Key Points
Paired immunoglobulin-like receptor B negatively regulates platelet activation.
Abstract
Murine paired immunoglobulin-like receptors B (PIRB), as the ortholog of human leukocyte immunoglobulin-like receptor B2 (LILRB2), is involved in a variety of biological functions. Here, we found that PIRB and LILRB2 were expressed in mouse and human platelets, respectively. PIRB intracellular domain deletion (PIRB-TM) mice had thrombocythemia and significantly higher proportions of megakaryocytes in bone marrow. Agonist-induced aggregation and spreading on immobilized fibrinogen were facilitated in PIRB-TM platelets. The rate of clot retraction in platelet-rich plasma containing PIRB-TM platelets was also increased. Characterization of signaling confirmed that PIRB associated with phosphatases Shp1/2 in platelets. The phosphorylation of Shp1/2 was significantly downregulated in PIRB-TM platelets stimulated with collagen-related peptide (CRP) or on spreading. The results further revealed that the phosphorylation levels of the linker for activation of T cells, SH2 domain-containing leukocyte protein of 76kDa, and phospholipase C were enhanced in PIRB-TM platelets stimulated with CRP. The phosphorylation levels of FAK Y397 and integrin β3 Y759 were also enhanced in PIRB-TM platelet spread on fibrinogen. The PIRB/LILRB2 ligand angiopoietin-like-protein 2 (ANGPTL2) was expressed and stored in platelet α-granules. ANGPTL2 inhibited agonist-induced platelet aggregation and spreading on fibrinogen. The data presented here reveal that PIRB and its ligand ANGPTL2 possess an antithrombotic function by suppressing collagen receptor glycoprotein VI and integrin αIIbβ3-mediated signaling.
Introduction
Platelets, which are derived from megakaryocytes, circulate in mammalian blood and play essential roles in hemostasis, angiogenesis, inflammation, and metastasis,1-3 contain a variety of receptors on their surface. The immunoglobulin superfamily (IgSF) is a large group of cell surface proteins that are involved in the adhesion, binding, or recognition of cells.4 Several IgSF members expressed on the platelet surface regulate platelet adhesion, activation, and aggregation. Among those receptors, platelet collagen receptor glycoprotein VI (GPVI) has short cytoplasmic domains lacking signaling motifs, but it transmits activating signals by linking to immunoreceptor tyrosine-based activation motif (ITAM) of the Fc receptor γ chain (FcRγ chain).5 The GPVI/FcRγ chain complex can propagate potent signaling causing αIIbβ3 activation and platelet aggregation and thereby play an important role in hemostasis and thrombosis formation.6 In contrast to GPVI, platelet endothelial cell adhesion molecule-1, a platelet surface IgSF member with 6 extracellular Ig domains and a cytoplasmic immunoreceptor tyrosine-based inhibitory motif (ITIM), mildly inhibits human or mouse platelet activation by collagen, adenosine 5′-diphosphate (ADP), or thrombin.7 Similarly, antibody-mediated cross-linking of G6B, a platelet surface IgSF member that contains 1 extracellular Ig-like domain and 2 ITIMs, has a significant inhibitory effect on human platelet activation and aggregation in response to the agonists ADP and the collagen-related peptide (CRP).8 Contrary to expectations, deletion of G6B inhibited and did not enhance agonist-induced mouse platelet activation, possibly by increasing GPVI and glycoprotein Ib (GPIb)α shedding.9 These studies revealed the complex effects of IgSF proteins on platelet function.
The leukocyte immunoglobulin-like receptors (LILRs), as a type of IgSF, include members of LILR subfamilies A (LILRA) and B (LILRB) that contain ITAMs and ITIMs, respectively. LILRs can affect a broad variety of biological functions and serve as potential therapeutic targets for a wide range of diseases.10 As the human LILRB2 homolog,11-13 the murine paired immunoglobulin-like receptor B (PIRB) contains 6 extracellular immunoglobulin domains and 4 cytoplasmic ITIMs.14 Phosphorylation of PIRB cytoplasmic ITIMs is known to recruit Shp1 and Shp2 phosphatases,15 which in turn negatively modulate signal transduction pathways in the immune system. Although the roles of PIRB or its human ortholog in immune responses, neuron axonal regeneration,12 and hematopoietic processes13 have been well studied, the expression and functions of PIRB in platelets remains unknown.
Here, we found that PIRB and LILRB2 were expressed in mouse and human platelets, respectively, and the mutation of PIRB upregulated mouse platelet activation. The PIRB/LILRB2 ligand angiopoietin-like-protein 2 (ANGPTL2) was expressed in platelets, and purified ANGPTL2 inhibited platelet activation. The data presented here suggest that LILRB2 may prove to be a potential target for antiplatelet therapy.
Methods
Materials
ADP, apyrase, prostaglandin E1, fibrinogen (Fg), the proteinase-activated receptor 4 (PAR4) agonist peptide AYPGKF, and anti-Flag M2 affinity gel were purchased from Sigma-Aldrich (St. Louis, MO). Thrombin was from Enzyme Research Laboratories (South Bend, IN). The fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD41 and rat anti-mouse CD62P monoclonal antibodies were from BD Biosciences (San Jose, CA). The mouse antiphosphorylated tyrosine monoclonal antibody 4G10 and rabbit antifocal adhesion kinase (FAK) polyclonal antibody were from Millipore (Billerica, MA). The goat anti-ANGPTL2 polyclonal antibody and peridinin-chlorophyll-protein complex (PerCP)-conjugated rat anti-mouse PIRB monoclonal antibody were from R&D Systems (Minneapolis, MN). The rabbit anti-Shp1, anti-Shp2, antiphospho-Shp1 tyrosine (Y) 564, antiphospho-Shp2 Y580, antiphospho-FAK Y397, anti-β3, antilinker for activation of T cells (LAT), and anti-SLP76 polyclonal antibodies and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody were from Cell Signaling Technology (Danvers, MA). The rabbit anti-phospho-β3 Y747 and antiphospho-β3 Y759 polyclonal antibodies were from Abcam (Cambridge, MA). The goat anti-human LILRB2 and anti-mouse PIRB (C19), rabbit anti-von Willebrand factor (VWF) and anti-PLCγ2 (Q-20) polyclonal antibodies, and control IgG were from Santa Cruz Biotechnology (Dallas, TX). The FITC-conjugated rat anti-mouse GPVI antibody (JAQ1) was from Emfret Analytics (Wurzburg, Germany). FITC-conjugated rat IgG2b control was from Biolegend. Phycoerythrin (PE)-conjugated rat anti-human LILRB2 monoclonal antibody was from eBioscience (San Diego, CA). The β3 Y759 antibody for detection of cleavage at β3 Y759 was a gift from Xiaodong Xi (Shanghai Jiao Tong University School of Medicine). The Alexa 488-conjugated Fg, Alexa 488-conjugated phalloidin, rhodamine-conjugated phalloidin, Alexa 594-conjagated donkey anti-goat antibody, and Alexa 488-conjugated donkey anti-rabbit antibody were from Life Technologies (Gaithersburg, MD). Secondary horseradish peroxidase-conjugated antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Supersignal chemiluminescent substrate was from Pierce (Rockford, IL).
Mice
PIRB mutant (PIRB-TM) C57BL/6 mice16 were used to study the function of PIRB in mouse platelet activation. In this mutant mouse, PIRB-mediated signaling across the plasma membrane does not occur because 4 of the exons that encode part of the PIRB intracellular domain have been removed from the PIRB gene. C57BL/6 mice were used as controls. The Shanghai Jiao Tong University School of Medicine Animal Care and Use Committee approved the animal research.
Platelet preparation and aggregation
Mouse and human washed platelets were prepared as described.17,18 Institutional Review Board approval was obtained from the Shanghai Jiao Tong University School of Medicine, and informed consent was obtained from volunteers in accordance with the Declaration of Helsinki. Inhibitor was incubated with the platelets for 3 minutes prior to stimulation.
Leukocytes preparation
Five volumes of Red Blood Cell Lysis Buffer (Thermo Scientific) were mixed with whole human or mouse blood collected with EDTA and incubated at room temperature for 5 minutes. The sample was then centrifuged at 300g for 5 minutes, and the supernatant was decanted. Two additional volumes of Red Blood Cell Lysis Buffer were then added and mixed to the pelleted white blood cells. The cells were collected by centrifugation at 300g for 5 minutes and resuspended in phosphate-buffered saline (PBS) for further analysis. The cell purity was examined by staining with 4′,6 diamidino-2-phenylindole and flow cytometry.
Immunoprecipitation and western blotting
For immunoprecipitation, the platelet samples were incubated in the Pierce IP lysis buffer with protease and phosphatase inhibitor cocktails (Thermo Scientific) on ice for 30 minutes, followed by binding with 2 µg/mL 4G10 or anti-PIRB antibody (C19) at 4°C overnight. Thirty-microliter protein A/G agarose beads were then added to each sample prior to incubation for 2 hours at 4°C. The beads were harvested by centrifugation at 3000g for 2 minutes and rinsed 3 times with 1 mL lysis buffer.
For western blotting, the immunoprecipitated beads, platelets, or leukocytes samples were boiled in sample buffer, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane as previously described.19 After detection of target proteins, the membranes were stripped and incubated with anti-mouse IgG, anti-goat IgG, or anti-GAPDH antibodies to demonstrate the equal loading.
Measurement of ANGPTLs expression levels in human and mouse platelets
Total mRNA was extracted from washed human or mouse platelets (for each group, n = 3). CD41 antibody staining was performed to assess the purity of the platelet population by flow cytometry. The expression levels of ANGPTL1 (A1), A2, A3, A4, A5, A6, and A7 in human platelets or A1, A2, A3, A4, A6, and A7 in mouse platelets20 were measured using real-time quantitative polymerase chain reaction (PCR; ABI 7500 Real-Time PCR system). Relative mRNA expression levels were normalized to GAPDH expression level. The sequences of PCR primers were as follows—for human GAPDH: forward 5′CTCAAGGGCATCCTGGGCTA3′, reverse 5′ATGAGGTCCACCACCCTGTT3′; for human A1: forward 5′GCATTCGGTCAGTGGGATTTA3′, reverse 5′CAAGCCAGTATTCTCCGTCAA3′; for human A2: forward 5′ACGACACCAGCTCCATCTACCT3′, reverse 5′AGTATTCGCCGTCAATGTTCC3′; for human A3: forward 5′ATGGTTTTGGGAGGCTTGATG3′, reverse 5′GAAGTGTCCTTTTGCTTTGTG3′; for human A4: forward 5′CAAGGCGGGGTTTGGGGAT3′, reverse 5′TGGGTGGGACGGTGGTGGC3′; for human A5: forward 5′GATGCATTCCGGGGTCTCAA3′, reverse 5′ACCAGCCGGTCTTGTTATGG3′. For human A6: forward 5′CGTGGATAGGGACCGAGAC3′, reverse 5′TCAGCCCAGTAGACACCATC3′; for human A7: forward 5′ CTGGGGAACGAACACATCCA 3′, reverse 5′TTCCCCAGGAAGAGGCGATA3′; for mouse GAPDH: forward 5′TGTGTCCGTCGTGGATCTGA3′, reverse 5′TTGCTGTTGAAGTCGCAGGAG3′; for mouse A1: forward 5′GGAATGCTGGGGACTCTATG3′, reverse 5′CCATCTTGGTGCTTGCTTCT3′; for mouse A2: forward 5′CCAGTCTCCCATCTTCCACT3′, reverse 5′CATCAGGCGGTTGGTATTCT3′; for mouse A3: forward 5′CGAAACCAACTACACGCTACA3′, reverse 5′TCCCTTTGCTCTGTGATTCC3′; for mouse A4: forward 5′GCACAGCATCACAGGGAAC3′, reverse 5′TAGAGAAGGGCAGGGAAAGG3′; for mouse A6: forward 5′CAGAGCACCAGAGAGAGCAG3′, reverse 5′ACCACACGGCTACTACACGA3′; for mouse A7: forward 5′GCCTCATAAACGCAAGACAC3′, reverse 5′CTGACCCAGTCGCTCTCCT3′.
Localization of ANGPTL2 in platelets
Washed human platelets attached to immobilized Fg or clotted platelet aggregates were fixed and stained with anti-ANGPTL2 antibodies, anti-VWF antibodies, control IgG, or Alexa 488-phalloidin, and the fluorescent images were captured.
Flag-ANGPTL2 purification and binding to platelets
Plasmid-expressing human ANGPTL2 with Flag tags at the C terminus was transfected into 293T cells, and Flag-ANGPTL2 was purified as previously described.13 Alexa 488-conjugated Flag-ANGPTL2 was prepared using an Alexa Fluor 488 Protein Labeling Kit (Life Technologies) according to the manufacturer's protocol. Washed human and mouse platelets at a concentration of 3 × 107/mL were incubated with Alexa 488-conjugated Flag-ANGPTL2 at room temperature for 20 minutes. The binding of Alexa 488-conjugated Flag-ANGPTL2 was measured by flow cytometry. To test the effects of goat anti-human LILRB2 antibody or rat anti-mouse PIRB monoclonal antibody on the binding of Alexa 488-conjugated Flag-ANGPTL2 to human or mouse platelets, the antibodies were incubated with the platelets for 15 minutes at room temperature prior to addition of Alexa 488-conjugated Flag-ANGPTL2.
Fg binding assay
Washed wild-type (WT) and PIRB-TM platelets at a concentration of 3 × 107/mL were incubated for 20 minutes at room temperature with 40 μg/mL Alexa 488-conjugated Fg and CRP or PAR4 peptide AYPGKF in a final volume of 50 μL Tyrode’s buffer containing 1 mM CaCl2, and Fg binding was measured by flow cytometry.
Platelet spreading on immobilized Fg
Analysis of platelet spreading on immobilized Fg was done as previously described.21 Platelets were stained by rhodamine-conjugated phalloidin and visualized by an upright fluorescent microscope AXIO ScopeA1 (ZEISS Group, Jena, Germany) equipped with a 100×/1.30 oil objective lens, an X-cite 120Q light source (EXFO, Mississauga, CA), and a digital camera. Up to 4 images were chosen at random per experiment and analyzed under blind conditions. The platelet surface area was analyzed using National Institutes of Health Image J software (National Institutes of Health, Bethesda, MD). Statistical significance was evaluated by the Student t test.
Clot retraction
Clot retraction using mouse platelets was processed as previously described.22 Clot size was quantified from photographs using National Institutes of Health Image J software, and retraction was expressed as retraction ratio (1 − [final clot size/initial clot size]).
Results
Mutation of PIRB causes mild thrombocythemia
The expression of PIRB or its human homolog LILRB2 in mouse or human platelets was measured prior to study of the function of PIRB in platelets. The results demonstrated that LILRB2 exists in human leukocytes and platelets as an ∼90-kDa protein (Figure 1A).23 Western blotting of PIRB from the mouse leukocytes and platelets samples demonstrated that PIRB exists as an ∼125-kDa protein (Figure 1B), consistent with previous reports.16,24
Mutation of PIRB causes mild thrombocythemia. (A) The expression of LILRB2 in human peripheral leukocytes (H-Leu) and human platelets (H-Plt). (B) The expression of PIRB in mouse peripheral leukocytes (M-Leu) and mouse platelets (M-Plt). (C) The expression of PIRB in WT mouse leukocytes (M-Leu) and platelets (M-Plt). Washed WT or PIRB-TM platelets at a concentration of 3 × 107/mL were incubated with PerCP-conjugated rat anti-mouse PIRB monoclonal antibody or PerCP-conjugated rat IgG control at 25°C for 30 minutes. The expression of PIRB on WT and PIRB-TM platelets was analyzed using flow cytometer. (D) The platelet counts in the peripheral blood of WT and PIRB-TM mice (WT, n = 10; PIRB-TM, n = 10; mean ± standard error of the mean [SEM]; **P < .01). (E) The percentage of CD41b-positive cells in WT and PIRB-TM mice bone marrow (BM) (WT, n = 5; PIRB-TM, n = 5; mean ± SEM; **P < .01).
Mutation of PIRB causes mild thrombocythemia. (A) The expression of LILRB2 in human peripheral leukocytes (H-Leu) and human platelets (H-Plt). (B) The expression of PIRB in mouse peripheral leukocytes (M-Leu) and mouse platelets (M-Plt). (C) The expression of PIRB in WT mouse leukocytes (M-Leu) and platelets (M-Plt). Washed WT or PIRB-TM platelets at a concentration of 3 × 107/mL were incubated with PerCP-conjugated rat anti-mouse PIRB monoclonal antibody or PerCP-conjugated rat IgG control at 25°C for 30 minutes. The expression of PIRB on WT and PIRB-TM platelets was analyzed using flow cytometer. (D) The platelet counts in the peripheral blood of WT and PIRB-TM mice (WT, n = 10; PIRB-TM, n = 10; mean ± standard error of the mean [SEM]; **P < .01). (E) The percentage of CD41b-positive cells in WT and PIRB-TM mice bone marrow (BM) (WT, n = 5; PIRB-TM, n = 5; mean ± SEM; **P < .01).
Although the function of PIRB in maintenance of hematopoitic stem cell (HSC) activity is well understood,13 the function of PIRB in thrombopoiesis has not been described. First, the blood platelet numbers in WT control and PIRB-TM mice were measured. Platelet counting revealed that WT mice had 612 ± 64 × 109 platelets/L, whereas the PIRB-TM mice had 897 ± 90.4 × 109 platelets/L (N = 10 mice/group). The blood of PIRB-TM mice contains ∼47% more platelets than that of WT mice (P < .01; Figure 1D), demonstrating that deficiency of PIRB function causes mild thrombocythemia. Because the circulating platelets were differentiated BM megakaryocytes derived from HSCs, we also measured the percentage of CD41 (integrin αIIb, as the specific marker of megakaryocytes and platelets)-positive cells in BM from both WT and PIRB-TM mice. We found that WT mouse BM had 0.61 ± 0.06% CD41-positive cells, whereas the PIRB-TM mice BM had 0.86 ± 0.15% CD41-positive cells. The BM of PIRB-TM mice contains ∼41% more megakaryocytes than that of WT mice (P < .01; Figure 1E), demonstrating that the mild thrombocythemia in PIRB-TM mice was due to an enhanced proportion of megakaryocytes in BM.
Aggregation and secretion were enhanced in PIRB-TM platelets in response to agonists
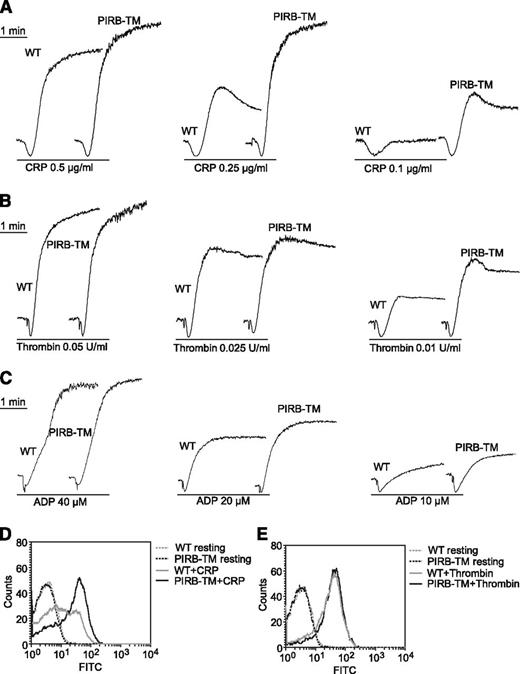
The role of PIRB in agonist-induced platelet aggregation and secretion was investigated by stimulating WT and PIRB-TM platelets with CRP, thrombin, or ADP. WT and PIRB-TM platelets were stimulated with 0.1, 0.25, and 0.5 μg/mL CRP separately to investigate the role of PIRB in collagen major receptor GPVI-mediated platelet activation. PIRB deficiency obviously enhanced CRP-induced platelet aggregation (Figure 2A). Aggregation by PIRB-TM platelets was also slightly enhanced in response to thrombin and ADP (Figure 2B-C).
The function of PIRB in platelet aggregation and secretion. (A-C) Aggregation of washed WT and PIRB-TM platelets was studied by aggregometery in response to agonists as indicated. (D-E) Washed WT and PIRB-TM platelets at a concentration of 3 × 107/mL were incubated with FITC-conjugated rat anti-mouse CD62P monoclonal antibody at 25°C for 20 minutes in the presence of (D) 0.5 μg/mL CRP or (E) 0.05 U/mL thrombin. The expression of CD62P was analyzed using a flow cytometer.
The function of PIRB in platelet aggregation and secretion. (A-C) Aggregation of washed WT and PIRB-TM platelets was studied by aggregometery in response to agonists as indicated. (D-E) Washed WT and PIRB-TM platelets at a concentration of 3 × 107/mL were incubated with FITC-conjugated rat anti-mouse CD62P monoclonal antibody at 25°C for 20 minutes in the presence of (D) 0.5 μg/mL CRP or (E) 0.05 U/mL thrombin. The expression of CD62P was analyzed using a flow cytometer.
p-Selectin is a transmembrane protein that resides within the α-granule membrane of unstimulated platelets. On platelet activation, p-selectin is translocated to the platelet surface via a secretory pathway.22 Expression of p-selectin on the platelet surface was investigated by flow cytometry in response to CRP or thrombin. Secretion was significantly enhanced in PIRB-TM platelets in response to CRP but not thrombin (Figure 2D-E). Thus, PIRB is an important regulator of CRP-induced platelet aggregation and secretion.
PIRB regulates GPVI-mediated platelet activation through recruiting Shp1/2 to attenuate the tyrosine phosphorylation of LAT, SLP76, and PLCγ2
GPVI associates with the FcRγ chain to initiate recruitment and activation of the LAT signal complex containing LAT, SLP76, PLCγ2, and Gads.25 Further activation of PLCγ2 results in integrin αIIbβ3 mediated inside-out signaling and activation. The activated αIIbβ3 binds to its ligand Fg and mediates outside-in signaling.26
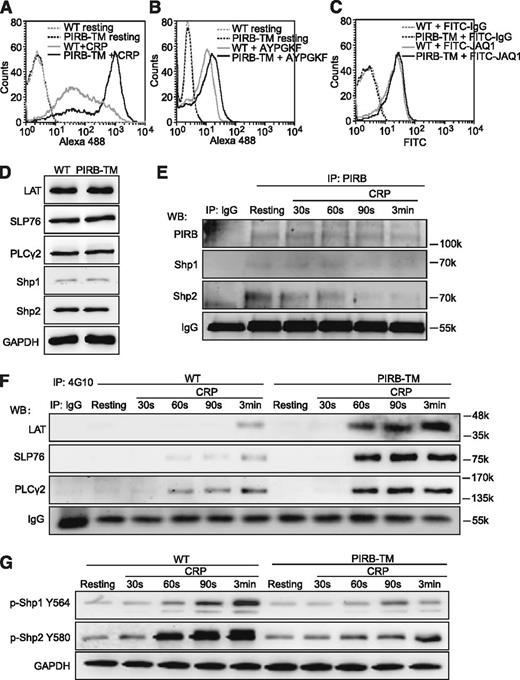
We first evaluated the function of PIRB in CRP or PAR4 peptide AYPGKF-induced αIIbβ3 activation using flow cytometric detection of Alexa 488-conjugated Fg binding to platelets. The results demonstrated that Fg binding was significantly enhanced in PIRB-TM platelets in response to CRP (Figure 3A), and PIRB deficiency slightly increased Fg binding induced by peptide AYPGKF under nonstirring conditions (Figure 3B). Because the PIRB mutation has no effects on GPVI expression in platelets (Figure 3C), these results apparently suggest that PIRB inhibits GPVI-mediated platelet activation mainly by suppressing αIIbβ3-mediated inside-out signaling.
PIRB regulates GPVI-mediated platelet activation. (A) Binding of Alexa 488-Fg to washed WT and PIRB-TM platelets stimulated with 0.5 μg/mL CRP. (B) Binding of Alexa 488-Fg to washed WT and PIRB-TM platelets stimulated with 100 μM PAR4 agonist peptide AYPGKF. (C) Washed WT and PIRB-TM platelets at a concentration of 3 × 107/mL were incubated with FITC-conjugated JAQ1 and FITC-conjugated rat IgG control at 25°C for 30 minutes. The expression levels of GPVI were detected using a flow cytometer. (D) The expression levels of LAT, SLP76, PLCγ2, Shp1, and Shp2 in WT and PIRB-TM platelets were examined by western blotting. GAPDH was used to verify equal gel loading. (E) The goat anti-mouse PIRB polyclonal antibody or goat IgG control was used to immunoprecipitate PIRB from lysate of washed WT platelets stimulated with 0.25 μg/mL CRP for indicated times. The immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with anti-PIRB, anti-Shp1, and anti-Shp2 antibodies for detection of Shp1/2 association with PIRB. IgG was used as a loading control. (F) The immunoprecipitations of tyrosine phosphorylated proteins from the lysates of WT and PIRB-TM platelets were treated with 0.25 μg/mL CRP for the indicated times, followed by the detections of tyrosine phosphorylation of LAT, SLP76, and PLCγ2. IgG was used as a loading control. (G) The time courses of phosphorylation levels of Shp1 Y564 and Shp2 Y580 in washed WT and PIRB-TM platelets in response to 0.25 μg/mL CRP. GAPDH was used to verify equal loading.
PIRB regulates GPVI-mediated platelet activation. (A) Binding of Alexa 488-Fg to washed WT and PIRB-TM platelets stimulated with 0.5 μg/mL CRP. (B) Binding of Alexa 488-Fg to washed WT and PIRB-TM platelets stimulated with 100 μM PAR4 agonist peptide AYPGKF. (C) Washed WT and PIRB-TM platelets at a concentration of 3 × 107/mL were incubated with FITC-conjugated JAQ1 and FITC-conjugated rat IgG control at 25°C for 30 minutes. The expression levels of GPVI were detected using a flow cytometer. (D) The expression levels of LAT, SLP76, PLCγ2, Shp1, and Shp2 in WT and PIRB-TM platelets were examined by western blotting. GAPDH was used to verify equal gel loading. (E) The goat anti-mouse PIRB polyclonal antibody or goat IgG control was used to immunoprecipitate PIRB from lysate of washed WT platelets stimulated with 0.25 μg/mL CRP for indicated times. The immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted with anti-PIRB, anti-Shp1, and anti-Shp2 antibodies for detection of Shp1/2 association with PIRB. IgG was used as a loading control. (F) The immunoprecipitations of tyrosine phosphorylated proteins from the lysates of WT and PIRB-TM platelets were treated with 0.25 μg/mL CRP for the indicated times, followed by the detections of tyrosine phosphorylation of LAT, SLP76, and PLCγ2. IgG was used as a loading control. (G) The time courses of phosphorylation levels of Shp1 Y564 and Shp2 Y580 in washed WT and PIRB-TM platelets in response to 0.25 μg/mL CRP. GAPDH was used to verify equal loading.
PIRB can form complexes with the tyrosine phosphotases Shp1 and Shp2 to carry out its negative regulation role.14 It is also known that T-cell receptor-mediated phosphorylation of LAT was rapidly dephosphorylated by activated Shp1, leading to inactivation of LAT and attenuation of subsequent downstream signaling events.27 To elucidate the role of PIRB in process of GPVI-mediated signaling pathways, the expression levels of potential signaling molecules in platelets were measured. We found that the PIRB mutation has no effect on the expression of LAT, SLP76, PLCγ2, Shp1, and Shp2 in platelets (Figure 3D). The time courses of the association between PIRB and Shp1/2 and the phosphorylation of Shp1/2, LAT, SLP76, and PLCγ2 in WT and PIRB-TM platelets in response to CRP were also examined. The tyrosine phosphorylation levels of LAT, SLP76, and PLCγ2 were comprehensively elevated in PIRB-TM platelets stimulated with CRP at the corresponding time points (Figure 3F). Co-immunoprecipitation results demonstrated that both Shp1 and Shp2 are associated with PIRB in resting WT platelets, and CRP stimulation caused a gradual dissociation of Shp2, but not Shp1, with PIRB in WT platelets (Figure 3E). The phosphorylation of Shp1 Y564 and Shp2 Y580 has been shown to reflect phosphatase activities of Shp1 and Shp2, respectively.28,29 The phosphorylation time courses of Shp1/2 in WT and PIRB-TM platelets stimulated with CRP were measured. The results in Figure 3G demonstrated that the phosphorylation levels of Shp1 Y564 and Shp2 Y580 significantly diminished in PIRB-TM platelets stimulated with CRP at the corresponding time points. These results suggest that PIRB-induced activation of Shp1/2 may negatively regulate GPVI-mediated platelet activation by attenuating the phosphorylation of LAT, SLP76, and PLCγ2.
PIRB regulates integrin αIIbβ3-mediated outside-in signaling
The platelet receptor integrin αIIbβ3-mediated bidirectional signaling is essential for platelet aggregation, clot retraction, and stable thrombus formation.30,31 Platelet spreading on immobilized Fg is dependent on cytoskeletal reorganization driven by αIIbβ3-mediated outside-in signaling.32 To characterize the role of PIRB in integrin αIIbβ3-mediated outside-in signaling, the spreading of WT and PIRB-TM platelets on immobilized Fg was assessed. The results in Figure 4A-B show that PIRB-TM platelets spread more extensively on immobilized Fg than did the WT platelets (the average size of WT platelets spread on Fg was 608 ± 38.3 pixels vs 831 ± 115.4 pixels for the PIRB-TM platelets spread on Fg for 30 minutes, 916.8 ± 86.6 pixels for WT platelets vs 1158 ± 75.1 pixels for the PIRB-TM platelet spread for 60 minutes, and 1492.6 ± 155.3 pixels for WT platelets vs 1583.4 ± 128.7 pixels for the PIRB-TM platelets spread for 90 minutes). Therefore, PIRB involves regulation of αIIbβ3-mediated outside-in signaling.
PIRB regulates integrin αIIbβ3-mediated outside-in signaling. (A) Representative phalloidin-stained images of washed WT and PIRB-TM platelets spreading on immobilized Fg for 30, 60, and 90 minutes, respectively. (B) Quantification of the areas (pixel number) of 4 random fields per experiment, and ≥3 independent experiments were performed. Statistical analyses were performed using the Student t test (mean ± standard deviation [SD]; *P < .05; **P < .01). (C) Washed WT or PIRB-TM mouse platelets spread on 40 µg/mL Fg for 90 minutes. The adherent platelets were solubilized and analyzed by western blotting for detection of the phosphorylation of FAK Y397, integrin β3 Y747 and Y759, and Shp1 Y564 and Shp2 Y580 and cleavage of integrin β3 Y759. GAPDH was used to verify equal loading. (D) Densitometry measurements from results in C. Values were normalized with respect to WT spreading for each immunoblot and are expressed as relative levels to spreading WT platelets. Statistical significance was determined using the Student t test. (n = 3, mean ± SD; **P < .01). (E) The expression levels of β3 and FAK in WT and PIRB-TM platelets. GAPDH was used to verify equal loading. (F) Clot retraction of PRP containing WT and PIRB-TM platelets in the presence of 0.5 U/mL thrombin. (G) Two-dimensional retraction of clots was measured, and the data were expressed as retraction ratios. Statistical significance was calculated using the Student t test (n = 5, mean ± SD; **P < .01).
PIRB regulates integrin αIIbβ3-mediated outside-in signaling. (A) Representative phalloidin-stained images of washed WT and PIRB-TM platelets spreading on immobilized Fg for 30, 60, and 90 minutes, respectively. (B) Quantification of the areas (pixel number) of 4 random fields per experiment, and ≥3 independent experiments were performed. Statistical analyses were performed using the Student t test (mean ± standard deviation [SD]; *P < .05; **P < .01). (C) Washed WT or PIRB-TM mouse platelets spread on 40 µg/mL Fg for 90 minutes. The adherent platelets were solubilized and analyzed by western blotting for detection of the phosphorylation of FAK Y397, integrin β3 Y747 and Y759, and Shp1 Y564 and Shp2 Y580 and cleavage of integrin β3 Y759. GAPDH was used to verify equal loading. (D) Densitometry measurements from results in C. Values were normalized with respect to WT spreading for each immunoblot and are expressed as relative levels to spreading WT platelets. Statistical significance was determined using the Student t test. (n = 3, mean ± SD; **P < .01). (E) The expression levels of β3 and FAK in WT and PIRB-TM platelets. GAPDH was used to verify equal loading. (F) Clot retraction of PRP containing WT and PIRB-TM platelets in the presence of 0.5 U/mL thrombin. (G) Two-dimensional retraction of clots was measured, and the data were expressed as retraction ratios. Statistical significance was calculated using the Student t test (n = 5, mean ± SD; **P < .01).
Integrin clustering leads to the activation of FAK by phosphorylation at Y397,33 which is a critical step in integrin-mediated outside-in signaling. The phosphorylation of integrin β3 cytoplasmic Y747 and Y759 is also important in αIIbβ3-mediated outside-in signaling.34 The dephosphorylation of Y759 is associated with calpain cleavage at Y759, which negatively regulates αIIbβ3-mediated outside-in signaling.35 Therefore, the phosphorylation of FAK Y397 and integrin β3 Y747 and Y759 and the cleavage of β3 Y759 were evaluated in WT and PIRB-TM mouse platelets spread on Fg for 90 minutes. The results in Figure 4C-D show that the PIRB mutation enhanced the levels of spreading-driven FAK Y397 phosphorylation. The phosphorylation levels of β3 Y759, but not Y747, were also significantly elevated in PIRB-TM platelets spread on Fg. Correspondingly, the cleavage of β3 Y759 was slightly diminished in PIRB-TM platelets spread on Fg. The phosphorylation levels of Shp1/2 were attenuated in PIRB-TM platelets spread on Fg. These results suggest that PIRB inhibits αIIbβ3-mediated outside-in signaling by suppressing the phosphorylation of β3 Y759 and FAK Y397 and also possibly by enhancing the cleavage of β3 Y759.
Integrin αIIbβ3-mediated outside-in signaling can also drive clot retraction.36 The results presented in Figure 4F-G show that the average ratio of clot retraction of platelet-rich plasma (PRP)-containing WT platelets was 0.10 ± 0.03 vs 0.25 ± 0.06 in PRP containing PIRB-TM platelets at 1 hour, PRP-containing WT platelets was 0.23 ± 0.04 vs 0.69 ± 0.04 in PRP-containing PIRB-TM platelets at 2 hours, and PRP-containing WT platelets was 0.51 ± 0.04 vs 0.81 ± 0.01 in PRP-containing PIRB-TM platelets at 3 hours. Therefore, PIRB deficiency in platelets accelerated clot retraction in PRP (P < .01).
Expression, storage, and secretion of PIRB ligand ANGPTL2 in human and mouse platelets
Although the function of PIRB in platelet activation was elucidated by the experiments described above, the ligand(s) of platelet PIRB remains unknown. Our previous work13 demonstrated that PIRB is a receptor of several ANGPTLs including ANGPTL2, and PIRB interaction with ANGPTLs plays important roles in ex vivo expansion of HSCs.
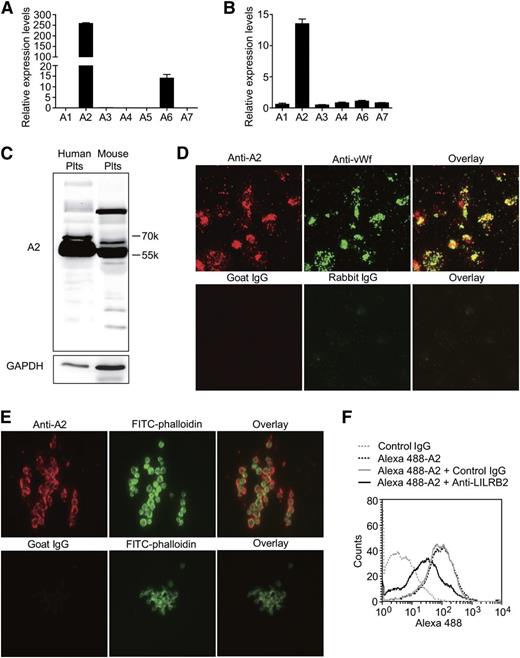
Here, we studied the expression and function of the ANGPTLs in human and mouse platelets. The ANGPTL family is composed of 7 proteins in humans (ANGPTL1-7) and 6 homologous (an ANGPTL5 homolog is missing) proteins in mice. First, we found that ANGPTL2 is highly expressed in human and mouse platelets using reverse transcriptase-PCR (Figure 5A-B); these results were confirmed using immunoblotting (Figure 5C). The immunofluorescence staining experiments demonstrated that ANGPTL2 colocalized with VWF in platelets (Figure 5D) and was released and bound to platelets on platelet aggregation (Figure 5E).
The expression, storage, and secretion of PIRB ligand ANGPTL2 in platelets. (A-B) The relative mRNA levels of ANGPTLs in human and mouse platelets separately (n = 5, mean ± SEM). (C) The expression levels of ANGPTL2 (A2) in human and mouse platelets. (D) Representative images of human platelets adhesion on immobilized Fg immunostained with goat anti-ANGPTL2 (A2, red) and rabbit anti-VWF (green) antibodies. Goat and rabbit IgG were used as negative controls, respectively. (E) Representative images of aggregated human platelets clots were stained with goat anti-ANGPTL2 (red), goat IgG negative control (red), and Alexa 488-phalloidin (green), respectively. (F) The effect of PE-conjugated rat anti-human LILRB2 monoclonal antibody on Alexa 488-conjugated Flag-ANGPTL2 binding to human platelets. PE-conjugated rat IgG was used as a negative control.
The expression, storage, and secretion of PIRB ligand ANGPTL2 in platelets. (A-B) The relative mRNA levels of ANGPTLs in human and mouse platelets separately (n = 5, mean ± SEM). (C) The expression levels of ANGPTL2 (A2) in human and mouse platelets. (D) Representative images of human platelets adhesion on immobilized Fg immunostained with goat anti-ANGPTL2 (A2, red) and rabbit anti-VWF (green) antibodies. Goat and rabbit IgG were used as negative controls, respectively. (E) Representative images of aggregated human platelets clots were stained with goat anti-ANGPTL2 (red), goat IgG negative control (red), and Alexa 488-phalloidin (green), respectively. (F) The effect of PE-conjugated rat anti-human LILRB2 monoclonal antibody on Alexa 488-conjugated Flag-ANGPTL2 binding to human platelets. PE-conjugated rat IgG was used as a negative control.
Flow cytometry and Alexa 488-conjugated purified Flag-ANGPTL2 were used to find out whether ANGPTL2 binding to human platelets is dependent on LILRB2. The results in Figure 5F demonstrated that anti-LILRB2 antibodies, but not control IgG, were able to partially inhibit ANGPTL2 binding to human platelets. These results confirmed that ANGPTL2 is the ligand of LILRB2 on human platelets.
Purified ANGPTL2 inhibited the activation of human platelets
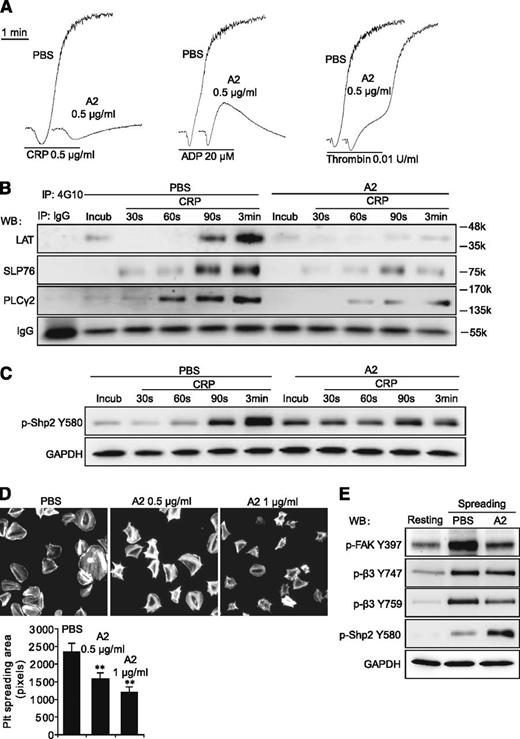
Purified Flag-ANGPTL2 was used to elucidate the function of ANGPTL2 in platelets. We found that 0.5 μg/mL of ANGPTL2 obviously inhibited the aggregation of human platelets stimulated with CRP, ADP, and thrombin (Figure 6A). The phosphorylation levels of LAT, SLP76, and PLCγ2 were significantly suppressed in response to CRP with 0.5 μg/mL ANGPTL2 (Figure 6B). ANGPTL2 significantly promoted the phosphorylation levels of Shp2 Y580 in the preincubation and early stages (30 and 60 seconds) but not in the late stages (90 seconds and 3 minutes) of human platelets in response to CRP (Figure 6C). The spreading of human platelets on immobilized Fg was dose-dependently inhibited by ANGPTL2 (Figure 6D). In contrast to the enhancement of Shp2 Y580 phosphorylation, spreading-driven phosphorylation of FAK Y397 and integrin β3 Y759 was significantly inhibited by ANGPTL2 (Figure 6E). These results clearly indicate that ANGPTL2 is one of the natural inhibitors of platelet activation that works by suppressing the tyrosine phosphorylation of multiple signaling molecules.
Purified ANGPTL2 inhibits human platelet activation. (A) The inhibitory effects of 0.5 μg/mL ANGPTL2 (A2) on human platelet aggregation in response to 0.5 μg/mL CRP, 20 μM ADP, and 0.01 U/mL thrombin, respectively. (B) The tyrosine phosphorylation time courses of LAT, SLP76, and PLCγ2 in washed human platelets in response to 0.5 μg/mL CRP with PBS control or 0.5 μg/mL ANGPTL2. IgG was used as a loading control. (C) The phosphorylation time courses of Shp2 Y580 in washed human platelets in response to 0.5 μg/mL CRP with PBS control or 0.5 μg/mL ANGPTL2. GAPDH was used to verify equal loading. (D) Representative phalloidin-stained images of washed human platelets spreading on immobilized Fg for 90 minutes in the presence of PBS, 0.5 μg/mL ANGPTL2, or 1 μg/mL ANGPTL2, respectively. Quantification of the areas (pixel number) of 4 random fields per experiment and ≥3 independent experiments were performed. Statistical analyses were performed using the Student t test (mean ± SD; **P < .01). (E) Phosphorylation of FAK Y397, integrin β3 Y747, Y759, and Shp2 Y580 in human platelets spread on Fg with or without 0.5 μg/mL ANGPTL2. GAPDH was used to verify equal loading.
Purified ANGPTL2 inhibits human platelet activation. (A) The inhibitory effects of 0.5 μg/mL ANGPTL2 (A2) on human platelet aggregation in response to 0.5 μg/mL CRP, 20 μM ADP, and 0.01 U/mL thrombin, respectively. (B) The tyrosine phosphorylation time courses of LAT, SLP76, and PLCγ2 in washed human platelets in response to 0.5 μg/mL CRP with PBS control or 0.5 μg/mL ANGPTL2. IgG was used as a loading control. (C) The phosphorylation time courses of Shp2 Y580 in washed human platelets in response to 0.5 μg/mL CRP with PBS control or 0.5 μg/mL ANGPTL2. GAPDH was used to verify equal loading. (D) Representative phalloidin-stained images of washed human platelets spreading on immobilized Fg for 90 minutes in the presence of PBS, 0.5 μg/mL ANGPTL2, or 1 μg/mL ANGPTL2, respectively. Quantification of the areas (pixel number) of 4 random fields per experiment and ≥3 independent experiments were performed. Statistical analyses were performed using the Student t test (mean ± SD; **P < .01). (E) Phosphorylation of FAK Y397, integrin β3 Y747, Y759, and Shp2 Y580 in human platelets spread on Fg with or without 0.5 μg/mL ANGPTL2. GAPDH was used to verify equal loading.
ANGPTL2 inhibited CRP-induced mouse platelet activation
The effects of ANGPTL2 on CRP-induced WT and PIRB-TM mouse platelet activation were also investigated. Aggregation of WT mouse platelets in response to CRP was dose-dependently inhibited by ANGPTL2, and the inhibitory effects of ANGPTL2 on aggregation were partially neutralized in PIRB-TM platelets (Figure 7A).
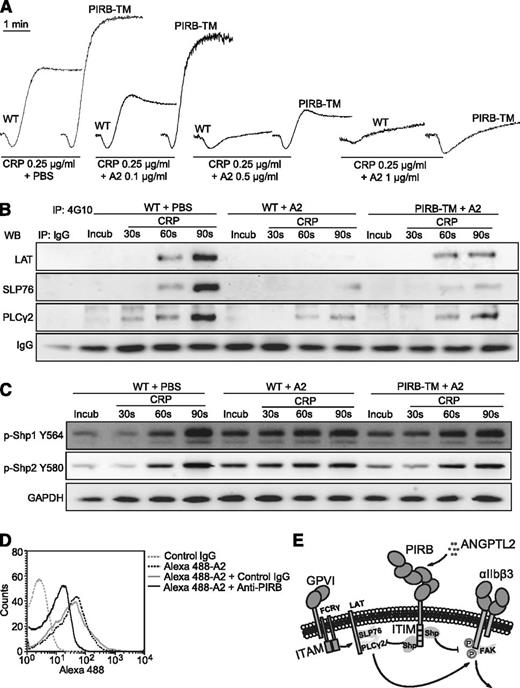
PIRB partially mediates ANGPTL2 elicited inhibition of CRP-induced activation of mouse platelets. (A) Aggregation of washed WT and PIRB-TM platelets in response to 0.25 μg/mL CRP with 0.1, 0.5, or 1 μg/mL ANGPTL2, respectively. (B) The tyrosine phosphorylation time courses of LAT, SLP76, and PLCγ2 in washed WT and PIRB-TM platelets preincubated with 0.5 μg/mL ANGPTL2 in the absence or presence of 0.25 μg/mL CRP. (C) Time course phosphorylation of Shp1 Y564 and Shp2 Y580 in washed WT and PIRB-TM platelets preincubated with 0.5 μg/mL ANGPTL2 in the absence or presence of 0.25 μg/mL CRP. GAPDH was used to verify equal loading. (D) The effects of PerCP-conjugated rat anti-mouse PIRB monoclonal antibody on Alexa 488-conjugated Flag-ANGPTL2 binding to WT mouse platelets. Rat IgG was used as a negative control. (E) A diagram of a plausible explanation for the inhibitory effects of PIRB/ANGPTL2 on platelet activation.
PIRB partially mediates ANGPTL2 elicited inhibition of CRP-induced activation of mouse platelets. (A) Aggregation of washed WT and PIRB-TM platelets in response to 0.25 μg/mL CRP with 0.1, 0.5, or 1 μg/mL ANGPTL2, respectively. (B) The tyrosine phosphorylation time courses of LAT, SLP76, and PLCγ2 in washed WT and PIRB-TM platelets preincubated with 0.5 μg/mL ANGPTL2 in the absence or presence of 0.25 μg/mL CRP. (C) Time course phosphorylation of Shp1 Y564 and Shp2 Y580 in washed WT and PIRB-TM platelets preincubated with 0.5 μg/mL ANGPTL2 in the absence or presence of 0.25 μg/mL CRP. GAPDH was used to verify equal loading. (D) The effects of PerCP-conjugated rat anti-mouse PIRB monoclonal antibody on Alexa 488-conjugated Flag-ANGPTL2 binding to WT mouse platelets. Rat IgG was used as a negative control. (E) A diagram of a plausible explanation for the inhibitory effects of PIRB/ANGPTL2 on platelet activation.
The phosphorylation of LAT, SLP76, and PLCγ2 was significantly suppressed in WT mouse platelets in response to CRP with ANGPTL2 (Figure 7B). The PIRB mutation was able to partially overcome the inhibitory effects of ANGPTL2 on the phosphorylation of LAT, SLP76, and PLCγ2 (Figure 7B). ANGPTL2 apparently promoted the phosphorylation levels of Shp1 Y564 and Shp2 Y580 in the preincubation and early stages (30 seconds) but not the late stage (90 seconds) of WT mouse platelets stimulated with CRP (Figure 7C). The PIRB mutation significantly suppressed ANGPTL2-facilitated early phosphorylation of Shp1 Y564 and Shp2 Y580 in mouse platelets in response to CRP (Figure 7C). Consistently, anti-PIRB antibodies were able to partially block ANGPTL2 binding to mouse platelets (Figure 7D). These results suggest that ANGPTL2-elicited inhibition of CRP-induced mouse platelet activation is mediated only in part through PIRB.
Discussion
The role of PIRB in platelet activation was investigated in this study. Deletion of PIRB intracellular domains obviously improved CRP-induced platelet aggregation in vitro. Further results demonstrated that Fg binding was greatly upregulated in PIRB-TM platelets in response to CRP, implying that PIRB negatively regulates GPVI-mediated signaling to activate integrin αIIbβ3. The tyrosine phosphorylation levels of LAT, SLP76, and PLCγ2 were significantly elevated in PIRB-TM platelets stimulated with CRP, further supporting that PIRB is the key negative regulator of GPVI-mediated platelet activation by suppressing the GPVI downstream activation signaling.
PIRB has been reported to associate with the tyrosine phosphatases Shp1 and Shp2. Here, we found that Shp1 and Shp2 were able to associate with PIRB in resting WT platelets, and Shp2, but not Shp1, gradually dissociated with PIRB in WT platelets on CRP stimulation. Alexandra et al recently showed that deletion of Shp1 in mice resulted in platelets being less responsive to CRP by reducing both GPVI expression and signaling via the Src-Syk-PLCγ2 pathway.37 On the contrary, deletion of Shp2 resulted in platelets being hyper-responsive to agonists.37 Although the phosphorylation of Shp1 Y564 and Shp2 Y580 was suppressed in PIRB-TM platelets in response to CRP, the distinct roles of Shp1 and Shp2 in platelet activation suggest that PIRB negatively regulates platelet activation, probably by enhancing the phosphatase activities of Shp2 instead of Shp1. However, further investigation will be needed to clarify this.
Bender et al recently reported the potential synergistic effects for GPVI and the ITAM-bearing C-type lectin-like receptor 2 (Clec-2) on human or mouse platelet reactivity and normal hemostasis.38 The role of PIRB on Clec-2–mediated platelet activation was preliminarily investigated in our study (see supplementary Figure 1, available on the Blood Web site). PIRB mutation also significantly enhanced low-concentration rat anti-mouse Clec-2 antibody–induced platelet aggregation, suggesting that PIRB may also negatively regulate Clec-2–mediated platelet activation. These in vitro results indicate that PIRB is presumably involved in thrombosis and hemostasis in vivo. Future experiments will still be required to elucidate the details of PIRB regulation of Clec-2–mediated platelet activation and to develop platelet-specific PIRB deletion mice to reveal the role of PIRB in thrombus formation.
