To the editor:
Approximately 90% of patients with primary myelofibrosis (PMF) harbor JAK2 (58%), CALR (25%), or MPL (7%) mutations.1-3 CALR-mutated PMF patients are younger than their JAK2-mutated counterparts, and they display higher platelet count, lower leukocyte count, higher hemoglobin level, lower incidence of spliceosome mutations, and longer survival.3 More than 80% of CALR mutations constitute 1 of 2 variants: type 1, a 52-bp deletion (p.L367fs*46), or type 2, a 5-bp TTGTC insertion (p.K385fs*47).1 Type 2, as opposed to type 1 CALR variant has been associated with higher platelet count in essential thrombocythemia4 and shorter survival in PMF.5 Furthermore, data are emerging that suggest functionally relevant structural differences between type 1 and type 2 CALR variants, including a higher α-helix content of the mutant C terminus in type 2, which is similar to what is seen with wild-type CALR, compared with type 1, which is significantly lower.6
In the current study, we used statistical models7 to calculate helix propensity for 31 unique amino acid sequences that were altered by CALR mutations, and we used the results to subclassify non-type 1/2 CALR mutations into “type 1-like” and “type 2-like” variants. Calculation of helix propensity was performed using AGADIR,7 which is a statistical approximation algorithm. Survival was calculated from the date of referral to date of death or last contact. Blast transformation (BT) replaced death as the uncensored variable to estimate leukemia-free survival. Cox proportional hazard regression model was used for multivariable analysis.
A total of 532 PMF patients were screened for JAK2, CALR, and MPL mutations; the respective mutational frequencies were 58%, 24.6%, and 7.3%. Among the 131 CALR-mutated cases, 98 (74.8%) harbored type 1, 15 (11.5%) type 2, and 18 (13.7%) other variants. Based on predicted helix propensity scale (supplemental Table 1, available on the Blood Web site), the “other” CALR mutations were subclassified as type 1-like (n = 12) or type 2-like (n = 6). The AGADIR-derived predicted helix propensity scale was 29.69 for wild-type CALR and 8.6 or 34.17 for type 1 and type 2 mutant CALR, respectively; accordingly, CALR variants with values close to or above the value for wild-type CALR were classified as type 2-like (range 26.47-36.12), and those with values close to or below the value for type 1 were classified as type 1-like (range 2.11-17.3).
A comparison of type 1/type 1-like (n = 110) and type 2/type 2-like (n = 21) CALR mutations showed the latter to be associated with higher dynamic international prognostic scoring system (DIPSS)-plus8 score (P = .01), EZH2 mutations (P = .0005), leukocyte count >25 × 109/L (P = .0003), higher circulating blast percentage (P = .02), and palpable spleen size >10 cm (P = .008) (supplemental Table 2). Comparison of type 1/type 1-like CALR and JAK2 mutations (n = 309) showed the former to be associated with younger age, higher platelet count, higher hemoglobin level, lower leukocyte count, and lower DIPSS-plus score (P < .01 for all comparisons; supplemental Table 2). None of these associations was evident during comparison of type 2/type 2-like CALR with JAK2 mutations.
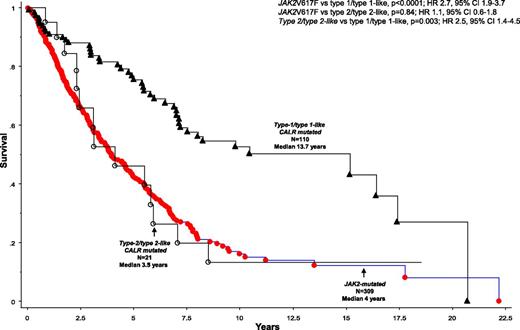
Median follow-up for the core group of 532 PMF patients was 3.4 years; during this period, 327 (61.5%) deaths and 54 BTs (10.2%) were recorded. Survival was similar between patients with type 1 and type 1-like (P = .8) and between type 2 and type 2-like (P = .63) CALR mutations; in contrast, survival was significantly shorter in patients with type 2 (HR 2.4, 95% CI 1.2-4.8) and type 2-like (HR 3.2, 95% CI 1.0-10.6), when compared with those with type 1 CALR mutations. Similarly, survival was shorter in patients with type 2/type 2-like vs type 1/type 1-like CALR mutations (P = .003; HR 2.5, 95% CI 1.4-4.5) (Figure 1), and the difference remained significant when analysis was adjusted for age (P = .047), ASXL1 (P = .003), or EZH2 (P = .001) mutations. Compared with JAK2-mutated cases (n = 309), survival was longer in patients with type 1/type 1-like (HR 0.4, 95% CI 0.3-0.5) but not in those with type 2/type 2-like (HR 0.9, 95% CI 0.5-1.6) CALR mutations (Figure 1); the difference in survival between JAK2 and type 1/type 1-like CALR-mutated cases remained significant (P < .01) when analysis was adjusted for age, ASXL1 or EZH2 mutations, or DIPSS-plus score.
Survival data on 440 patients with PMF stratified by their JAK2 and CALR mutational status. Patients with CALR mutations were further substratified into type 1/type 1-like and type 2/type 2-like, based on the helical propensity of their mutant CALR C terminus. Among these 440 patients, 264 (60%) deaths (205 JAK2, 45 type 1/type 1-like and 14 type 2/type 2-like CALR mutated) and 40 BTs (27 JAK2, 10 type 1/type 1-like and 3 type 2/type 2-like CALR-mutated) were documented.
Survival data on 440 patients with PMF stratified by their JAK2 and CALR mutational status. Patients with CALR mutations were further substratified into type 1/type 1-like and type 2/type 2-like, based on the helical propensity of their mutant CALR C terminus. Among these 440 patients, 264 (60%) deaths (205 JAK2, 45 type 1/type 1-like and 14 type 2/type 2-like CALR mutated) and 40 BTs (27 JAK2, 10 type 1/type 1-like and 3 type 2/type 2-like CALR-mutated) were documented.
Additional studies are needed to confirm or further refine our operational classification of CALR mutations into 2 prognostically distinct subgroups. In the meantime, our observations highlight the importance of clarifying the specific types of CALR mutations when discussing prognostic implication, especially with patients.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by the Mayo Clinic Harvey-Yulman Charitable Foundation for Myelofibrosis Tissue Bank and Clinical Database of Molecular and Biological Abnormalities.
Contribution: A. Tefferi designed the study, contributed patients, extracted data, performed statistical analysis, wrote the paper, and approved the final draft of the manuscript; A.D.P. contributed patients, provided bioinformatics analysis support, extracted data, and approved the final draft of the manuscript; T.L.L. performed mutation analysis, provided bioinformatics analysis support, and approved final draft of manuscript; A. Tischer provided bioinformatics analysis support; C.M.F., E.A.W., and A.A.B. performed mutation analysis, extracted data, and approved the final draft of manuscript; R.P.K. provided resources for cytogenetic analysis and expertise in result interpretation; and C.A.H. reviewed bone marrow pathology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayalew Tefferi, Division of Hematology, Department of Medicine, Mayo Clinic, Rochester, MN 55905; e-mail: tefferi.ayalew@mayo.edu.