In this issue of Blood, Hahn and Lowrey demonstrate enhanced translation of γ-globin messenger RNA (mRNA) upon activation of eukaryotic initiation factor 2αP (eIF2αP) stress signaling. This finding underscores translational control as a novel mechanism in regulating fetal hemoglobin production (HbF).1
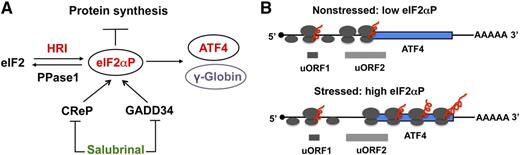
Selective enhancement of translation of activating transcription factor 4 (ATF4) and γ-globin mRNAs by eIF2αP in erythroid precursors. eIF2 is a heterotrimeric protein complex, which binds initiating methionyl transfer RNA and the 40S ribosomal subunit to start nascent polypeptide synthesis. HRI is the major eIF2α kinase in the erythroid lineage, and is indispensable in coordinating heme and globin synthesis as well as in combating oxidative stress. Phosphorylation of the α-subunit of eIF2 by HRI impairs the recycling of eIF2 for another round of initiation and thus inhibits translation of the vast majority of mRNAs. However, eIF2αP also selectively increases the translation of ATF4 mRNA. (A) In the 5′UTR of ATF4 mRNA, there are 2 uORFs that are preferentially translated under nonstressed conditions and prevent the downstream translational initiation in the coding sequence of ATF4 mRNA. As initiating 40S ribosomal subunits scan from the cap structure, translation starts at the uORF1. After termination of translation, the 40S subunit remains associated with mRNA and reinitiates efficiently at uORF2 under nonstressed conditions. Upon stress, elevated eIF2αP impairs the reinitiation of 40S at uORF2 due to limiting functional eIF2. Thus, 40S continues to scan downstream and initiates at the AUG codon of the coding sequence of ATF4 mRNA permitting the synthesis of ATF4 protein. (B) The homeostasis of the cellular eIF2αP level is controlled not only by the activation of HRI, but also by dephosphorylation of eIF2αP by PPase1 in order to regenerate active eIF2. CreP and GADD34 are the 2 regulatory proteins that recruit eIF2αP to PPase1 for dephosphorylation. Salubrinal, a small chemical molecule, is a selective inhibitor of dephosphorylation of eIF2αP by interfering with the recruitment of eIF2αP to PPase1. Thus, treatment of cells with salubrinal results in an increased eIF2αP level. In differentiating human erythroid cells, γ-globin translation is increased upon salubrinal treatment as shown in this Blood article.
Selective enhancement of translation of activating transcription factor 4 (ATF4) and γ-globin mRNAs by eIF2αP in erythroid precursors. eIF2 is a heterotrimeric protein complex, which binds initiating methionyl transfer RNA and the 40S ribosomal subunit to start nascent polypeptide synthesis. HRI is the major eIF2α kinase in the erythroid lineage, and is indispensable in coordinating heme and globin synthesis as well as in combating oxidative stress. Phosphorylation of the α-subunit of eIF2 by HRI impairs the recycling of eIF2 for another round of initiation and thus inhibits translation of the vast majority of mRNAs. However, eIF2αP also selectively increases the translation of ATF4 mRNA. (A) In the 5′UTR of ATF4 mRNA, there are 2 uORFs that are preferentially translated under nonstressed conditions and prevent the downstream translational initiation in the coding sequence of ATF4 mRNA. As initiating 40S ribosomal subunits scan from the cap structure, translation starts at the uORF1. After termination of translation, the 40S subunit remains associated with mRNA and reinitiates efficiently at uORF2 under nonstressed conditions. Upon stress, elevated eIF2αP impairs the reinitiation of 40S at uORF2 due to limiting functional eIF2. Thus, 40S continues to scan downstream and initiates at the AUG codon of the coding sequence of ATF4 mRNA permitting the synthesis of ATF4 protein. (B) The homeostasis of the cellular eIF2αP level is controlled not only by the activation of HRI, but also by dephosphorylation of eIF2αP by PPase1 in order to regenerate active eIF2. CreP and GADD34 are the 2 regulatory proteins that recruit eIF2αP to PPase1 for dephosphorylation. Salubrinal, a small chemical molecule, is a selective inhibitor of dephosphorylation of eIF2αP by interfering with the recruitment of eIF2αP to PPase1. Thus, treatment of cells with salubrinal results in an increased eIF2αP level. In differentiating human erythroid cells, γ-globin translation is increased upon salubrinal treatment as shown in this Blood article.
