In this issue of Blood, Manchev et al describe a consanguineous family with severe macrothrombocytopenia and bleeding symptoms where exome sequencing revealed a homozygous missense mutation in the PRKACG gene (p.74Ile>Met) encoding the γ-catalytic subunit of cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA).1
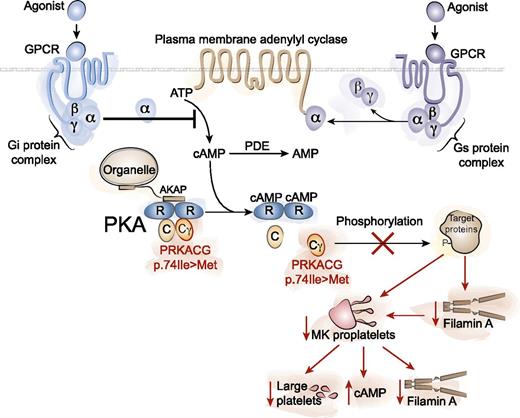
PKA is a tetrameric holoenzyme consisting of 2 regulatory subunits (R) and 2 catalytic subunits (C). Binding of cAMP to the regulatory subunits activates PKA by displacing the catalytic subunits, allowing target protein phosphorylation. PKAs are localized to specific intracellular regions by A kinase anchor proteins (AKAPs). G protein-coupled receptors (GPCRs) can stimulate or inhibit cAMP production via Gs (stimulatory) and Gi (inhibitory) α subunits of heterotrimeric G proteins coupled to adenylyl cyclases. Phosphodiesterases degrade cAMP to AMP. A homozygous missense mutation in the PRKACG gene (p.74Ile>Met) encoding the γ-catalytic subunit of PKA causes inherited macrothrombocytopenia. The inability of the defective PKA to phosphorylate target proteins results in decreased filamin A (FLNa) in MK and platelets, decreased MK proplatelet formation, increased platelet cAMP, and abnormal platelet function. Illustration by Yuriy Baglaenko, University of Toronto.
PKA is a tetrameric holoenzyme consisting of 2 regulatory subunits (R) and 2 catalytic subunits (C). Binding of cAMP to the regulatory subunits activates PKA by displacing the catalytic subunits, allowing target protein phosphorylation. PKAs are localized to specific intracellular regions by A kinase anchor proteins (AKAPs). G protein-coupled receptors (GPCRs) can stimulate or inhibit cAMP production via Gs (stimulatory) and Gi (inhibitory) α subunits of heterotrimeric G proteins coupled to adenylyl cyclases. Phosphodiesterases degrade cAMP to AMP. A homozygous missense mutation in the PRKACG gene (p.74Ile>Met) encoding the γ-catalytic subunit of PKA causes inherited macrothrombocytopenia. The inability of the defective PKA to phosphorylate target proteins results in decreased filamin A (FLNa) in MK and platelets, decreased MK proplatelet formation, increased platelet cAMP, and abnormal platelet function. Illustration by Yuriy Baglaenko, University of Toronto.
Inherited platelet disorders contribute significantly to bleeding problems. Glanzmann thrombasthenia patients with platelets deficient in fibrinogen receptor αIIβ3 integrin and Bernard-Soulier syndrome (BSS) patients with abnormalities of the von Willebrand factor (VWF) receptor complex GPIb-IX-V are readily diagnosed via abnormal platelet aggregation profiles and severe bleeding symptoms. However, subtler congenital platelet disorders often require specialized diagnosis. Recent advances in high-throughput DNA/RNA sequencing have facilitated the identification of several defects,2,3 but a clinically useful initial diagnostic approach is to look for thrombocytopenia and abnormalities in platelet size.2 Conditions associated with macrothrombocytopenia include BSS, MYH9-related disease, Gray platelet syndrome, Paris-Trousseau/Jacobsen syndrome, platelet-type von Willebrand disease, GATA1-related disease, GFI1B-related thrombocytopenia, ITGA2B/ITGB3-related thrombocytopenia, thrombocytopenia associated with sitosterolemia, FLNa-related thrombocytopenia, ACTN1-related thrombocytopenia, and TUBB1-related thrombocytopenia.4 Manchev et al now add another to this list: PRKACG-related macrothrombocytopenia.
They describe 2 siblings from consanguineous parents with a lifelong bleeding diathesis consisting of epistaxis, hematomas, menorrhagia, and bleeding with ruptured ovarian cysts. Platelet counts were 5 to 8 × 109/L, and blood films and electron microscopy revealed 90% of platelets large to giant. Abnormal platelet function was demonstrated by decreased GPIb-IX-V internalization, complete lack of P-selectin expression on platelet activation, decreased thrombin-induced Ca2+ mobilization, and a lower F-actin/G-actin ratio when spread on VWF.
Whole-exome sequencing resulted in identification of 2 homozygous missense mutations predicted to be pathogenic. One was in the gene GNE (p.559Gly>Arg) encoding a bifunctional enzyme involved in the biosynthesis of N-acetylneuramic acid, where mutations result in myopathy or sialuria but not thrombocytopenia—phenotypes not observed in the probands. The other homozygous missense mutation (c.222G>A; p.741Ile>Met) was in the PRKACG gene encoding the γ-catalytic subunit (Cγ; γ isoform) of PKA.
Megakaryopoiesis and platelet production was investigated by examining patient bone marrow, which revealed unusual megakaryocyte (MK) clusters, whereas differentiation from CD43+CD42+ cells in vitro showed normal MK development and ploidy. An important clue came when the investigators enumerated MKs elaborating 1 or more proplatelets at day 13 of culture, where the frequency of proplatelet-bearing MKs was 2.5-fold lower in patients compared with controls. To confirm that mutations in PRKACG are causative of decreased platelet production, they overexpressed wild-type (WT) PRKACG and mutant PRKACG (p.74Ile>Met) in patient CD34+ cells transduced with lentiviral constructs. Overexpression of WT PRKACG, but not mutant PRKACG, significantly increased proplatelet production and decreased the diameter of platelet-like cell bodies along proplatelets. This rescue confirmed that mutated γ-catalytic subunit of PKA contributes to the observed macrothrombocytopenia.
PKA holoenzyme complexes consist of 2 regulatory and 2 catalytic subunits specified by 4 regulatory subunit isoforms (RIα, RIβ, RIIα, RIIβ) and 3 catalytic subunit isoforms (Cα, Cβ, Cγ) (see figure).5 cAMP binding to the regulatory subunits causes catalytic subunit dissociation and target protein phosphorylation. In platelets, G protein-coupled receptors regulate intracellular cAMP levels via Gs and Gi α subunits of heterotrimeric G proteins coupled to adenylyl cyclases, to stimulate or inhibit cAMP production, respectively (see figure). PKA signaling specificity is not only determined by cell type-specific expression of the different regulatory and catalytic subunit isoforms but also by localization of PKAs (by A kinase anchor proteins) to receptors, ion channels, transporters, and organelles.5 Phosphodiesterases that degrade cAMP to AMP (see figure) are also localized to specific intracellular regions. In platelets, PKA and cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG) phosphorylate a broad range of target proteins (including FLNa and GPIbβ) resulting in the inactivation of Ras and Rho family G proteins, inhibition of Ca2+ release from intracellular stores, and alteration of actin cytoskeletal dynamics.6 To understand the basis of the defective PKA, the authors confirmed that the p.74Ile>Met mutation in PRKACG did not lead to proteolytic degradation of unstable protein in MKs and platelets. Interestingly, patient platelet cAMP levels were noted to be three- to fivefold higher compared with controls.1 Whether the increased patient platelet cAMP levels are due to an inactive PKA that fails to down-regulate cAMP or through another mechanism remains to be determined.
The PKA targets FLNa and GPIbβ, previously shown to cause macrothrombocytopenia,3 were further investigated. Total GPIbβ levels and phosphorylation at Ser166 on GPIbβ was unaltered in cultured patient MKs and platelets compared with controls, suggesting that GPIbβ is not involved. Because phosphorylation of FLNa at Ser2152 by PKA protects it from proteolysis,6 the authors assessed FLNa levels in patient MKs and platelets and observed greatly reduced levels compared with controls. These observations were consistent with findings in patients containing mutations in FLNa, demonstrating subpopulations of giant platelets and abnormalities in cultured patient MKs.7
Studies of mice lacking FLNa in the MK lineage suggest that defective platelet biogenesis and accelerated platelet clearance cause severe macrothrombocytopenia.8
As with all discoveries, many questions arise. FLNa-Ser2152 phosphorylation was not directly assessed, nor do we know anything about potential altered FLNa downstream signaling events such as Syk phosphorylation in response to platelet activation through the collagen receptor GPVI or C-type lectin-like receptor 2.9 Platelet FLNa has an important role in stabilizing the VWF receptor complex GPIb-IX-V by linking GPIbα to the actin cytoskeleton, so why are there increased GPIb-IX-V levels in patient resting platelets, whereas they were decreased in FLNa-null mouse platelets?8 The answer may lie in the requirement of a coordinated spatiotemporal expression of GPIbα and FLNa for normal intracellular protein trafficking and platelet biogenesis.10 Consequently FLNa-null MKs are fundamentally different from MKs that potentially degrade FLNa secondary to defective PKA. Is the observed platelet function defect in the patients due to their elevated platelet cAMP levels, known to be inhibitory, or due to altered receptor-cytoskeletal composition and dynamics on platelet stimulation? The finding that mutations in PRKACG cause bleeding and reduced platelet counts provides important insights into normal MK development and platelet function, as well as offering another target gene to interrogate when assessing patients with familial macrothrombocytopenia.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal