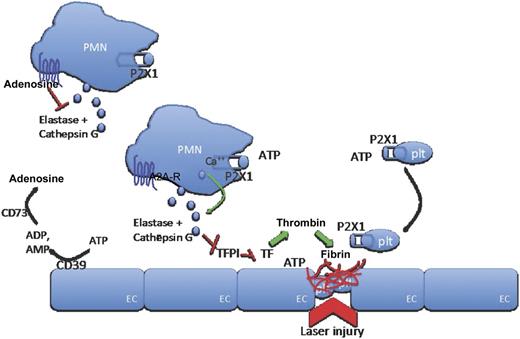
In this issue of Blood, Darbousset et al define opposing roles for adenosine triphosphate (ATP) and adenosine in regulating polymorphonuclear neutrophil (PMN) activation, fibrin formation, and thrombus growth following vascular injury.1
ATP, released at sites of vascular injury, interacts with P2X1 cation channels on PMNs and platelets to induce release of neutrophil elastase and cathepsin G and recruit platelets to the site of injury. Locally released elastase and cathepsin G inhibit TFPI, thereby releasing its inhibitory effect on locally activated tissue factor to allow coagulation generation of the fibrin clot to proceed. With increasing distance to the site of injury, ATP is hydrolyzed by ectonucleotidases (CD39) to ADP and AMP and further metabolized to adenosine. Adenosine inhibits PMN activation (and also elastase release) through interaction with A2A adenosine receptors on PMN surfaces to restrict PMN activation, and therefore coagulation, to the site of injury. A2A-R, adenosine receptor; EC, endothelial cell; plt, platelet.
ATP, released at sites of vascular injury, interacts with P2X1 cation channels on PMNs and platelets to induce release of neutrophil elastase and cathepsin G and recruit platelets to the site of injury. Locally released elastase and cathepsin G inhibit TFPI, thereby releasing its inhibitory effect on locally activated tissue factor to allow coagulation generation of the fibrin clot to proceed. With increasing distance to the site of injury, ATP is hydrolyzed by ectonucleotidases (CD39) to ADP and AMP and further metabolized to adenosine. Adenosine inhibits PMN activation (and also elastase release) through interaction with A2A adenosine receptors on PMN surfaces to restrict PMN activation, and therefore coagulation, to the site of injury. A2A-R, adenosine receptor; EC, endothelial cell; plt, platelet.
It has previously been appreciated that PMNs accumulate rapidly at sites of endothelial injury2 and that their arrival aids generation of the fibrin clot by (1) expression of tissue factor (TF)2 and (2) inhibition of TF pathway inhibitor (TFPI).3 However, how PMNs become activated to provide these clot-generating signals and how these responses integrate with those of platelets, long thought to be the first responders to vascular injury, remained to be elucidated. In this work, the authors demonstrate a new role in thrombus formation for the ATP-responsive Ca2+ channel, P2X1, on neutrophils and platelets.
ATP, widely recognized as an intracellular energy source, is also an important extracellular signaling molecule. It is released from the dense granules of activated platelets following their contact with matrix components lining injured vascular endothelium,4 but it is also released by neutrophils5 and endothelial cells.6 The interaction of ATP with its sole receptor on platelets, P2X1, is known to be important for thrombus growth, because P2X1−/− mice have a clear defect in thrombus formation after vascular injury.7 However, whether the loss of P2X1 on neutrophils, in addition to platelets, contributed to this defect was not appreciated, because the expression of P2X1 on neutrophils has only recently been accepted.8 Darbousset et al show that injection of wild-type (WT) neutrophils into P2X1−/− mice is sufficient to partially reconstitute fibrin generation after vascular laser injury, suggesting that P2X1-dependent activation of neutrophils plays an important role in the formation of the fibrin clot.1 This is a newly appreciated mechanism for ATP in the regulation of fibrin formation, as P2X7 expression on myeloid cells was previously shown to be required for the decryption of TF and release of TF+ microparticles leading to thrombin generation.9 Conversely, injection of WT neutrophils into injured P2X1−/− mice was not sufficient to reconstitute platelet accumulation into the growing thrombus; rather, P2X1 expression on both platelets and neutrophils is required for full platelet- and fibrin-rich hemostatic plug formation.
Although release of circulating nucleotides ATP and adenosine 5′-diphosphate (ADP; an important platelet agonist) positively regulates generation of the hemostatic plug close to the site of injury, an extra level of regulation is achieved by the metabolism of these extracellular nucleotides with increasing distance from the injured site. Endothelial and plasma ectonucleotidases, chiefly CD39 and CD73, hydrolyze ATP and ADP sequentially to adenosine 5′-monophosphate (AMP) and adenosine, respectively. In contrast to the neutrophil-activating properties of ATP, this study demonstrates that a selective agonist for the A2A adenosine receptor on neutrophils inhibits both neutrophil accumulation at the site of injury and elastase release from the activated neutrophils. This suggests that complete hydrolysis of ATP and ADP through AMP to adenosine may constrain fibrin deposition close to the site of injury by activating neutrophil A2A receptors as concentrations of adenosine (relative to ATP) rise further from the injury site, thereby inhibiting neutrophil elastase release and consequent inhibition of TFPI. Whether in vivo levels of adenosine are consistent with this model is at present unclear; nevertheless, such a mechanism would be consistent with studies that have shown enhanced fibrin deposition in CD39−/− mice.10
In summary, this study demonstrates a novel role for the ionotropic ATP receptor P2X1 on neutrophils in the generation of fibrin at sites of vascular injury through the release of neutrophil elastase (to inactivate TFPI) and confirms a requirement for neutrophil P2X1 to work together with P2X1 on platelets to generate the hemostatic plug (see figure). Because nucleotide:nucleoside ratios are important for regulating both thrombotic and inflammatory events, P2X1 may be an attractive target for the development of antagonists to limit thrombo-inflammatory disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.