Persistent HbF expression is known to lessen the severity of β-thalassemia and sickle cell anemia (SCA),2,3 2 of the most prevalent β-hemoglobinopathies. Reactivation of HbF has since been an active area of research. These research efforts have led to the discovery of hydroxyurea (HU) as a successful treatment of some, but not all, patients with SCA and β-thalassemia.4 Recent discoveries of Bcl11A as a transcription repressor of HbF expression and a silencer of γ-globin expression during development5 have reinvigorated the field of globin switching and reactivation of HbF. To date, the majority of these studies have been focused on the transcriptional regulation with anticipation of discovering new therapeutic targets for treating β-thalassemia and SCA.6 Last year, Hahn and Lowrey reported that induction of γ-globin expression could also be achieved posttranscriptionally via phosphorylation of eIF2α.7 In the current article, they have extended this study further and demonstrated that the activation of fetal globin occurs at the level of translation.
During late-stage erythroid differentiation, heme-regulated eIF2α kinase (HRI) is predominant and responsible for >90% of eIF2α phosphorylation (see figure, panel A).8 The steady state of eIF2αP in vivo is regulated by the equilibrium of eIF2α kinases and eIF2αP phosphatase (PPase1). Salubrinal is a selective inhibitor of eIF2αP dephosphorylation by interfering with the recruitment of eIF2αP to PPase1 through growth arrest and DNA damage–inducible protein 34 (GADD34) and constitutive repressor of eIF2α phosphorylation (CReP). Hahn and Lowrey found that treatment of differentiating human CD34+ cells with salubrinal did not affect either stability or the cytoplasmic-to-nuclear ratio of γ-globin or β-globin mRNA. They further investigated the effect of salubrinal on translation of globin mRNAs by polysome profiling, which measures the loading of ribosomes onto specific mRNAs and thus the translational efficiency of each mRNA. At 24 hours of salubrinal treatment, eIF2αP returned to near basal level of untreated controls. During this time, it was observed that both γ-globin and β-globin mRNAs from salubrinal-treated cells were translated more efficiently relative to actin mRNA. This was revealed by the shift of globin mRNAs toward the heavier polyribosomes after salubrinal treatment. By comparing the ratios of heavy to light polysomes, γ-globin mRNA was determined to be translated approximately twice as efficiently as β-globin mRNA. They also demonstrated directly an increase in γ-globin protein synthesis in salubrinal-treated K562 cells by measuring puromycin-released nascent polypeptides that were reactive to anti-HbF antibody.
Weinberg et al have shown earlier that butyrate treatment of SCA patient blood samples increased efficiency of translation of γ-globin mRNA.9 The current study by Hahn and Lowrey demonstrated that increased translational of γ-globin mRNA is mediated by eIF2αP signaling. What might be the mechanism by which salubrinal and eIF2αP increase the translational efficiency of γ-globin mRNA? The one well-established cardinal feature for the selective upregulation of translation by eIF2αP is the presence of upstream open reading frames (uORFs) in the 5′ untranslated region (UTR) of unique classes of mRNAs such as ATF4 mRNA. Translation of ATF4 mRNA is selectively enhanced by activation of HRI-eIF2αP signaling in normal and β-thalassemic erythroid precursors.8,10 Under nonstressed conditions, these uORFs restrict the translation at the downstream-initiating AUG codon encoding ATF4 protein. Upon stress, phosphorylation of eIF2α reduces the pool of functional eIF2 and slows down the initiation to permit the translation start site at the coding sequence of ATF4 mRNA (see figure, panel B). It will be of great interest to determine whether uORFs are involved in the increased translational efficiency of γ-globin mRNA upon salubrinal treatment. Although there is no cognate AUG codon in the 5′UTR of annotated γ-globin mRNA, there are non-AUG initiation codons, which can generate uORFs. Alternatively, the downstream targets of eIF2αP signaling may participate in this event. It is also possible that a yet-to-be-discovered novel mechanism may regulate γ-globin mRNA translation.
By identifying eIF2αP and translation initiation as a mechanism for induction of HbF, the study of Hahn and Lowrey provides the impetus for more effective combinational therapy targeting both transcription and translation in treating β-hemoglobinopathies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal