4 (20) |
| Thrombocytopenia | 4 (31) | 3 (15) |
| Pyrexia | 3 (23) | 4 (20) |
| Lymphopenia | 4 (31) | 2 (10) |
| Anemia | 1 (8) | 5 (25) |
| Febrile neutropenia | 1 (8) | 2 (10) |
| Patients with at least 1 AE (grade 3), n (%) | 8 (62) | 13 (65) |
| IRR | 2 (15) | 5 (25) |
| Neutropenia | 2 (15) | 1 (5) |
| Febrile neutropenia | 0 (0) | 0 (0) |
| Lymphopenia | 2 (15) | 2 (10) |
| Thrombocytopenia | 2 (15) | 0 (0) |
| Leukopenia | 2 (15) | 1 (5) |
| Anemia | 0 (0) | 2 (10) |
| Patients with at least 1 AE (grade 4), n (%) | 6 (46) | 7 (35) |
| Neutropenia | 5 (38) | 3 (15) |
| Febrile neutropenia | 1 (8) | 1 (5) |
| Thrombocytopenia | 0 (0) | 3 (15) |
| . | Phase 1, n = 13 . | Phase 2, n = 20 . |
|---|---|---|
| Patients with at least 1 AE (all grades), n (%) | 13 (100) | 20 (100) |
| IRR* | 13 (100) | 19 (95) |
| Hypotension | 10 (77) | 12 (60) |
| Pyrexia | 8 (62) | 13 (65) |
| Chills | 7 (54) | 6 (30) |
| Vomiting | 6 (46) | 11 (55) |
| Neutropenia | 7 (54) | 4 (20) |
| Thrombocytopenia | 4 (31) | 3 (15) |
| Pyrexia | 3 (23) | 4 (20) |
| Lymphopenia | 4 (31) | 2 (10) |
| Anemia | 1 (8) | 5 (25) |
| Febrile neutropenia | 1 (8) | 2 (10) |
| Patients with at least 1 AE (grade 3), n (%) | 8 (62) | 13 (65) |
| IRR | 2 (15) | 5 (25) |
| Neutropenia | 2 (15) | 1 (5) |
| Febrile neutropenia | 0 (0) | 0 (0) |
| Lymphopenia | 2 (15) | 2 (10) |
| Thrombocytopenia | 2 (15) | 0 (0) |
| Leukopenia | 2 (15) | 1 (5) |
| Anemia | 0 (0) | 2 (10) |
| Patients with at least 1 AE (grade 4), n (%) | 6 (46) | 7 (35) |
| Neutropenia | 5 (38) | 3 (15) |
| Febrile neutropenia | 1 (8) | 1 (5) |
| Thrombocytopenia | 0 (0) | 3 (15) |
Events occurring during or within 24 hours of an infusion for which there was a reasonable suspected causal relationship with that infusion.
Eight patients (62%) reported 9 infections; only bronchitis and influenza occurred in >1 patient. Two of the 9 were grade 3 (bronchitis and oral herpes); both were considered treatment-related by the investigator and resolved without sequelae. Four patients (31%) reported 7 serious adverse events (SAEs: febrile neutropenia, neutropenia, thrombocytopenia, gingivitis, IRR, bronchitis, and TLS). TLS was grade 3, considered to be obinutuzumab-related and resolved after treatment. No AEs led to withdrawal, and no deaths or dose-limiting toxicities were observed during phase 1.
No significant complement activation was observed; a slight decrease in C3 and C4 (Figure 2A), without a significant increase in C3a and C5a (data not shown), was seen immediately after first infusion. Relative to baseline and as expected, significant increases in cytokines were detected midway through the first obinutuzumab infusion (Figure 2B), coinciding with the emergence of IRRs.
Complement and cytokine levels over time among phase 1 and 2 study participants. (A) Complement C3 and C4 (mean ± standard deviation). (B) Cytokines (mean ± standard deviation). C, cycle; D, day.