Deletion of PIRB intracellular domains significantly facilitated platelet spreading on immobilized Fg and accelerated clot retraction, thereby indicating that PIRB also negatively affects αIIbβ3-mediated outside-in signaling. It is well known that platelet adhesion to immobilized Fg induces αIIbβ3 outside-in signaling and the phosphorylation of β3 at Y747 and Y759,39 which regulates the binding of talin, kindlins, and Src to β3 cytoplasmic tail and the cytoskeleton reorganization, resulting in platelet spreading and retraction.40 In our study, we found that the phosphorylation levels of β3 Y759, but not β3 Y747, were increased in PIRB-TM platelet spreading on Fg. Conversely, the phosphorylation levels of Shp1 Y564 and Shp2 Y580 and the cleavage of β3 at Y759 were significantly suppressed in PIRB-TM platelet spreading on Fg. These results suggested that PIRB negatively regulates integrin αIIbβ3-mediated outside-in signaling, probably via suppressing the phosphorylation of β3 Y759 and FAK Y397 and promoting the cleavage of β3 Y759.
ANGPTL2 was shown to be a ligand of PIRB and LILRB2. Here, we found that ANGPTL2 is expressed in both human and mouse platelets and is localized in platelet α-granule. Purified ANGPTL2 apparently inhibited agonist-induced human and mouse platelet aggregation and spreading. Interestingly, we found the binding of ANGPTL2 to human (or mouse) platelets partially depends on LILRB2 (or PIRB). ANGPTL2 was also probably able to bind to other inhibitory receptors such as LILRB3 and platelet endothelial cell adhesion molecule-1 in human platelets (supplementary Figure 1). The PIRB mutation significantly suppressed ANGPTL2-facilitated Shp1/2 phosphorylation and partially reversed the inhibitory effects of ANGPTL2 on CRP-induced phosphorylation of LAT, SLP-76, and PLCγ2, suggesting that ANGPTL2 inhibits platelet activation at least partially through the PIRB-dependent pathway.
In summary, the data presented here demonstrate the previously undocumented and critical role of PIRB in platelet activation in vitro. PIRB apparently attenuates platelet activation by suppression of both GPVI and integrin αIIbβ3-mediated outside-in signaling (Figure 7E). ANGPTL2, through one of its receptors, PIRB, exerts an inhibitory effect on platelet activation. The results presented here provide preliminary data demonstrating that LILRB2 and ANGPTL2 are promising antiplatelet targets.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Program of National Natural Science Foundation of China grants 81170479 and 81030039 (J.L.), National Key Basic Research Program of China grant 2012CB518000, the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, Shanghai Municipal Education Commission and Shanghai Education Development Foundation grant 10SG21, Pujiang Program grant 13PJ1405600, the 1000-Youth Elite Program, and National Institutes of Health, National Cancer Institute grant 1R01CA172268.
Authorship
Contribution: X.F., J. Zheng, and J.L. designed the experiments, analyzed data, and wrote the paper; X.F., P.S., J.D., Y.L., X.C., X.L., K.Z., X.W., Y.S., and K.W. performed the experiments; and L.Z., C.C.Z., J. Zhang, and G.-q.C. helped with the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junke Zheng, 280 South Chongqing Rd, Shanghai 200025, China; e-mail: zhengjunke@shsmu.edu.cn; or Junling Liu, 280 South Chongqing Rd, Shanghai 200025, China; e-mail: liujl@shsmu.edu.cn.

![Figure 1. Mutation of PIRB causes mild thrombocythemia. (A) The expression of LILRB2 in human peripheral leukocytes (H-Leu) and human platelets (H-Plt). (B) The expression of PIRB in mouse peripheral leukocytes (M-Leu) and mouse platelets (M-Plt). (C) The expression of PIRB in WT mouse leukocytes (M-Leu) and platelets (M-Plt). Washed WT or PIRB-TM platelets at a concentration of 3 × 107/mL were incubated with PerCP-conjugated rat anti-mouse PIRB monoclonal antibody or PerCP-conjugated rat IgG control at 25°C for 30 minutes. The expression of PIRB on WT and PIRB-TM platelets was analyzed using flow cytometer. (D) The platelet counts in the peripheral blood of WT and PIRB-TM mice (WT, n = 10; PIRB-TM, n = 10; mean ± standard error of the mean [SEM]; **P < .01). (E) The percentage of CD41b-positive cells in WT and PIRB-TM mice bone marrow (BM) (WT, n = 5; PIRB-TM, n = 5; mean ± SEM; **P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/15/10.1182_blood-2014-03-557645/4/m_2421f1.jpeg?Expires=1767807205&Signature=PM4eClSizyU6rkoOZpkun-A7vGgF7S638rCLGOHkWUi5uFJhk6Uafij9ZgPB2AgyTiKJkGfNC~lOsmBWYYpWR6jyINlnUSgYZGPUPbYflVM7BpH4Dwx8hMWDmpv7ihPnKIH2idf6D97ZVo2T1Pko65mEEk5PQ8styQK6OVE7tX1Mpu0oXQgbE5IyyGlDeT9JEyAy3wRPRf4UpALmKPRwjcA4HhEi1xnx7ps6KSMc3h9eBGzIsyhjXHgAKkWADBG4du2IIeZ~ddLpOBjRzkQ2fFrewSpFlLtVrU2UnDtkhr~IpR6x5C84kQbTuLfOUfBWNWD4JwjknScglc8JLvddGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. PIRB regulates integrin αIIbβ3-mediated outside-in signaling. (A) Representative phalloidin-stained images of washed WT and PIRB-TM platelets spreading on immobilized Fg for 30, 60, and 90 minutes, respectively. (B) Quantification of the areas (pixel number) of 4 random fields per experiment, and ≥3 independent experiments were performed. Statistical analyses were performed using the Student t test (mean ± standard deviation [SD]; *P < .05; **P < .01). (C) Washed WT or PIRB-TM mouse platelets spread on 40 µg/mL Fg for 90 minutes. The adherent platelets were solubilized and analyzed by western blotting for detection of the phosphorylation of FAK Y397, integrin β3 Y747 and Y759, and Shp1 Y564 and Shp2 Y580 and cleavage of integrin β3 Y759. GAPDH was used to verify equal loading. (D) Densitometry measurements from results in C. Values were normalized with respect to WT spreading for each immunoblot and are expressed as relative levels to spreading WT platelets. Statistical significance was determined using the Student t test. (n = 3, mean ± SD; **P < .01). (E) The expression levels of β3 and FAK in WT and PIRB-TM platelets. GAPDH was used to verify equal loading. (F) Clot retraction of PRP containing WT and PIRB-TM platelets in the presence of 0.5 U/mL thrombin. (G) Two-dimensional retraction of clots was measured, and the data were expressed as retraction ratios. Statistical significance was calculated using the Student t test (n = 5, mean ± SD; **P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/15/10.1182_blood-2014-03-557645/4/m_2421f4.jpeg?Expires=1767807205&Signature=2BSgPlJz4-nOUAn102uzGo1nronym8-WFNtm5DvsnUGb0yBUpW89eDOEVgKCdg0PNTLPJQmVq87ISGQ4oiCtOHiV3x9y6Qisg7wUDE~9z8uqCYiklbBz-iDarWcN7q1Hp~EtB8yHJlIZUcrHdHwHr6jFpRp-ULEPuWc5GD3JVC7Z9AfSiIm6w1jgZc-Vdwj9ZGCXI16CoilYv760kwAePa3BZXjW4mg4DqNA4s8nuryvvkmQnEH0wzjyAK9IW1W8GK~ggb7oDRN9ru3N-hWK-7Mc22dpvD-tL0f9YmECJew4z1C~Sj3mtWMMTkJ6YNhCp-o8YWTZBTVfSU0iSf5-Og__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal