In this issue of Blood, Alshahrani et al demonstrate that carcinoembryonic antigen-related cell adhesion molecule 2 (CEACAM2) is expressed on platelets and negatively regulates the collagen receptor glycoprotein (GP)VI-FcRγ chain and C-type lectin-like receptor 2 (CLEC-2)-mediated platelet activation.1
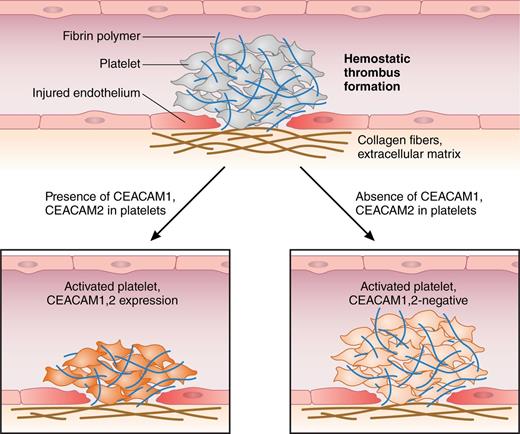
Arterial thromboembolism is initiated by hemostatic thrombus formation. Injured endothelium, exposure of subendothelial collagen I, and local concentration of hemostatic factors that bind to platelet surface receptors trigger their shape change, adherence, and activation. Both Ig-ITIM receptors CEACAM1 and CEACAM2 negatively regulate platelet-collagen I interactions, attenuate platelet activation, and attenuate thrombus growth and stability in vitro and in vivo.1,7 Professional illustration by Patrick Lane, ScEYEnce Studios.
Arterial thromboembolism is initiated by hemostatic thrombus formation. Injured endothelium, exposure of subendothelial collagen I, and local concentration of hemostatic factors that bind to platelet surface receptors trigger their shape change, adherence, and activation. Both Ig-ITIM receptors CEACAM1 and CEACAM2 negatively regulate platelet-collagen I interactions, attenuate platelet activation, and attenuate thrombus growth and stability in vitro and in vivo.1,7 Professional illustration by Patrick Lane, ScEYEnce Studios.
The authors show in a set of in vitro and in vivo experiments that CEACAM2−/− platelets are hyperactivated in response to collagen I and laser coagulation-induced arterial injury, leading to enhanced thrombogenesis. This is the first report demonstrating that the platelet immunoglobulin (Ig)-immunoreceptor tyrosine-based inhibitory motif (ITIM) receptor CEACAM2 regulates thrombus stability and size and blocks signaling of GPVI-FcRγ and CLEC-2 pathways.
Arterial thromboembolism following endothelial injury is a major clinical complication that occurs in atherosclerosis, myocardial, and cerebral infarction. Immediately after vascular injury, platelets become activated by newly exposed subendothelial collagen, form aggregates, and produce a thrombus within seconds or minutes. Although platelet adhesion is intended to shield the injured endothelial layer, excessive platelet aggregation results in vessel occlusion. Binding of collagen via collagen receptors, such as integrins, or immunoreceptors, such as the GPVI-FcRγ chain complex, elicits powerful platelet activation. Hence, antithrombotic drugs target activatory platelet receptors. In addition, CLEC-2 triggers platelet activation after binding to podoplanin or rhodocytin. These receptors either contain an immunoreceptor tyrosine-based activation motif (ITAM; in the GPVI-FcRγ chain complex) or a hemi-ITAM (HemITAM; in CLEC-2) with a single YxxL sequence that bind src kinase family members or spleen tyrosine kinase (Syk) and activate phospholipase Cγ2 (PLCγ2) signaling.2,3
To prevent excessive thrombus formation, platelet aggregation is autoregulated by down-modulating immunoglobulin domain-containing receptors. These Ig-ITIM–bearing receptors bind tyrosine phosphatases, especially the Src homology region 2 domain-containing phosphatases-1 (SHP-1) or SHP-2, to counterbalance ITAM receptor signaling. Therefore, it remains challenging to define inhibitory receptors that control platelet activation, thrombogenesis, and the size of thrombi.
Now, it is required to address the question of whether and how many inhibitory platelet receptors are essential or redundant for platelet aggregation. Furthermore, their individual or mutual contributions for the upkeep of the delicate threshold between vessel-protective homeostasis and formation of a vessel-blocking thrombus need to be defined. In knockout mice, hemostasis-related phenotypes often remain obscure until more than one of the key regulators are inactivated. For example, in the FcRγ chain complex−/− mouse, thrombus growth is reduced but not ablated, indicating that the GPVI-FcRγ chain complex is required but not sufficient to control thrombus formation. Also, FcRγ chain complex deficiency does not prevail unless thrombin generation is impaired or CLEC-2 is depleted.3,4 Similarly, lack of the platelet Ig-ITIM receptors, CEACAM1 and CEACAM2, produces no overt hemostatic phenotype in knockout mice, whereas platelet-endothelial cell adhesion molecule 1−/− (Pecam1−/−) mice exhibit prolonged bleeding time in vivo, although this phenotype results from alterations in endothelial rather than platelet function.5
Evidence exists for the role of the Ig-ITIM receptors CEACAM1 and PECAM1 on the inhibition of platelet activation: CEACAM1 and PECAM1 share their regulatory effects on platelet-collagen interactions. Remarkably, CEACAM1- and PECAM1-deficient platelets are hyper-responsive toward subthreshold doses of collagen, and collagen-induced thrombi are more stable and larger compared with controls.1,6,7 Alshahrani et al address the issue of how a close relative of CEACAM1, CEACAM2, regulates platelet activation. CEACAM2 expression has not been identified on platelets thus far. They used Ceacam2−/− mice to examine platelet activation or thrombogenesis under conditions of collagen I exposure and enforced thrombin formation.1 Whereas CEACAM1 is conserved in humans and rodents, CEACAM2 was identified only in mice.1 Strikingly, CEACAM2 and CEACAM1 contain an almost identical ITIM-containing long cytoplasmic tail (L) in their platelet-expressed isoforms; conversely, they differ in their extracellular domain organization in that platelet CEACAM1-L contains 4 extracellular Ig-like domains (CEACAM1-4L), whereas CEACAM2-L only possesses 2 extracellular domains (CEACAM2-2L), with limited homology to CEACAM1.1 In their comparison of the phenotypes of the Ceacam1−/− and the Ceacam2−/− model, the authors reveal that CEACAM2 deficiency produces a phenocopy of the CEACAM1 knockout; indeed, both CEACAMs are upregulated after platelet activation and negatively modulate platelet-collagen interactions. They prevent platelet hyperactivation and limit thrombogenesis both in vitro and in vivo1,7 (see figure). In addition, CEACAM1- and CEACAM2-dependent regulation of thrombin-induced thrombogenesis is suspected, although definite proof needs to be identified. For CEACAM2-expressing platelets, the authors report that platelet aggregation is reduced and CEACAM2−/− thrombi were more stable compared with controls. Moreover, CEACAM2 deficiency and antibody-mediated depletion of GPVI induce reversal of thrombus growth, whereas depletion of GPVI alone affected thrombus stability to a lesser extent.1 Hence, CEACAM2 interferes with platelet-platelet interaction, probably by competing with adhesion molecules or hemostatic factors that facilitate buildup of platelet aggregates.
Furthermore, the authors identify novel cross-talk between CEACAM2-2L and CLEC-2, which relies on Syk signaling for platelet activation. Both CEACAM1 and CEACAM2 interfere with Syk and PLCγ2 signaling in platelets, and CEACAM1-4L also downregulates TLR4-dependent Syk signaling in myeloid cells, thereby dampening inflammation.1,7,8 This makes CEACAM1 and CEACAM2 an interesting link that connects hemostasis and innate immunity via Syk signaling. Notably, in platelets, CLEC-2–mediated activation depends more on Syk activity than on activation of the GPVI-FcRγ chain complex.3
Members of the CEACAM family of adhesion molecules mediate homophilic and heterophilic ligand binding in cis and trans in intercellular adhesion to elicit their regulatory roles in cellular proliferation, adhesion, and motility, as well as in insulin metabolism, immunity, tumor development, and vascular remodeling.9 It is of note that both CEACAM1 and CEACAM2 bind murine coronavirus, although with different efficacy.1 Both CEACAM1 and CEACAM2 are capable of differential ligand binding that is also controlled by their dimerization status, which in turn, regulates their src and SHP binding capacities.9,10 Because GPVI and CLEC-2 rely on clustering for platelet activation synapse formation, CEACAM (homo-)dimers could thus dilute activation receptor microclusters in the platelet signaling synapse and interfere with efficient GPVI-FcRγ complex and CLEC-2 microdomain formation. Future studies will have to be conducted to identify a putative human homolog of CEACAM2-2L. Furthermore, the potential of Ig-ITIMs as therapeutic targets for antiplatelet therapy needs to be evaluated.
Conflict-of-interest disclosure: The author declares no competing financial interests.