Complement and cytokine levels over time among phase 1 and 2 study participants. (A) Complement C3 and C4 (mean ± standard deviation). (B) Cytokines (mean ± standard deviation). C, cycle; D, day.
In phase 2, 12 patients (60%) received all 10 planned infusions. The remainder received 1 (3 patients), 4 (1 patient), 6 (3 patients), and 9 (1 patient) infusions. The first obinutuzumab infusion was slowed, interrupted, or permanently discontinued 3, 13, and 3 times in 14 patients, respectively. Three patients subsequently required dose interruptions (2 during cycle 2 and 1 during cycle 8), all due to IRRs. Only 1 patient missed 1 obinutuzumab infusion (day 15, cycle 1). All patients experienced ≥1 AE during phase 2, most frequently IRRs, anemia, neutropenia, and pyrexia (Table 2).
Most AEs were grade 1/2 (74 of 101, 73%). Fifteen patients (75%) experienced 27 grade 3/4 events. Grade 3/4 neutropenia was reported in 4 patients during treatment; 3 experienced a single episode, with onset on days 27, 27, and 155, 1 experienced 4 episodes of grade 3/4 neutropenia, with onset on days 3, 37, 114, and 150. All patients received G-CSF per institutional practice at least once. All but 1 episode of grade 3/4 neutropenia resolved within 30 days (median duration, 17 days; range, 8-71 days). As in phase 1, all episodes of grade 3/4 neutropenia were considered treatment-related. The patient who experienced 4 episodes of grade 3/4 neutropenia also reported 1 episode of grade 4 febrile neutropenia (onset day 15).
Grade 4 neutropenia and thrombocytopenia were each experienced by 3 patients. No infections or bleeding episodes accompanied grade 4 neutropenia or thrombocytopenia. Two patients with grade 4 thrombocytopenia received platelet infusions. Six phase 2 participants (30%) reported 8 infections (herpes zoster [2 patients], bacterial infection, lung infection, nasopharyngitis, oral herpes, pharyngitis, testicular abscess). Of these, 3 were grade 3 (bacterial infection, herpes zoster, testicular abscess); none were grade 4. Herpes zoster was considered by the investigator to be treatment-related and resolved without sequelae.
Nine patients (45%) reported 11 SAEs (4 IRRs and 1 each of pyrexia, pure red cell aplasia, febrile neutropenia, pancytopenia, abdominal pain, bacterial infection, interstitial lung disease). SAEs led to 3 treatment discontinuations (2 IRRs, 1 interstitial lung disease; all 3 events resolved). No patient experienced TLS or died during treatment in phase 2.
Recommended premedication during GAUGUIN comprised oral acetaminophen and an antihistamine; steroid premedication was not recommended before the first infusion and was used as a curative treatment of IRRs, or as prophylaxis for those with a history of IRRs. In phase 1, 1 of 13 patients received steroid premedication on day 1 (2 patients in total received steroid premedication in phase 1, including 1 patient after day 1); in phase 2, 6 of 20 patients received steroid premedication on day 1 (8 patients in total received steroid premedication in phase 2, including 6 patients after day 1).
Safety: follow-up period
The median observation time for treatment and follow-up in phase 1 was 38.7 months (range, 14.4-44.5 months). From 28 days after last obinutuzumab dose to end of follow-up, 8 of 13 patients (62%) reported 22 AEs. Only cough (3 patients), nasopharyngitis (2), and sinusitis (2) occurred in >1 patient. Three grade 3/4 AEs (febrile neutropenia, cataract, lung cancer) and 3 SAEs (febrile neutropenia, pneumococcal sepsis, lung cancer) were reported. One episode of febrile neutropenia (onset day 295) was reported as both a grade 3/4 AE and SAE; this patient also experienced grade 4 febrile neutropenia during treatment. Of 8 infections during follow-up, only nasopharyngitis and sinusitis occurred in >1 patient (2 patients each). There were 5 deaths during follow-up: 4 progressive disease (PD) and 1 metastatic lung cancer.
Of the 20 phase 2 participants, 1 did not enter follow-up because of new anti-leukemia therapy. The median observation time in phase 2 was 28.8 months (range, 8.8-31.3 months). From 28 days after last obinutuzumab dose to the end of follow-up, 9 of 19 patients (47%) reported 20 AEs, most commonly nasopharyngitis (3 patients), cough (2), lung infection (2), and diarrhea (2). Two patients (11%) experienced 5 grade 3/4 AEs (lung infection, septic shock, neutropenia, pyrexia, renal cancer); all were considered treatment-related. The patient whose renal cancer was considered to be treatment-related had lesions present before obinutuzumab initiation. The grade 4 neutropenia during follow-up had its onset on day 371 and lasted 12 days; G-CSF was administered. Another 4 patients had low neutrophil counts reported during follow-up, 3 at the time of B-cell recovery.
Nasopharyngitis (3 patients; 16%) and lung infection (2 patients; 11%) were the only infections occurring in >1 patient. Two patients (11%) reported 4 treatment-related SAEs (lung infection, septic shock, pyrexia, renal cancer). There were 7 deaths (37%) during follow-up, attributed to PD (4 deaths), colon cancer (1), lung adenocarcinoma (1), and septic shock (1). Septic shock was the only treatment-related AE listed as leading to death. This patient received his last dose of obinutuzumab on day 148, initiated new anti-leukemia treatment (ofatumumab) on day 483, and died on day 823.
Efficacy
Both the ETR and BOR to obinutuzumab monotherapy during phase 1 were 62%. At end of treatment, all 8 responders (62%) had PR, 3 patients had SD, and 2 had PD. For BOR, 8 patients had PR and 5 had SD. Once the highest dose (1200/2000 mg) was analyzed, there appeared to be a dose-response relationship: 1 of 3 patients in each of the 400/800 mg and 1000/1000 mg cohorts, 2 of 3 patients in the 800/1200 mg cohort, and all 4 patients in the 1200/2000 mg cohort achieved PR. With a median follow-up of 38.7 months (range, 14.4-44.5 months), the median duration of response was 10.5 months (range, 8.5-37.0 months); 1 patient was still in remission at clinical cutoff.
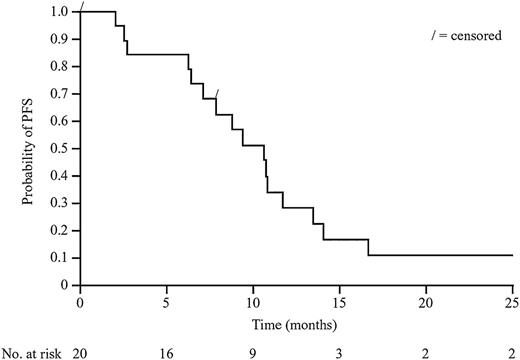
The ETR in phase 2 was 15% (3 PR, 5 SD, 8 PD, and 4 patients with missing data [1 eligibility criteria violation, 2 IRRs during first cycle, and 1 treatment refusal after 2 cycles]) and BOR was 30% (1 CR, 5 PR, 5 SD, 6 PD, and 3 missing data). With a median follow-up of 28.8 months (range, 8.8-31.3 months), median PFS was 10.7 months (95% confidence interval, 7.1-11.7). The Kaplan-Meier plot for PFS in phase 2 is shown in Figure 3. Median duration of response for the 6 patients with CR or PR at any point during treatment was 8.9 months (range, 0.8-26.1 months).
Eight and 10 patients in phase 1 and phase 2, respectively, had previously received rituximab. In rituximab-experienced patients, ETR and BOR were both 62.5% in phase 1 (n = 5 responders, all PR). In phase 2, there were no responders at end of treatment, but BOR was 20% (n = 2, both PR). This pattern broadly mirrors that in the overall cohorts for phase 1 and phase 2, although the patient numbers are small.
The influence of baseline tumor burden was examined (Figure 4). Nine of the 11 phase 1/2 participants with PR at treatment end had baseline SPD <2100 cm2. Among responders with baseline tumor burden >2100 cm2, 1 each received treatment with 400/800 mg and 1200/2000 mg. All responders with baseline SPD <2100 cm2 received an obinutuzumab dose ≥800/1200 mg (800/1200 mg [n = 2]; 1000/1000 mg [n = 4]; 1200/2000 mg [n = 3]). In general, patients with lower baseline tumor burden were more likely to respond to treatment. Median baseline tumor burden was higher in phase 2 than phase 1.
ETR by baseline tumor burden in phase 1 and 2 of the GAUGUIN study. *In phase 1 of GAUGUIN, 4 different doses of obinutuzumab were evaluated. In phase 2, all patients received obinutuzumab 1000/1000 mg. The size of the bar below the x-axis reflects the dose received by the patient.
ETR by baseline tumor burden in phase 1 and 2 of the GAUGUIN study. *In phase 1 of GAUGUIN, 4 different doses of obinutuzumab were evaluated. In phase 2, all patients received obinutuzumab 1000/1000 mg. The size of the bar below the x-axis reflects the dose received by the patient.
Pharmacokinetics
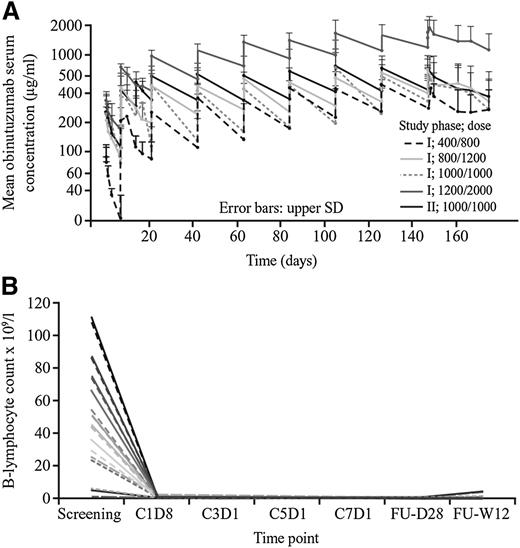
In phase 1, mean obinutuzumab serum concentrations (Cmax and Ctrough values) increased with increasing dose, with patients administered obinutuzumab 1200/2000 mg achieving the highest concentrations (Figure 5A). Mean Cmax and Ctrough values increased each day from the first infusion to the last, but steady state was not reached for any dose regimen. In phase 2, however, 1 additional infusion of 1000 mg in the 1000/1000 mg regimen during the first treatment cycle resulted in steady-state concentrations from cycle 2 onwards. It was not possible to assess the relationship between pharmacokinetics and clinical response or tumor burden due to the small number of patients in the phase 1 dose cohorts and low number of responders in phase 2.
Pharmacokinetic and pharmacodynamic properties of obinutuzumab. (A) Mean serum concentrations of obinutuzumab over time. (B) B-lymphocyte count over time in all GAUGUIN study participants. Reported lymphocyte counts are preinfusion for each. FU, follow-up; SD, standard deviation; W, week. The 2 patients who were B-cell depleted at screening were included based on prior CLL diagnoses and lymph node progression.
Pharmacokinetic and pharmacodynamic properties of obinutuzumab. (A) Mean serum concentrations of obinutuzumab over time. (B) B-lymphocyte count over time in all GAUGUIN study participants. Reported lymphocyte counts are preinfusion for each. FU, follow-up; SD, standard deviation; W, week. The 2 patients who were B-cell depleted at screening were included based on prior CLL diagnoses and lymph node progression.
Pharmacodynamics
Pharmacodynamic data are given in supplemental Methods. B-cell count data, illustrating a rapid and sustained elimination of B cells, are shown in Figure 5B; recovery occurred in the majority of patients within 24 months after last dose.
Discussion
The phase 1/2 GAUGUIN study demonstrated that obinutuzumab can be safely administered to patients with relapsed/refractory CLL at doses up to 2000 mg, with most AEs (commonly IRRs), being of grade 1/2. AE incidence was dose independent, with no dose-limiting toxicities over the 400 to 2000 mg dose range. The incidence of grade 3/4 IRRs was higher in the GAUGUIN study (15% phase 1; 30% phase 2) than in a phase 1 study of obinutuzumab monotherapy in NHL patients (10%)11 and in CLL patients receiving high-dose rituximab.12 Circulating tumor cells have been implicated in rituximab-associated IRRs in CLL patients.17 IRRs after mAb infusions have been associated with CDC activation and/or cytokine release.18,19
Obinutuzumab was not associated with significant complement activation. Cytokine levels were significantly elevated after first obinutuzumab infusion, suggesting that IRRs may be related to cytokine release from malignant B cells rather than CDC; rapid B-cell depletion supports this explanation. However, cytokine release could not be correlated with IRR incidence or severity due to small sample size. Lack of CDC did not prevent IRRs, and B-cell depletion was rapid and profound with obinutuzumab; the rate of B-cell depletion was faster and more complete than that with ofatumumab,20 an anti-CD20 mAb that induces higher levels of CDC than rituximab.21
Both early- (7 patients in phase 1 and 4 in phase 2) and late-onset (1 patient in phase 1 [febrile neutropenia] and 1 during phase 2 follow-up) neutropenia were reported with obinutuzumab. Of the 13 patients with grade 3/4 neutropenia, 6 (46%) had first onset within 30 days of first treatment exposure. Early-onset neutropenia is uncommon with rituximab, and was observed in only 10% and 8% of indolent NHL patients in phase 1 and 2 of GAUGUIN, respectively.11,15 However, late-onset neutropenia with rituximab has an incidence of 8% to 27%, median time to onset of 70 to 190 days, and median duration of 5 to 77 days.22 As median time to late-onset rituximab-associated neutropenia parallels the time to B-cell recovery, it is possible that stromal cell-derived factor 1/chemokine (C-X-C motif) ligand 12 (involved in both lymphopoiesis and granulopoiesis) is implicated in late-onset neutropenia.22 Three patients in GAUGUIN experienced low neutrophil counts at the time of B-cell recovery. Irrespective of the time to onset, clinical aspects and outcomes of neutropenia were similar for patients treated with rituximab and obinutuzumab.
Obinutuzumab was effective in eliminating circulating B cells (Figure 5B). The phase 1 BOR of 62% compares favorably with previous data: weekly rituximab 375 mg/m2 for 4 weeks in patients with previously untreated CLL yielded an objective response of 51% (22 of 43 patients).23 The objective response in a dose-escalating study of ofatumumab monotherapy (administered weekly for 4 weeks at 100-2000 mg) in patients with relapsed/refractory CLL was 44% (14 of 32 patients).20
In a larger phase 2 study in fludarabine-refractory CLL, the overall response rate with ofatumumab 2000 mg was 47% with a PFS of 5.9 months.24 We observed a trend for a dose-response relationship in phase 1: 1 of 3 patients in the 400/800 mg and 1000/1000 mg dose groups, 2 of 3 patients in the 800/1200 mg dose group, and all 4 patients in the 1200/2000 mg dose group experienced responses. However, there were fewer responses during phase 2 (BOR 30%), likely due to higher baseline tumor burden of the participants.
In phase 2, 12 of 16 patients (75%) with an evaluable ETR had baseline SPD >2100 mm2; all 3 phase 2 participants with PR had baseline SPD <2100 mm2. In phase 1, 6 of 13 patients (46%) had baseline SPD <2100 mm2. Considering both study phases, 10 of 11 patients (91%) with PR received ≥1000 mg obinutuzumab. Thus, 100% of patients with low baseline tumor burden (ie, <2100 mm2) treated with obinutuzumab ≥1000 mg obtained PR.
Animal studies have demonstrated a correlation between tumor burden, rituximab pharmacokinetics, and tumor response.25 The lower ETR in patients with higher tumor burden suggests that higher obinutuzumab concentrations may be required for maximum efficacy as monotherapy. This hypothesis is tested in GAGE (NCT01414205), a randomized phase 2 study in which 80 individuals with untreated CLL received obinutuzumab 1000 mg or 2000 mg.26 Initial data from GAGE found a trend toward increased objective response rate with the higher dose of obinutuzumab (49% vs 67%; P = .08), which may support the trend observed in phase 1 of GAUGUIN.
GAUGUIN showed that obinutuzumab, a novel type II anti-CD20 mAb, is active as monotherapy in patients with heavily pretreated relapsed/refractory CLL, and was consistent with previous data in patients with relapsed CD20-positive B-cell malignancies.10 Recently, the CLL11 trial (NCT01010061) confirmed the activity of obinutuzumab (1000 mg) plus chlorambucil in unfit patients with CLL and other comorbidities, with an increased PFS compared with rituximab plus chlorambucil.27 Compared with rituximab, obinutuzumab demonstrated a higher incidence of neutropenia and IRRs. Further trials combining obinutuzumab with chemotherapy should therefore monitor such toxicities. Data from CLL11 confirmed a higher incidence of IRRs with obinutuzumab, highlighting the need for first-dose administration strategies with obinutuzumab (currently under investigation in the GREEN Study; NCT01905943) to avoid premature treatment discontinuation.
For clinical use, obinutuzumab monotherapy is likely to be suitable in situations where rituximab or ofatumumab monotherapy are currently used, due to its higher propensity for B-cell depletion, and in patients not suitable for cytotoxic chemotherapy. To further optimize monotherapy dosing in CLL, studies of the effects of tumor burden on the pharmacokinetics and pharmacodynamics of obinutuzumab are needed. However, arguably the most exciting development for obinutuzumab will lie in its potential for combination with new kinase inhibitors such as ibrutinib or idelalisib, where its efficiency with regard to eliminating circulating B cells may be synergistic with the strong ability of such kinase inhibitors to induce the migration of clonal cells from lymph node and bone marrow to the blood.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The GAUGUIN study was sponsored by F. Hoffmann–La Roche. Third-party writing assistance was provided by Martin Quinn at Gardiner-Caldwell Communications (Macclesfield, United Kingdom) and funded by F. Hoffmann–La Roche.
Authorship
Contribution: G.C. and F.M. designed and performed the research and collected, analyzed, and interpreted data; S.d.G. and V.L. performed the research and collected data; J.D. and B.M. performed the research; G.L. analyzed and interpreted data and performed statistical analyses; M.W. and E.W.-F. designed the research and collected, analyzed, and interpreted data; M.H. performed research and collected, analyzed, and interpreted data; M.-S.D. and R.B. performed research and collected data; and all authors made the final decision to submit this manuscript for publication and wrote/reviewed and provided final approval on the manuscript.
Conflict-of-interest disclosure: G.C. has served as a consultant for, and has received honoraria from, F. Hoffmann–La Roche. S.d.G. has received honoraria from F. Hoffmann–La Roche France for participation in advisory boards. M.-S.D. and F.M. have received honoraria from F. Hoffmann–La Roche. V.L. has served as combination a consultant for Cellectis, on advisory committees for F. Hoffmann–La Roche, Gilead, GlaxoSmithKline, and Janssen Pharmaceuticals, and as a member of speaker bureaus for F. Hoffmann–La Roche, Gilead, Janssen Pharmaceuticals, and Mundipharma. G.L. and E.W.-F. are salaried employees of F. Hoffmann–La Roche. M.W. is a salaried employee of Genentech, was a salaried employee of F. Hoffmann–La Roche, and owns stock in F. Hoffmann–La Roche. M.H. served as a consultant for, and has received research funding from, F. Hoffmann–La Roche. The remaining authors declare no competing financial interests.
The current affiliation for M.W. is Genentech, San Francisco, CA.
Correspondence: Guillaume Cartron, Département d’Hématologie Clinique, Centre Hospitalier Universitaire Montpellier, 80 Avenue Augustin Fliche, Montpellier, France 34295; e-mail: g-cartron@chu-montpellier.fr.